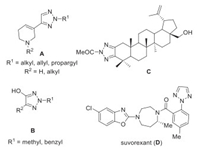
Among the numerous known heterocyclic systems, the 1, 2, 3-triazole ring is inarguably one of the most useful and successful ones which has exhibited ubiquitous and distinctive applications in a variety of scientific and industrial areas, including but not limited in drug discovery, chemical biology, science of functional materials as well as diversity & target oriented organic synthesis [1-4]. Accordingly, tremendous efforts have been afforded to the development of synthetic methodology towards 1, 2, 3-triazoles [5-8]. Representatively, the click alkyne azide cycloaddition [9-15], organocatalytic 1, 2, 3-triazole annulation [16, 17], nonmetal reagent-catalyzed 1, 2, 3-triazole annulation [18-20] and even azide-free 1, 2, 3-triazole annulation [21-24] have contributed significantly to the great success of 1, 2, 3-triazole chemistry. On the contrary, owing to the internally higher molecular stability, most of the reported methods on 1, 2, 3-triazoles provide either N1-substituted or N1-H 1, 2, 3-triazole as products. Comparing with the N1-1, 2, 3-triazole synthesis, equivalent synthetic methods toward N2-H or N2-substituted 1, 2, 3-triazoles are much less available [25-31]. Many N2-substituted 1, 2, 3-triazoles, however, have been proved to be highly valuable for their individual role as central backbone in biologically functional molecules. For example (Fig. 1), the N2-substituted 1, 2, 3-triazoles A are reported with muscarinic agonist activity wherein the N2-substitutent is found to be crucial [32]. The N2-substituted 4-hydroxyl 1, 2, 3-trialzoles B are bioisosteres of glutamic acid which exhibit promise in new drug design with enhanced binding affinity [33]. The N2-acetyl triazole of betulin (C) is a compound possessing potent cytotoxic activity to different cancer cell lines and low toxicity to normal cells [34]. Moreover, suvorexant (D) is a potent, brain penetrant dual orexin receptor antagonist with potential application in the treatment of primary insomnia (Fig. 1) [35]. Considering the high application space of N2-substituted 1, 2, 3-triazoles, developing and designing practical method for the synthesis of such compounds is thus an issue of high urgency.
|
Download:
|
| Fig. 1. Some valuable N2-substituted 1, 2, 3-triazole derivatives. | |
As easily accessible synthons with versatile versions of presence, enaminones and analogous enaminoesters have been identified with extraordinary broad application in the synthesis of numerous organic products in recent decade [36-44]. Specifically, employing these stable enamines as the donors of either C4-C5 or N3-C4-C5 fragment have successfully enabled the synthesis of 1, 2, 3-triazole with a broad array of structural features, including the 1, 5-disubstitued 1, 2, 3-triazoles [45, 46], 1, 4-disubstitued 1, 2, 3-triazoles [47, 48], free N1-H 1, 2, 3-triazoles [49, 50], and full substituted 1, 2, 3-triazoles [51-53]. However, such enamines have not previously been observed to be capable of participating the synthesis of N2-substituted or N2-H 1, 2, 3-triazoles [54]. During our recent efforts in developing novel and sustainable synthesis, we have disclosed that the NH- or NH2-functionalized enaminones/enaminoesters could be used for designing water-mediated green synthetic by making use of enamines' inherent hydrophilicity resulting from the hydrogen bond effect between enamines and water [55-58]. Herein, we report our work on the hydrophilic enamine-based reactions towards the synthesis of N2-subtituted 1, 2, 3-triazoles in water without using any metal reagent. In addition, using DMSO as medium has led to the selective synthesis of N2-H 1, 2, 3-triazoles via similar metal-free operation. Notably, this is the first example on the N2-sulfonyl 1, 2, 3-triazole synthesis via direct triazole annulation, and previous methods were based on the N-sulfonylation of prior prepared N-H-1, 2, 3-triazoles [59-61].
At the beginning, the reaction of enaminoester 1a and tosyl azide 2a were employed with t-BuONa, which gave rise to N2-tosyl 1, 2, 3-triazole 3a with 42% yield by heating at 40 ℃ in water medium (Table 1, entry 1). In a series of parallel entries employing different organic and inorganic base species, TMEDA exhibited among the best effect in promoting this reaction (entries 2–7). Notably, organic solvents such as MeCN, EtOH, toluene, and DCM all displayed poor applicability as medium for the reaction, disclosing the specific advantage of water in the present reaction (entries 8–11). Screening the reaction temperature indicated that enhancing the temperature to 60 ℃ was most favorable (entries 12–14). Later on, the loadings of 2a as well as TMEDA catalyst were varied, respectively. However, not evident improvement was observed (entries 15–17). Further increased yield of 3a was reached by prolonging the reaction time to 12 h (entry 18, Table 1).
|
|
Table 1 Optimization on reaction conditions.a |
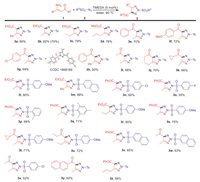
With the satisfactory result given by the optimized reaction conditions, the scope of the reaction in synthesizing N2-substituted 1, 2, 3-triazoles were then investigated. Based on the results afforded by the experiments, the present method for the synthesis of N2-sulfonyl 1, 2, 3-triazoles was generally applicable to the reactions of NH2-functionalied enamines and sulfonyl azides. First, for the enamine substrate, the enamines featured with ester (3a-3d, 3l-3m and 3r, Scheme 1), aryl ketone (3e-3h, 3n-3q, 3s, 3v and 3y-3z, Scheme 1) as well as alkyl ketone fragment (3i-3k, 3t-3u and 3w-3x, Scheme 1) all displayed smooth tolerance to the expected synthesis. On the other hand, besides tosyl azide, the sulfonyl azides functionalized with alkoxylphenyl (3l and 3r-3u, Scheme 1), halophenyl (3n, 3o and 3x, Scheme 1), alkylphenyl (3p-3q, Scheme 1) and naphthyl (3m and 3v-3w, Scheme 1) were also practical reactants for the titled synthesis. The electron withdrawing group functionalized phenyl sulfonyl azides gave lower yield of the target products (3o and 3x, Scheme 1). Using methyl sulfonyl azide to react with 1a, however, did not afford the product. Performing the reaction for 3b synthesis in 4 mmol scale gave satisfactory yield. No such 1, 2, 3-triazole was observed in the reaction of phenyl azide with 1a, either. The structure of N2-substituted 1, 2, 3-triazoles was confirmed by the single crystal analysis on 3d (CCDC: 1868189).
|
Download:
|
| Scheme 1. Scope of the water-mediated N2-sulfonyl 1, 2, 3-triazole synthesis (Yield in parenthesis was obtained from 4 mmol scale reaction). | |
To further investigate the effect of reaction medium to the reaction outcome, the reactions in DMSO, a non-proton organic solvent, were then conducted. Interestingly, the selective reaction pathway providing N2-H 1, 2, 3-triazole was observed. The brief examination on this reaction showed that different NH2-enamines and sulfonyl azides could be independently used for the synthesis of N2-H 1, 2, 3-triazoles 4 with generally good to excellent yields (Scheme 2).

|
Download:
|
| Scheme 2. Brief scope on the tunable synthesis of N2-H 1, 2, 3-triazoles in DMSO. | |
In the process of exploring reaction mechanism, the isotope labeling experiment using 15N-labelled enaminone 1a was employed to react with 2a to the standard conditions. The isolation of 15N-labelled product 15N-3a confirmed that the C-N bond in enaminone substrate was not broken (Scheme 3a). Similar result was obtained from the reaction synthesizing product 15N-4a in DMSO (Scheme 3b), which further supported the above conclusion. Another experiment subjecting N-H-1, 2, 3-triazole 4a with TsNH2 did not provide product 3a under the water-based standard conditions (Scheme 3c), indicating that 4a was not formed during the generation of N2-sulfonyl triazole product.

|
Download:
|
| Scheme 3. Control experiments. | |
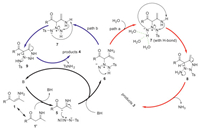
According to the information given by control experiments and the selective formation of different products, a plausible reaction mechanism involving hydrogen bond is proposed. As outlined in Scheme 4, initially, the deprotonation of enaminone 1 takes place via its isomeric form 1′ in the presence of base, leading to the formation of anion intermediate 5. The incorporation of 5 with tosyl azide and the in situ generated BH provides intermediate 6 and regenerates the base. The tautomerization of 6 gave rise to intermediate 7. Because of the presence of unsymmetrical N=N=N structure in this intermediate, the cyclization based on the nucleophilic addition of the amino group to the N=N bond may take place via two different site selective pathways. For the reactions in water, the NH site might be masked by the hydrogen bond, which induces the addition of NH2 group to the N-site connected to the Ts group (path a) [21]. This selective cyclization gives intermediate 8 which eliminates NH3 (observed by GC-MS, see Supporting information) to provide products 3. On the other hand, when performing the reactions in non-protonic DMSO medium, without the effect of hydrogen bond in the =N–H site, the attack of the NH2 group takes place selectively to this less steric site (path b), which enables the formation of cyclic intermediate 9. The elimination of TsNH2 (observed by GC-MS, see Supporting information) from this intermediate then provides products 4.
|
Download:
|
| Scheme 4. The plausible reaction mechanism. | |
In summary, by employing enaminones and sulfonyl azide as starting materials, we have established a new method for the synthesis of rarely accessed N2-sulfonyl 1, 2, 3-triazoles in pure water medium. In addition, the reactions have been identified with broad substrate scope and high efficiency using only low loading amine (5 mol% TMEDA) as catalyst without using any metal reagent. Notably, switching the reaction medium to DMSO leads to the selective synthesis of N2-H 1, 2, 3-triazoles with identical substrates. The unprecedented outcome for enaminone chemistry, the incomparable green reaction conditions as well as the selectivity tunable synthesis of N2-H 1, 2, 3-triazoles constituted the specific advantages of the present work.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is financially supported by the National Natural Science Foundation of China (No. 21861019) and Natural Science Foundation of Jiangxi Province (No. 20202ACBL203006).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.003.
| [1] |
K. Bozorov, J. Zhao, H.A. Aisa, Bioorg. Med. Chem. 27 (2019) 3511-3531. DOI:10.1016/j.bmc.2019.07.005 |
| [2] |
J. Totobenazara, A.J. Burke, Tetrahedron Lett. 56 (2015) 2853-2859. DOI:10.1016/j.tetlet.2015.03.136 |
| [3] |
H.M.L. Davies, J.S. Alford, Chem. Soc. Rev. 43 (2014) 5151-5162. DOI:10.1039/C4CS00072B |
| [4] |
D.T.G. Gonzaga, D.R. da Rocha, F.C. da Silva, et al.
, Curr. Top. Med. Chem. 13 (2013) 2850-2865. DOI:10.2174/15680266113136660202 |
| [5] |
J.S.S. Neto, G. Zeni, Coord. Chem. Rev. 409 (2020) 213-217. |
| [6] |
Z. Chen, Z. Liu, G. Cao, et al.
, Adv. Synth. Catal. 359 (2017) 202-224. DOI:10.1002/adsc.201600918 |
| [7] |
S. Noriega, E. Leyva, E. Moctezuma, et al.
, Curr. Org. Chem. 24 (2020) 536-549. DOI:10.2174/1385272824666200226120135 |
| [8] |
D. Alves, B. Goldani, E.J. Lenardão, et al.
, Chem. Rec. 18 (2018) 527-542. DOI:10.1002/tcr.201700058 |
| [9] |
V.V. Rostovtsev, L.G. Green, V.V. Fokin, et al.
, Angew. Chem. Int. Ed. 41 (2002) 2596-2599. DOI:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| [10] |
C.W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 67 (2002) 3057-3064. DOI:10.1021/jo011148j |
| [11] |
E.J. Yoo, M. Ahlquist, S.H. Kim, et al.
, Angew. Chem. Int. Ed. 46 (2007) 1730-1733. DOI:10.1002/anie.200604241 |
| [12] |
L. Zhang, X. Chen, P. Xue, et al.
, J. Am. Chem. Soc. 127 (2005) 15998-15999. DOI:10.1021/ja054114s |
| [13] |
W. Song, N. Zheng, Org. Lett. 19 (2017) 6200-6203. DOI:10.1021/acs.orglett.7b03123 |
| [14] |
M. Li, N. Zheng, J. Li, et al.
, Green Chem. 22 (2020) 2394-2398. DOI:10.1039/d0gc00555j |
| [15] |
X. Duan, N. Zheng, M. Li, et al.
, Chin. Chem. Lett. 32 (2021) 10.1016/j.cclet.2021.05.037. DOI:10.1016/j.cclet.2021.05.037 |
| [16] |
S.S.V. Ramasastry, Angew. Chem. Int. Ed. 53 (2014) 14310-14312. DOI:10.1002/anie.201409410 |
| [17] |
D.B. Ramachary, A.B. Shashank, S.S. Karthik, Angew. Chem. Int. Ed. 53 (2014) 10420-10424. DOI:10.1002/anie.201406721 |
| [18] |
X. Zhang, K.P. Rakesh, H.L. Qin, Chem. Commun. 55 (2019) 2845-2848. DOI:10.1039/c8cc09693g |
| [19] |
L. Deng, Y. Liu, J.P. Wan, Eur. J. Org. Chem. (2020) 5606-5609. DOI:10.1002/ejoc.202000938 |
| [20] |
D. Zhang, Y. Fan, Z. Yan, et al.
, Green Chem. 21 (2019) 4211-4216. DOI:10.1039/c9gc01129c |
| [21] |
J.P. Wan, D. Hu, Y. Liu, et al.
, ChemCatChem 7 (2015) 901-903. DOI:10.1002/cctc.201500001 |
| [22] |
Z. Chen, G. Cao, J. Song, et al.
, Chin. J. Chem. 35 (2017) 1797-1807. DOI:10.1002/cjoc.201700459 |
| [23] |
Z. Chen, Q. Yan, Z. Liu, et al.
, Angew. Chem. Int. Ed. 52 (2013) 13324-13328. DOI:10.1002/anie.201306416 |
| [24] |
Z.J. Cai, X.M. Lu, Y. Zi, et al.
, Org. Lett. 16 (2014) 5108-5111. DOI:10.1021/ol502431b |
| [25] |
H.N. Liu, H.Q. Cao, C.W. Chung, et al.
, Org. Lett. 22 (2020) 1396-1401. DOI:10.1021/acs.orglett.0c00006 |
| [26] |
P. Wu, Y. He, H. Wang, et al.
, Org. Lett. 22 (2020) 310-315. DOI:10.1021/acs.orglett.9b04335 |
| [27] |
P. Bao, H. Yue, N. Meng, et al.
, Org. Lett. 21 (2019) 7218-7222. DOI:10.1021/acs.orglett.9b02295 |
| [28] |
S. Roshandel, M.J. Lunn, G. Rasul, et al.
, Org. Lett. 21 (2019) 6255-6258. DOI:10.1021/acs.orglett.9b02140 |
| [29] |
X. Deng, X. Lei, G. Nie, et al.
, J. Org. Chem. 82 (2017) 6163-6171. DOI:10.1021/acs.joc.7b00752 |
| [30] |
S. Mamijo, T. Jin, Z. Huo, et al.
, J. Am. Chem. Soc. 125 (2003) 7786-7787. DOI:10.1021/ja034191s |
| [31] |
Z. Li, Q. Wei, L. Song, et al.
, Org. Lett. 21 (2019) 6413-6417. DOI:10.1021/acs.orglett.9b02269 |
| [32] |
E.K. Moltzen, H. Pedersen, K.P. Bøgesø, et al.
, J. Med. Chem. 37 (1994) 4085-4099. DOI:10.1021/jm00050a006 |
| [33] |
A.C. Pippione, F. Dosio, A. Ducime, et al.
, MedChemCommun 6 (2015) 1285-1292. DOI:10.1039/C5MD00182J |
| [34] |
V.V. Grishko, I.A. Tolmacheva, V.O. Nebogatikov, et al.
, Eur. J. Med. Chem. 127 (2017) 629-639. |
| [35] |
C.D. Cox, M.J. Breslin, D.B. Whitman, et al.
, J. Med. Chem. 53 (2010) 5320-5332. DOI:10.1021/jm100541c |
| [36] |
L. Gan, Y. Liu, J.P. Wan, et al.
, J. Org. Chem. 86 (2021) 1231-1237. DOI:10.1021/acs.joc.0c02431 |
| [37] |
Q. Yu, Y. Zhang, J.P. Wan, Green Chem. 21 (2019) 3436-3441. DOI:10.1039/c9gc01357a |
| [38] |
L. Fu, Z. Xu, J.P. Wan, et al.
, Org. Lett. 22 (2020) 9518-9523. DOI:10.1021/acs.orglett.0c03548 |
| [39] |
L. Fu, Y. Liu, J.P. Wan, Org. Lett. 23 (2021) 4363-4367. DOI:10.1021/acs.orglett.1c01301 |
| [40] |
D. Hu, L. Yang, J.P. Wan, Green Chem. 22 (2020) 6773-6777. DOI:10.1039/d0gc02806a |
| [41] |
D. Ba, Y. Chen, W. Lv, et al.
, Org. Lett. 21 (2019) 8603-8606. DOI:10.1021/acs.orglett.9b03189 |
| [42] |
Y. Yuan, W. Hou, D. Zhang-Negrerie, et al.
, Org. Lett. 16 (2014) 5410-5413. DOI:10.1021/ol5026525 |
| [43] |
F. Gu., W. Yao, Chin. J. Org. Chem. 40 (2020) 4384-4386. DOI:10.6023/cjoc202000091 |
| [44] |
Q. Yu, Y. Liu, J.P. Wan, Chin. Chem. Lett. 32 (2021) 3514-3517. DOI:10.1016/j.cclet.2021.04.037 |
| [45] |
G. Cheng, X. Zeng, J. Shen, et al.
, Angew. Chem. Int. Ed. 52 (2013) 13265-13268. DOI:10.1002/anie.201307499 |
| [46] |
J.P. Wan, S. Cao, Y. Liu, J. Org. Chem. 80 (2015) 9028-9033. DOI:10.1021/acs.joc.5b01121 |
| [47] |
J.P. Wan, S. Cao, Y. Liu, Org. Lett. 18 (2016) 6034-6037. DOI:10.1021/acs.orglett.6b02975 |
| [48] |
S. Cao, Y. Liu, J.P. Wan, et al.
, ChemCatChem 10 (2018) 5007-5011. DOI:10.1002/cctc.201801366 |
| [49] |
J. Thomas, V. Goyvaerts, S. Liekens, et al.
, Chem. Eur. J. 22 (2016) 9966-9970. DOI:10.1002/chem.201601928 |
| [50] |
L. Yang, Y. Wu, J.P. Wan, et al.
, Beilstein J. Org. Chem. 14 (2018) 2348-2353. DOI:10.3762/bjoc.14.210 |
| [51] |
L. Deng, Y. Liu, J.P. Wan, et al.
, J. Org. Chem. 84 (2019) 14179-14186. DOI:10.1021/acs.joc.9b01817 |
| [52] |
A. De Nino, V. Algieri, M.A. Tallarida, et al.
, Eur. J. Org. Chem. (2019) 5725-5731. DOI:10.1002/ejoc.201900889 |
| [53] |
N. Guo, X. Lin, H. Xu, et al.
, Org. Biomol. Chem. 17 (2019) 6148-6152. DOI:10.1039/c9ob01156k |
| [54] |
T. Opsomer, W. Dehaen, Chem. Commun. 57 (2021) 1568-1590. DOI:10.1039/d0cc06654k |
| [55] |
Y. Guo, L. Wei, J.P. Wan, et al.
, J. Org. Chem. 84 (2019) 2984-2990. DOI:10.1021/acs.joc.8b02897 |
| [56] |
X. Zheng, J.P. Wan, Adv. Synth. Catal. 361 (2019) 5690-5694. DOI:10.1002/adsc.201901054 |
| [57] |
L. Gan, L. Wei, J.P. Wan, ChemistrySelect 5 (2020) 7822-7825. DOI:10.1002/slct.202002247 |
| [58] |
I. Elghmry, Y. Al-Faiyz, Tetrahedron Lett. 57 (2016) 110-112. DOI:10.1016/j.tetlet.2015.11.070 |
| [59] |
R.J. Reddy, A. Shankar, M. Waheed, et al.
, Tetrahedron Lett. 59 (2018) 2014-2017. DOI:10.1016/j.tetlet.2018.04.023 |
| [60] |
M. Yamauchi, T. Miura, M. Murakami, Heterocycles 80 (2010) 177-181. DOI:10.3987/COM-09-S(S)44 |
| [61] |
T. Beryozkina, I.V. Efinov, W.F. Fabian, et al.
, Tetrahedron 71 (2015) 6189-6195. DOI:10.1016/j.tet.2015.06.088 |
 2022, Vol. 33
2022, Vol. 33 


