b Fujian Key Laboratory of Advanced Manufacturing Technology of Specialty Chemicals, Fuzhou University, Fuzhou 350116, China
In the past decades, cyclic polymers, as one of the oldest topological polymers, have attracted large attention from scientists and researchers because of their unique physical properties [1-7]. Due to their endless molecular topology, cyclic polymers present different properties from linear polymers, including higher glass transition temperature and critical solution temperature, smaller hydrodynamic volume and radius of gyration, lower intrinsic viscosity and cytotoxicity [8-15]. Further studies have shown that cyclic polymers have stronger drug loading and release capacity, longer cycle time, lower cytotoxicity, and higher gene transfection efficiency than linear precursors, making them be widely used in biomedical applications [4,16,17]. Numerous literatures have been reported on the synthesis of cyclic polymers, such as, conventional step-growth polycondensation (SGP) [18]. Generally, the synthetic strategies of cyclic polymers were divided into two categories, ring-closure and ring-expansion techniques [3,5,19].
The ring-closure technique is a traditional route to synthesize cyclic polymers, including the synthesis of linear polymer precursors and the cyclization of them under extremely diluted conditions [4,18,20,21]. The linear polymer precursors contain functional groups that can bond to each other at the end of polymer chain [10,22,23]. The cyclization of the linear polymer precursors includes three methodologies: unimolecular homodifunctional cyclization, bimolecular homodifunctional (Scheme 1a) and unimolecular heterodifunctional cyclization (Scheme 1b) [3,19]. Among them, bimolecular cyclization was first studied because of the synthesis of homodifunctional polymer precursors is more convenient [24]. However, the drawback of this method is that one of the homodifunctional polymer might react with two of the other homodifunctional polymer to produce linear by-product or multiple molecular weight cyclic polymer [18,22]. Therefore, unimolecular homodifunctional and unimolecular heterodifunctional approach have been expended to overcome the shortcoming [25]. In addition, unimolecular heterodifunctional cyclization attracts more attentions due to the synthesis of cyclic polymer is relatively easy by click reaction [26-30]. Meanwhile, in order to avoid produce multiple-cyclic polymer, the cyclization is taken out at extremely low concentration of 10−5 mol/L [3,13,18,31]. In a word, ring-closure approach inevitably requires highly diluted reaction condition and via a time-consuming dropwise addition process, which provides diversity of cyclic polymer but with quite low synthetic efficiencies.

|
Download:
|
| Scheme 1. Schematic representation of synthetic pathways for the preparation of cyclic polymers. (a) bimolecular ring closure, (b) unimolecular ring closure for the generation of cyclic polymer and by-products. (c) ring-expansion approach for the preparation of cyclic polymers, and (d) this work adopts the simple synthesis method without catalyst. | |
Another technique to obtain a cyclic polymer is ring-expansion (Scheme 1c), which refers to the insertion of monomer into a ring living species, such as ring catalyst and initiator [32,33]. Many approaches play crucial roles in ring-expansion, such as, ring-expansion metathesis polymerization (REMP), reversible addition fragmentation chain-transfer polymerization (RAFT), and zwitterionic polymerization [34-39]. Ring-expansion technique can avoid the appearance of linear by-products, so the efficiency is greatly improved compared with ring-closure technique [39]. In addition, the cyclic polymer obtained by this technique owns narrow polydispersity index (Đ) and high number-average molecule weight (Mn) [3,13,22]. Nonetheless, the synthesis of living species presents significant challenges, which cannot meet most of the requirements [3].
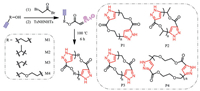
Our previous work has reported a convenient, safe, and green approach to synthesize polypyrazoles via the 1,3-dipolar cycloaddition of α-substituted electron-deficient bisdiazo compounds to bisalkynes without catalyst [40]. Both of the chain end groups of the obtained polypyrazoles are alkynes, which suggests that the diazo groups are easily react with alkyne. The results strongly motivate us to come up with a hypothesis that an AB type monomer consisted of substituted electron-deficient diazo group and alkyne group can easily undergo 1,3-dipolar cycloaddition to form cyclic polypyrazole (Scheme 2). Herein, to prove that, we have synthesized four kinds of diazo monomers (M1-M4) contained alkyne group as well, and then polymerization of these four monomers was taken out by heating without any catalyst and solvent. As expected, the obtained polypyrazoles were cyclic polymers, which were proved by LC-QTOF-MS. Comparing to other methods, this work provides a new and more convenient way for the synthesis of cyclic polymer. Interestingly, the obtained cyclic polypyrazoles self-assemble in methanol to form vesicles during the reprecipitation process driven by hydrogen bond.
|
Download:
|
| Scheme 2. The illustration of the synthesis of four diazo monomers (M1-M4) and corresponding four cyclic polymers (P1-P4). | |
Monomers (M1-M4) contain both diazo group and alkyne group are synthesized from corresponding alkynol and their 1H NMR spectra are shown in Fig. 1a. Sharp peaks appeared closely at 4.79 ppm are assigned to the proton adjacent to -C=N2 group, which proves the successfully introduce of the diazo group [41]. The other sharp peaks appeared closely at 2.49 ppm are belonged to the proton connected to -C≡C- group, indicating the existence of alkyne group [42]. Polymerization of M1-M4 was carried out under 100 ℃ heating, producing the polypyrazoles (P1-P4) with high yields in the range of 78.8-97.9% without any catalyst (Table S1 in Supporting information). The Mn and the Đ of the obtained polypyrazoles characterized by GPC are in the range of 3800-4400 g/mol and 1.63-1.81, respectively (Fig. S2 in Supporting information). The 1H NMR spectra of P1-P4 are presented in Fig. 1b. Peaks belonged to methylene protons adjacent to the diazo groups and protons connect to alkyne groups disappear, proving the complete conversion of the monomer. The 1H NMR spectra of P1-P4 show two separate peaks at δ 13.5 and δ 14.4, which suggest the formation of -NH- group belonged to the pyrazole rings [43,44]. Moreover, the broad resonance band at δ 6.4-6.9 is assigned to the protons in the pyrazole rings [45]. In addition, resonance band at δ 3.8-4.3 is belonged to the -OCH2- groups on the polymer main chain [46]. Fig. 1c shows the 13C NMR spectra of the obtained polypyrazoles. Peaks appeared at δ 160.0-163.1 are belonged to the carbonyl groups [47]. The signals for the carbons of olefins originated by 1,3-diploar cycloaddition of diazo groups to alkynes on the pyrazole ring arise at δ 125.3-150.6 and δ 106.2-110.5, respectively [48]. All the NMR results of the obtained polymers prove the successfully synthesis of polypyrazoles.

|
Download:
|
| Fig. 1. (a) The 1H NMR spectra of monomers M1-M4 in CDCl3 (●=1, ▼=2). (b) The 1H NMR (■=3, ☆=4) and (c) 13C NMR spectra of polypyrazoles in (CD3)2SO (◀=5, ▶=6). (d) The FT-IR spectra of the resulting polypyrazoles. | |
Furthermore, the FT-IR spectra of the obtained polymers are detected and shown in Fig. 1d. The pyrazole rings in the main chain of polymers are certified by the broad bands at 3400 cm−1, which corresponds to the -NH- stretch vibration [49]. Bands belonging to -C=C- vibrations cannot be assigned precisely because of the interference from -NH- stretch vibration. Bands of -CH-, -CH2-, and -CH3 stretch of aliphatic groups are noticed at around 3000 cm−1. Importantly, sharp bands near 2100 cm−1 belonged to the -C≡C- stretch vibration from alkynes totally disappeared [42], further proving that alkynes and diazo groups of the monomers are transformed into the pyrazole rings through 1,3-dipolar cycloaddition reaction, which in agreement to the results of 1H NMR. Besides, the intense bands around at 1720 cm−1 are ascribed to the carbonyl bonds [41]. Weak absorption bands around at 1635 cm−l and 1570 cm−l correspond to -C=C- and -C=N- stretching, respectively [50].
A conclusion could be drawn by the above-mentioned results is that neither diazo group nor alkyne group is the chain end group of the obtained polypyrazoles. Therefore, quadrupole time-of-flight LC/MS (LC-QTOF-MS) was introduced to analyze the detail structure of polypyrazoles and P3 was chosen as the study object. The polymerization of M3 was carried out in 2 h to obtain P3 with low Mn. The LC-QTOF-MS spectrum of P3 with m/z from 1300 to 1900 is shown in Fig. 2. Regular repeating patterns spaced by m/z 124.03 are recorded, which is in agreement with the mass weight of M3 (m/z 124.03), as well as the repeat unit of P3. The main m/z peaks in the range of 1380 to 1770 can be sorted as [Na + P3]+ series, which match quite well with the following mass formulae:

|

|
Download:
|
| Fig. 2. LC-QTOF-MS spectrum of P3. | |
where m means the number of the repeat unit of P3. Importantly, no molecular weight of the end groups is found, which proves P3 is a cyclic polymer.
The thermal properties of the obtained polypyrazoles were examined by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Fig. S3 (Supporting information) shows the DSC curves of the obtained polymers, and glass transition temperatures (Tg) between 42.3 ℃ and 55 ℃ were observed (Table S2 in Supporting informaiton). Comparing to the linear polypyrazoles, the Tg of the cyclic polypyrazoles sharply decreases [40]. The TGA curves (Fig. S4 in Supporting information) of the polypyrazoles indicated that all the obtained polymers were thermally stable, losing merely 5% of their weights at temperatures higher than 181 ℃ (Table S2). The degradation of the obtained polymers was divided into two main stages when they are heated under nitrogen from room temperature to 600 ℃, as evidenced by the derivative thermogravimetric (DTG) analysis (Fig. S4). The UV-vis absorption spectra of diluted solution of P3 in DMSO are also shown (Fig. S5 in Supporting information). Remarkable absorption bands in the range of 260-400 nm are presented, which belong to the π-π transition and n-π transition [40].
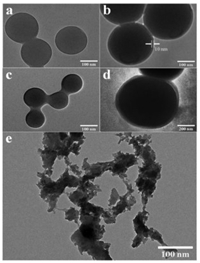
After the crude products are purified by reprecipitation with DMF/methanol, scanning electron microscopy (SEM) is used to investigate the morphologies of the produced polymers (P1-P4), where particles with wrinkle surface and diameters of 100-220 nm are observed (Fig. S6 in Supporting information). Then, TEM is introduced to investigate the inner structure of the particles (Figs. 3a-d). Surprisingly, the obtained particles are not micelles but vesicles, whose thickness are around 10 nm and diameters are in the range of 130-750 nm. This is the first example of the self-assemble of non-amphiphilic cyclic polymer to form vesicle. To prove the self-assemble of P3 during reprecipitation process, quantum dots (QD) and P3 are dissolved by DMF and then precipitated in methanol (Scheme 3). In this process, the self-assemble of P3 generates vesicles contained QD inside. The obtained white solid (named P3-QD) is washed with abundant methanol to remove any dissociative QD. Then, the fluorescence spectra of P3, QD and P3-QD are characterized and shown in Scheme 3. P3 shows broad emission band in the range of 380-700 nm. Two sharp peaks are detected in 416 and 339 nm, respectively. QD presents a strong emission band in the range of 520-800 nm, and the strongest emission peak locates at 582 nm. P3-QD presents almost the same emission band, proving the incorporation of QD in P3 vesicles during the reprecipitation process.
|
Download:
|
| Fig. 3. TEM images of (a) P1, (b) P2, (c) P3, (d) P4, and (e) P3 precipitated in methanol contained trace amount of TFA. | |

|
Download:
|
| Scheme 3. The process of P3 package QD and the fluorescence spectra of P3 (blue line), QD (black line) and P3-QD (red line). | |
It is worthwhile to ensure how the self-assemble of P3 occur during the reprecipitation. In that case, we repeat the reprecipitation process again. The difference compared to previous experiment is the additional of trace amount of TFA in methanol (The pH of methanol is 6.5). The FT-IR is used to check the obtained polymer (P3) and the spectrum is shown in Fig. S7 (Supporting information). Comparing to the P3 obtained by pure methanol, it is found that a new and intense band around 1785 cm−1 arises, which causes by the partially broken of hydrogen bond formed by the N-H and ester group on the main chain of P3. It is proved that the broken of hydrogen bond induce the partial increasement in wavenumber [51]. Besides, TEM is used to further confirm the hypothesis. As shown in Fig. 3e, the vesicle structure of P3 is destroyed in presence of TFA. These results suggest that the formation of vesicle is induced by the hydrogen bond formed by the N-H and ester group of polypyrazoles.
In summary, one-step synthesis of polypyrazole by the 1,3-diploar cycloaddition of AB type monomers that contain diazo and alkyne groups is carried out, which provides a convenient process to obtain cyclic polymer without any catalyst and solvent. The successfully synthesis of cyclic polypyrazoles is proved by the results of 1H NMR, 13C NMR, FT-IR and LC-QTOF-MS. Furthermore, the polypyrazoles self-assemble into vesicles with diameter in the range of 130-750 nm during the reprecipitation process with DMF/methanol. The reason for that is the formation of intermolecular hydrogen bond between -NH- and ester groups. The detailed mechanism will be further studied in our future work.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21504025), Natural Science Foundation of Fujian Province (No. 2019J05040), Fujian Provincial Department of Education (No. JT180038), Talent program (No. GXRC-18041) and '111' program of Fuzhou University.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.033.
| [1] |
S. Zhang, X. Cheng, J. Wang, et al., Polym. Chem. 9 (2018) 5155-5163. DOI:10.1039/C8PY01014E |
| [2] |
W. Ryu, L. Xiang, T. Jeon, M. Ree, Polymer 207 (2020) 122899. DOI:10.1016/j.polymer.2020.122899 |
| [3] |
J. Chen, H. Li, H. Zhang, et al., Nat. Commun. 9 (2018) 5310. DOI:10.1038/s41467-018-07754-1 |
| [4] |
Z. Liu, Y. Huang, X. Zhang, et al., Macromolecules 51 (2018) 7672-7679. DOI:10.1021/acs.macromol.8b00950 |
| [5] |
Y. Jiang, Z. Zhang, D. Wang, N. Hadjichristidis, Macromolecules 51 (2018) 3193-3202. DOI:10.1021/acs.macromol.8b00333 |
| [6] |
L. Guo, D. Zhang, J. Am. Chem. Soc. 131 (2009) 18072-18074. DOI:10.1021/ja907380d |
| [7] |
D. Tan, X. Hu, Z. Cao, M. Luo, D.J. Darensbourg, ACS Macro Lett. 9 (2020) 866-871. DOI:10.1021/acsmacrolett.0c00302 |
| [8] |
Y. Cai, J. Lu, F. Zhou, et al., Macromol. Rapid Commun. 35 (2014) 901-907. DOI:10.1002/marc.201300913 |
| [9] |
S. Zhang, Y. Tezuka, Z. Zhang, et al., Polym. Chem. 9 (2018) 677-686. DOI:10.1039/C7PY01544E |
| [10] |
W. Zhu, Z. Li, Y. Zhao, K. Zhang, Macromol. Rapid Commun. 36 (2015) 1987-1993. DOI:10.1002/marc.201500367 |
| [11] |
T.E. Gartner Ⅲ, F.M. Haque, A.M. Gomi, et al., Macromolecules 52 (2019) 4579-4589. DOI:10.1021/acs.macromol.9b00600 |
| [12] |
Y. Jeong, Y. Jin, T. Chang, F. Uhlik, J. Roovers, Macromolecules 50 (2017) 7770-7776. DOI:10.1021/acs.macromol.7b01511 |
| [13] |
Z. Jia, M.J. Monteiro, J. Polym. Sci. Part A: Polym. Chem. 50 (2012) 2085-2097. DOI:10.1002/pola.25999 |
| [14] |
L. Gao, J. Oh, Y. Tu, T. Chang, C.Y. Li, Polymer 170 (2019) 198-203. DOI:10.1016/j.polymer.2019.03.018 |
| [15] |
M.A. Cortez, W.T. Godbey, Y. Fang, et al., J. Am. Chem. Soc. 137 (2015) 6541-6549. DOI:10.1021/jacs.5b00980 |
| [16] |
G. Kang, L. Sun, Y. Liu, et al., Langmuir 35 (2019) 12509-12517. DOI:10.1021/acs.langmuir.9b02346 |
| [17] |
X.Y. Tu, M.Z. Liu, H. Wei, J. Polym. Sci. Part A: Polym. Chem. 54 (2016) 1447-1458. DOI:10.1002/pola.28051 |
| [18] |
T. Josse, J. De Winter, P. Gerbaux, O. Coulembier, Angew. Chem. Int. Ed. 55 (2016) 13944-13958. DOI:10.1002/anie.201601677 |
| [19] |
F.M. Haque, S.M. Grayson, Nat. Chem. 12 (2020) 433-444. DOI:10.1038/s41557-020-0440-5 |
| [20] |
P. Sun, J.A. Liu, Z. Zhang, K. Zhang, Polym. Chem. 7 (2016) 2239-2244. DOI:10.1039/C6PY00165C |
| [21] |
H. Zhang, W. Wu, X. Zhao, Y. Zhao, Macromolecules 50 (2017) 3411-3423. DOI:10.1021/acs.macromol.7b00220 |
| [22] |
B.A. Laurent, S.M. Grayson, Chem. Soc. Rev. 38 (2009) 2202-2213. DOI:10.1039/b809916m |
| [23] |
M.W. Ali, Z. Muhammad, Q. Jia, et al., Polym. Chem. 11 (2020) 4164-4171. DOI:10.1039/D0PY00599A |
| [24] |
T. He, G.H. Zheng, C.Y. Pan, Macromolecules 36 (2003) 5960-5966. DOI:10.1021/ma021371y |
| [25] |
L. Gao, Z. Ji, Y. Zhao, et al., ACS Macro Lett. 8 (2019) 1564-1569. DOI:10.1021/acsmacrolett.9b00747 |
| [26] |
J.N. Hoskins, S.M. Grayson, Macromolecules 42 (2009) 6406-6413. DOI:10.1021/ma9011076 |
| [27] |
H. Wei, D.S.H. Chu, J. Zhao, J.A. Pahang, S.H. Pun, ACS Macro Lett. 2 (2013) 1047-1050. DOI:10.1021/mz400560y |
| [28] |
X. Wan, T. Liu, S. Liu, Biomacromolecules 12 (2011) 1146-1154. DOI:10.1021/bm101463d |
| [29] |
M. Cai, J. Hu, J.L. Tian, et al., Chin. Chem. Lett. 26 (2015) 675-680. DOI:10.1016/j.cclet.2015.03.015 |
| [30] |
J.C. Chen, Y.P. Wang, H.D. Wang, et al., Chin. Chem. Lett. 21 (2010) 496-500. DOI:10.1016/j.cclet.2009.11.025 |
| [31] |
Y. Oga, Y. Hosoi, A. Takasu, Polymer 186 (2020) 122019. DOI:10.1016/j.polymer.2019.122019 |
| [32] |
Y.A. Chang, R.M. Waymouth, J. Polym. Sci. Part A: Polym. Chem. 55 (2017) 2892-2902. DOI:10.1002/pola.28635 |
| [33] |
S. Lv, Y. Sun, Y. Xu, S. Yang, L. Wang, Chin. Chem. Lett. 31 (2020) 1568-1571. DOI:10.1016/j.cclet.2019.11.027 |
| [34] |
C.W. Bielawski, D. Benitez, R.H. Grubbs, Science 297 (2002) 2041-2044. DOI:10.1126/science.1075401 |
| [35] |
S.A. Gonsales, T. Kubo, M.K. Flint, et al., J. Am. Chem. Soc. 138 (2016) 4996-4999. DOI:10.1021/jacs.6b00014 |
| [36] |
A. Bunha, P.F. Cao, J.D. Mangadlao, R.C. Advincula, React. Funct. Polym. 80 (2014) 33-39. DOI:10.1016/j.reactfunctpolym.2014.03.001 |
| [37] |
K. Zhang, M.A. Lackey, Y. Wu, G.N. Tew, J. Am. Chem. Soc. 133 (2011) 6906-6909. DOI:10.1021/ja2007559 |
| [38] |
E.J. Shin, H.A. Brown, S. Gonzalez, et al., Angew. Chem. Int. Ed. 50 (2011) 6388-6391. DOI:10.1002/anie.201101853 |
| [39] |
H.A. Brown, Y.A. Chang, R.M. Waymouth, J. Am. Chem. Soc. 135 (2013) 18738-18741. DOI:10.1021/ja409843v |
| [40] |
L. Xiao, S. Cai, Q. Liu, et al., Polym. Chem. 5 (2014) 607-613. DOI:10.1039/C3PY01105D |
| [41] |
T. Toma, J. Shimokawa, T. Fukuyama, Org. Lett. 9 (2007) 3195-3197. DOI:10.1021/ol701432k |
| [42] |
F. Sanda, T. Kawano, T. Masuda, Polym. Bull. 55 (2005) 341-347. DOI:10.1007/s00289-005-0445-7 |
| [43] |
S. Wang, B. Cheng, Sci. Rep. 7 (2017) 12712. DOI:10.1038/s41598-017-12727-3 |
| [44] |
M.R. Bhosle, L.D. Khillare, S.T. Dhumal, R.A. Mane, Chin. Chem. Lett. 27 (2016) 370-374. DOI:10.1016/j.cclet.2015.12.005 |
| [45] |
X. Tang, C. Zheng, Y. Chen, et al., Macromolecules 49 (2016) 9291-9300. DOI:10.1021/acs.macromol.6b02192 |
| [46] |
C. Chen, C.G. Wang, L. Xiao, A. Goto, Chem. Commun. 54 (2018) 13738-13741. DOI:10.1039/C8CC08157C |
| [47] |
G. Feng, S. Xu, R. Chen, et al., Tetrahedron Lett. 61 (2020) 152622. DOI:10.1016/j.tetlet.2020.152622 |
| [48] |
L.L. Bengel, B. Aberle, A.N. Egler-Kemmerer, et al., Angew. Chem. Int. Ed. 60 (2020) 5554-5560. DOI:10.1002/anie.201914775 |
| [49] |
J.P. Castaneda, G.S. Denisov, S.Y. Kucherov, V.M. Schreiber, A. Shurukhina, J. Mol. Struct. 660 (2003) 25-40. DOI:10.1016/j.molstruc.2003.07.010 |
| [50] |
Y. Cui, Z. Xu, H.Y. Li, et al., ACS Appl. Polym. Mater. 2 (2020) 4512-4520. DOI:10.1021/acsapm.0c00592 |
| [51] |
M.R. Ghadiri, J.R. Granja, R.A. Milligan, D.E. McRee, N. Khazanovich, Nature 366 (1993) 324-327. DOI:10.1038/366324a0 |
 2022, Vol. 33
2022, Vol. 33 
