b Qian Xuesen Collaborative Research Center of Astrochemistry and Space Life Sciences, Ningbo University, Ningbo 315211, China;
c Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China;
d Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China
Natural biological molecules have a unique characteristic of chiral selection such as amino acids of l-configuration in proteins and nucleosides of d-configuration in nucleic acids. Current mechanisms affording chiral selection of biomolecules implicate possible roles of force fields [1-5], crystallization [6], adsorption [7], magnetization [8], circular polarized light [9-11] and self-assembly [12-14]. However, the question of chiral selectivity in natural amino acid and nucleoside is still a mystery. According to the "RNA world" hypothesis, chemical evolution prepares conditions for the emergence of life, prompting subsequent biological evolution [15,16]. Thus, we proposed that chiral selection results from some simple intrinsic chemical reaction in the prebiotic environment.
In contemporary biochemistry, aminoacyl-tRNA (aa-tRNA) is produced by the transfer of aminoacyl group from 5′-aminoacyl-adenylates (5′-aa-AMPs) to the 2′/3′-OH terminus of tRNA (Fig. 1A) [17-19]. Tamura and Schimmel have reported that the RNA minihelix acts as a carrier for chiral selection of amino acids by simulating the aminoacylation of RNA. And the RNA minihelix is thought to be the precursor of the tRNA (Fig. 1B) [20-22]. It is possible that chiral selection happened in the formation of 5′-aa-AMP. However, to our best knowledge, such chiral selection has not been reported.

|
Download:
|
| Fig. 1. Biosynthesis of aa-tRNA and chiral selection of plausible prebiotic process. (A) Formation of 5′-aa-AMPs from a mixture of l-aa and ATP lead to aa-tRNA forming in the biosynthesis. (B) The chiral selectivity of l-/d-amino acid comes during the formation of aa-RNA minihelix. (C) The chiral selectivity of l-/d-amino acid in our experimental scheme. aa: amino acid; 5′-aa-AMPs: 5′-aminoacyl-adenylates; tRNA: transfer RNA; aa-RS: aminoacyl-tRNA synthases; Nu: nucleoside. | |
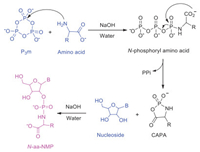
5′-aa-AMPs are the pivotal activated intermediates used by all living organisms in peptide synthesis. Remarkably, they are highly unstable and easily undergo hydrolysis [23-25]. Recently, we have investigated nucleotide amidate (N-aa-NMP, including N-aa-AMP, N-aa-GMP, N-aa-CMP and N-aa-UMP), which are the analogs of 5′-aa-AMPs, obtained from the reaction of amino acid, nucleoside and trimetaphosphate (P3m) under prebiotic conditions (Scheme 1) [26,27]. In particular, the Lost City hydrothermal field, where there are 40-91℃ alkaline hydrothermal fluids (pH 9~11), might be appropriate as potential milieu for driving the origin of life [28,29].
|
Download:
|
| Scheme 1. Reaction of amino acid, nucleoside and P3m for N-aa-NMP synthesis. B represents the nucleobase, namely adenine, guanine, cytosine and uracil. CAPA: Cyclic acylphosphoramidate. | |
As the chemical and physical characteristics of early evolutionary biochemical module may not be related to contemporary biosystem [30], we believed that the N-aa-NMP might be precursors of 5′-aa-AMPs. Thus, N-aa-NMP were used as models to probe the chiral selection between l-/d-amino acid (l-/d-aa) and d-/l-nucleoside (d-/l-Nu) (Fig. 1C).
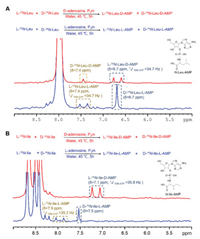
One-pot synthesis of chiral N-aa-NMPs using equal amounts of l-15N-aa and d-14N-aa with the nucleoside and P3m were carried out at an initial pH of 11.7 and 45 ℃ for simulating the alkaline aqueous solution of the Lost City hydrothermal field as the plausible prebiotic conditions. The pH value of the reaction eventually dropped to 8~9 after 5 h. The resulting reaction was monitored by 31P NMR spectroscopy. It was found that the 31P NMR signal of l-15N-aa-NMP was split into a doublet signal due to the coupling by 15N. The corresponding signal for that of d-14N-aa-NMP appeared as a singlet (Fig. 2).
|
Download:
|
| Fig. 2. Tracing chiral N-aa-AMP in the presence of adenosine by 31P NMR. (A) The formation of l-15N-Leu-d-AMP was preferred over that of d-14N-Leu-d-AMP in the presence of d-adenosine (red). The l-adenosine chose d-Leu more than l-Leu (blue). (B) In the presence of d-adenosine, the formation of l-15N-Ile-d-AMP was the only product observed (red). The l-adenosine chose d-Ile more than l-Ile (blue). | |
For example, reaction of l-15N-Leu and d-14N-Leu with d-adenosine showed a 31P doublet resonance at 6.7 ppm with coupling constant 1J15N-31P = 34.7 Hz and a singlet at 7.4 ppm attributable to l-15N-Leu-d-AMP and d-14N-Leu-d-AMP, respectively (red line of Fig. 2A and Table 1). It showed that the formation of l-15N-Leu-d-AMP was preferred over that of d-14N-Leu-d-AMP by a ratio of about 2.6:1 (Fig. 3A).
|
|
Table 1 31P chemical shift (ppm) and 1J15N-31P (Hz) in parentheses of N-aa-NMPs. |

|
Download:
|
| Fig. 3. The relative intensity of 31P peaks of the l-15N-aa-NMP and d-14N-aa-NMP forming in these reactions. Bar graph representation of the reaction with d-adenosine (A), l-adenosine (B), d-guanosine (C) and l-guanosine (D). | |
Moreover, when the chirality of adenosine was changed into the l-configuration, the result showed that the dominant product was d-14N-Leu-l-AMP by a factor of 3.3 (blue line of Figs. 2A and 3B). It is noteworthy that the 31P NMR spectra gave perfect two pairs of mirror image products with identical chemical shift (l-15N-Leu-d-AMP vs. d-14N-Leu-l-AMP and d-14N-Leu-d-AMP vs. l-15N-Leu-l-AMP, Fig. 2A and Table 1).
Similarly, analogous experiments were also performed with other four l-/d-amino acids (Ala, Val, Phe and Ile) revealing similar selectivity for the l-15N-aa-AMP and d-14N-aa-AMP species. Thus d-adenosine preferentially reacted with the l-amino acids while l-adenosine chose d-amino acid more than l-amino acid (Figs. 2B, 3A, 3B and Figs. S21–S23 in Supporting informaiton).
In the case of isoleucine (Ile), the reaction with d-adenosine showed l-15N-Ile-d-AMP as the only product (red line of Fig. 2B). Conversely, d-14N-Ile-l-AMP was favored in the reaction with l-adenosine by a factor of 2.1 (blue line of Fig. 2B). The unusual behavior for Ile is attributed to the presence of one more chiral center (Fig. 2B). Hence, l-N-Ile-d-AMP (δ 7.1 ppm) and d-N-Ile-l-AMP (δ 7.5 ppm) are not mirror isomers, as evidenced by the differing chemical shifts of 31P NMR, indicating that 31P NMR is senstive enough to reflect chiral variation on the surroundings (Table 1, Fig. 2B). The CD spectra of l-Ile-d-AMP and d-Ile-l-AMP were showed at Fig. S29 (Supporting information).
Using d-guanosine, a clear preference for the reaction of the l-amino acid over d-amino acid was also observed. Conversely, the d-amino acid showed significant preference for reaction with l-guanosine (Figs. 3C and D, Figs. S24-S28 in Supporting information).
N-Leu-AMP (Figs. S1–S8 in Supporting information), N-Ile-AMP (Figs. S9–S14 in Supporting information) and N-Val-GMP (Figs. S15–S20 in Supporting information) were isolated by HPLC, and characterized by 1D and 2D NMR and HR-MS, respectively. The results confirmed that the structure of these compounds were covalent bound to the phosphate group via the 2′-OH of ribose and amino group of amino acid (2′-N-aa-NMP). This is consistent with our previous result [26].
In general, our data show each pair l-/d-aa reaction with d- or l-nucleoside respectively generate mixtures of two stereoisomers of chiral N-aa-NMPs, as well as the corresponding mirror isomers. Nonetheless, these results showed that reactions of l-/d-amino acid and d-/l-nucleoside exhibit an intrinsic chiral selection at the molecular level. Fig. 3 showed that the chiral selectivity was about 1.2~7.3-fold for each case, respectively. Although this iterated chiral selection was under weak pressure, it could lead to an overpowering preference to use l-amino acids in biological system over the long period of prebiotic evolution in "RNA world".
Table 1 presented very important and interesting data that each pair l-/d-aa reaction with d- or l-nucleoside respectively can generate a mixture of two stereoisomers of chiral N-aa-NMPs, where showed two sets of mirror image isomers. For example, adenosine reacted with amino acid (leucine, alanine, valine or phenylalanine) to give two sets of mirror image isomers (1a vs. 2a and 1b vs. 2b), respectively, in which both the chirality of adenosine and amino acid were simultaneous opposite. And they showed perfect two pairs of mirror image products with identical 31P NMR chemial shift and 15N-31P coupling constant, respectively. For the case of guanosine, 3a vs. 4a and 3b vs. 4b also gave the very neat mirror image isomers with identical NMR data.
Based on the above observations and the known dominance of l-amino acids and d-nucleosides in life systems on Earth, it is tempting to speculate that mirror image biopolymers might also exhibit biological activity. In this regard, we noted that Wang et al. have reported synthetic biopolymer molecular system capable of mirror image genetic replication and transcription [31,32].
In conclusion, the chiral selection of amino acids and nucleosides were observed at the molecular level during the formation of N-aa-NMP. While this selectivity provides insights relevant to prebiotic chemistry, it also suggests the concept of a mirror image life system in which the building blocks are the mirror image isomers of those on Earth.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 91856126, 42003062, 21778042 and 41876072), Scientific Research Grant of Ningbo University (No. 215-432000282), and Ningbo Top Talent Project (No. 215-432094250). We are indebted to Prof. Douglas W. Stephan (University of Toronto), Prof. Yuejin Hua (Zhejiang University) for many constructive comments on this manuscript.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.031.
| [1] |
G.L. Rikken, E. Raupach, Nature 405 (2000) 932-935. DOI:10.1038/35016043 |
| [2] |
M.A. Famiano, R.N. Boyd, T. Kajino, T. Onaka, Astrobiology 18 (2018) 190-206. DOI:10.1089/ast.2017.1686 |
| [3] |
A. Oda, T. Nakayoshi, S. Fukuyoshi, et al., Chirality 30 (2018) 332-341. DOI:10.1002/chir.22821 |
| [4] |
M.F. Guasti, Phys. Lett. A 383 (2019) 3180-3186. DOI:10.1016/j.physleta.2019.06.002 |
| [5] |
A. Yachmenev, J. Onvlee, E. Zak, A. Owens, J. Kupper, Phys. Rev. Lett. 123 (2019) 243202. DOI:10.1103/PhysRevLett.123.243202 |
| [6] |
C. Viedma, Phys. Rev. Lett. 94 (2005) 065504. DOI:10.1103/PhysRevLett.94.065504 |
| [7] |
R.M. Hazen, T.R. Filley, G.A. Goodfriend, Proc. Natl. Acad. Sci. 98 (2001) 5487-5490. DOI:10.1073/pnas.101085998 |
| [8] |
X.C. Ye, J.X. Cui, B.W. Li, et al., Nat. Commun. 10 (2019) 1964. DOI:10.1038/s41467-019-09997-y |
| [9] |
J. Bailey, A. Chrysostomou, J.H. Hough, et al., Science 281 (1998) 672-674. DOI:10.1126/science.281.5377.672 |
| [10] |
J.Y. Wang, G.L. Zhuang, M.Q. Chen, et al., Angew. Chem. Int. Ed. 59 (2020) 1619-1626. DOI:10.1002/anie.201909401 |
| [11] |
J.Y. Kim, J. Yeom, G.P. Zhao, et al., J. Am. Chem. Soc. 141 (2019) 11739-11744. DOI:10.1021/jacs.9b00700 |
| [12] |
J. Yeom, B. Yeom, H. Chan, et al., Nat. Mater. 14 (2015) 66-72. DOI:10.1038/nmat4125 |
| [13] |
P. Yin, Z.M. Zhang, H. Lv, et al., Nat. Commun. 6 (2015) 6475. DOI:10.1038/ncomms7475 |
| [14] |
C. Viedma, J.M. McBride, B. Kahr, P. Cintas, Angew. Chem. Int. Ed. 52 (2013) 10545-10548. DOI:10.1002/anie.201303915 |
| [15] |
P.G. Higgs, N. Lehman, Nat. Rev. Genet. 16 (2015) 7-17. DOI:10.1038/nrg3841 |
| [16] |
W. Gilbert, Nature 319 (1986) 618-618. |
| [17] |
P. Schimmel, Annu. Rev. Biochem. 56 (1987) 125-158. DOI:10.1146/annurev.bi.56.070187.001013 |
| [18] |
M. Ibba, D. Soll, Annu. Rev. Biochem. 69 (2000) 617-650. DOI:10.1146/annurev.biochem.69.1.617 |
| [19] |
D. Soll, Nucleic Acids Symp. Ser. (Oxf) (2004) 283-284. |
| [20] |
K. Tamura, P. Schimmel, Science 305 (2004) 1253-1253. DOI:10.1126/science.1099141 |
| [21] |
K. Tamura, P.R. Schimmel, Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 13750-13752. DOI:10.1073/pnas.0606070103 |
| [22] |
K. Tamura, Int. J. Mol. Sci. 12 (2011) 4745-4757. DOI:10.3390/ijms12074745 |
| [23] |
H. Griesser, M. Bechthold, P. Tremmel, E. Kervio, C. Richert, Angew. Chem. Int. Ed. 129 (2017) 1244-1248. DOI:10.1002/ange.201610651 |
| [24] |
N.S. Wickramasinghe, J.C. Lacey, Orig. Life Evol. Biospheres 22 (1992) 361-368. DOI:10.1007/BF01809372 |
| [25] |
J.P. Biron, A.L. Parkes, R. Pascal, J.D. Sutherland, Angew. Chem. Int. Ed. 44 (2005) 6731-6734. DOI:10.1002/anie.200501591 |
| [26] |
J. Ying, S. Fu, X. Li, et al., Chem. Commun. 54 (2018) 8598-8601. DOI:10.1039/C8CC04767G |
| [27] |
T. Wang, P. Zhang, G. Hu, et al., ChemistrySelect 3 (2018) 7849-7855. DOI:10.1002/slct.201800965 |
| [28] |
D.S. Kelley, J.A. Karson, D.K. Blackman, et al., Nature 412 (2001) 145-149. DOI:10.1038/35084000 |
| [29] |
W. Martin, J. Baross, D. Kelley, M.J. Russell, Nat. Rev. Microbiol. 6 (2008) 805-814. DOI:10.1038/nrmicro1991 |
| [30] |
J. Lacey Jr, D. Mullins, Orig. Life 13 (1983) 3-42. DOI:10.1007/BF00928761 |
| [31] |
Z. Wang, W. Xu, L. Liu, T.F. Zhu, Nat. Chem. 8 (2016) 698-704. DOI:10.1038/nchem.2517 |
| [32] |
J.J. Ling, C. Fan, H. Qin, et al., Angew. Chem. Int. Ed. 59 (2020) 3724-3731. DOI:10.1002/anie.201914799 |
 2022, Vol. 33
2022, Vol. 33 

