b Department of Organic Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden
Fluorine has the second smallest atomic radius, the highest electronegativity and it forms the strongest single bond with carbon. The introduction of fluorine into a bioactive molecule can dramatically modify its physico-chemical properties, such as acidity, lipophilicity and stability [1-4]. These modifications usually enhance the biological activity by affecting any parameters, such as binding to target receptors or enzymes and transporting bioactive molecules from the point of application to the target site, as well as preventing metabolic deactivation.
Even though there are so many advantages of fluorine-containing molecules, the introduction of fluorine into a molecule always causes a significant increase in cost. This is because of the lack of simple, efficient and cheap fluorine introduction method in agrochemical industry. As we all know, the most common method for fluorine introduction is to use fluorine-containing building blocks [5].
The dramatic effect of fluorine on the biological activity of agrochemicals such as fungicides, insecticides, herbicides, acaricides, and nematicides has earned fluorine a unique place in the toolbox of the agrochemical chemist [6-10]. Recently, Shibata and coworkers reviewed almost all the existing fluorine-containing agrochemicals by subdividing them into several categories, including agrochemical type, chemotype, mode of action, heterocycle and chirality [11]. However, the fluorine introduction methods of these pesticides have not been properly discussed. We believe the discussion of this issue is very critical, because it may provide ideas for the development of new and economical pesticide synthetic routes, and it may also promote agricultural chemists to develop new fluorine introduction methods and create new pesticides.
This review aims to present the fluorine introduction approaches for the agrochemicals that received an ISO name during the last decade (January 2011 to December 2020) [12]. In fact, it is basically equivalent to summarize the introduction methods of fluorine-containing building blocks that have been used for these agrochemicals. There are 54 fluorine containing molecules that received an ISO name in that period, we selected 40 of them based on diversity of chemical or synthetic methods.
Since the authors pay more attention to the fluorine introduction step, the complete synthetic route is sometimes not presented in this review. For example, the synthesis of fluorine-free building blocks is not included in this review. In most cases, the exact synthetic route of an agrochemical is not fully disclosed by the manufacturer. The authors extracted synthetic routes from published papers or patents. Consequently, the fluorine introduction methods described herein might be close to the manufacturing methods, but will unlikely be exactly the same. Nevertheless, this review intends to show the type and scope of fluorine-containing building blocks and small molecules most likely to be used for the synthesis of latest agrochemicals, as well as how exactly they have been used.
Generally, the synthesis of easily available fluorine-containing building blocks, such as fluorobenzene, trifluoromethylbenzene derivatives and small nonaromatic raw materials will not be discussed in this review. The general method for preparing fluorobenzene derivatives is the Balz-Schiemann reaction [13], in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate by using HBF4 as fluorine source (Scheme 1a). Alternatively, the Halex reaction [14] has been widely used to convert electron poor aromatic chlorides to the corresponding aromatic fluorides by using spray-dried KF as a fluorine source (Scheme 1b). Though there are a number of approaches for the preparation of the trifluoromethylbenzene derivatives, the most common industrial approach is the halogen exchange reaction involving the conversion of trichloromethylbenzene (Scheme 1c).

|
Download:
|
| Scheme 1. The general methods for preparing fluorobenzene and trifluoromethylbenzene derivatives. | |
This review is classified by type of agrochemical, including fungicides, herbicides, insecticides, acaricides and nematicides. In the fungicide section, fluorine introduction methods of 15 representative fungicides are described (Fig. 1).
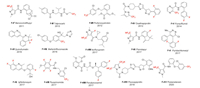
|
Download:
|
| Fig. 1. Structure of 15 fungicides featured in this review. a F-Ⅰ means fungicide Ⅰ. | |
Benzovindiflupyr (F-Ⅰ) was developed by Syngenta and received its ISO name in 2011. It is a pyrazole carboxamide fungicide, belongs to the class of succinate dehydrogenase inhibitors (SDHIs) [15]. It is active on a broad spectrum of diseases in cereals, especially efficient for soybean rust. The key step of the synthesis of benzovindiflupyr is the combination of oxime 8 and 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbonyl chloride 7 via Semmler-Wolff type aromatization and amidation (Scheme 2) [16]. Since 4, 4-difluoroacetoacetate 4 is not available in bulk quantities, the preparation of 7 starts from dichloroacetyl choloride 1. The fluorine introduction is achieved by Halex reaction of dichloroacetamide 2 with KF [17]. Then the difluoroacetamide 3 is converted to the ketoester 4 through an amido-Claisen approach discovered by Syngenta researchers. The reaction of 4 with triethyl orthoformate gives a mixture of the vinylether 5 in excellent yield [18], which reacts with methylhydrazine to obtain the pyrazolate 6 [19]. Compound 6 can be transferred to acid chloride 7 through sequential hydrolysis and chlorination. Intermediates 6 and 7 are both useful fluorine-containing building blocks in the synthesis of agrochemicals containing difluoromethyl pyrazole carboxamide moiety, such as pydiflumetofen, inpyrfluxam and pyrapropoyne (Scheme 3) [20-22].
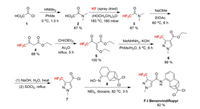
|
Download:
|
| Scheme 2. Fluorine introduction of benzovindiflupyr (F-Ⅰ). | |
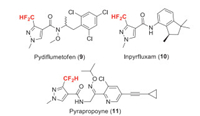
|
Download:
|
| Scheme 3. Representative fungicides containing difluoromethyl pyrazole carboxamide moiety. | |
2.2. Tolprocarb (F-Ⅱ)
Tolprocarb (F-Ⅱ) was developed by Mitsui Chemicals and received its ISO name in 2012. It is a carbamate fungicide developed for controlling rice blast, which is caused by the fungal pathogen Magnaporthe grisea. It is been recognized as a melanin biosynthesis polyketide synthase inhibitor (MBI-P) [23, 24]. The synthesis of tolprocarb starts from 2, 2, 2-trifluoroethanol 12, which is a commercially available fluorine source in bulk quantities (Scheme 4). The transformation from 12 to trifluoroethyl chloroformate 13 can be considered as the fluorine introduction step [25]. The carbamate coupling of valine 14 with trifluoroethyl chloroformate 13 affords 15 followed by a couple of transformations finally lead to tolprocarb [26].

|
Download:
|
| Scheme 4. Fluorine introduction of tolprocarb (F-Ⅱ). | |
2.3. Flufenoxystrobin (F-Ⅲ)
Flufenoxystrobin (F-Ⅲ) was developed by Shenyang Research Institute of Chemical Industry (SYRICI) and received its ISO name in 2012. It belongs to the group of strobilurin analogues, is a quinone outside inhibitor (QoI) fungicide [27]. Flufenoxystrobin exhibits excellent fungicidal activity against Erysiphe graminis and moderately high acaricidal activity against Tetranychus cinnabarinus [28]. Flufenoxystrobin is synthesized by alkylation of 2-chloro-4-(trifluoromethyl)phenol 17 with intermediate 18 (Scheme 5) [29]. Compound 17 can be manufactured by hydroxyl substitution of easily available building block 3, 4-dichlorobenzotrifluoride 16 [30].
|
Download:
|
| Scheme 5. Fluorine introduction of flufenoxystrobin (F-Ⅲ). | |
2.4. Oxathiapiprolin (F-Ⅳ)
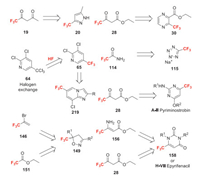
Oxathiapiprolin (F-Ⅳ) was developed by DuPont and received the ISO name in 2012. It belongs to the class of piperidinyl thiazole isoxazolines, and has extremely high efficacy against oomycete plant pathogens. Its mode of action involves binding to the oxysterol-binding protein in oomycetes [27]. Oxathiapiprolin has two fluorine-containing moieties [31, 32]. Trifluoromethyl group is introduced through condensation of trifluoroacetylacetone 19 with hydrazine, in which trifluoromethyl pyrazole 20 can be obtained in excellent yield (Scheme 6). The alkylation of 20 followed by nitrile group conversion affords the important intermediate 23. Compound 23 reacts with the chloroacetylisoxazoline derivative 27 to yield oxathiapiprolin. The required intermediate 27 is obtained in two steps from 2, 6-difluorobenzaldehyde 24. First, the Wittig reaction of 24 affords styrene 25. Then, 25 undergoes a 1, 3-dipolar cycloaddition with oxime 26 to obtain intermediate 27. Trifluoroacetylacetone 19 and difluorobenzaldehyde 24 used in the synthesis are both easily available raw materials.
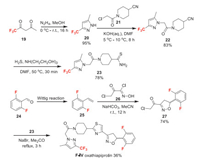
|
Download:
|
| Scheme 6. Fluorine introduction of oxathiapiprolin (F-Ⅳ). | |
2.5. Pyraziflumid (F-Ⅴ)
Pyraziflumid (F-Ⅴ) was discovered by Nihon Nohyaku and received the ISO name in 2014. It has unique pyrazine carboxamide skeleton, belongs to the class of succinate dehydrogenase inhibitors (SDHIs). Pyraziflumid shows excellent biological activity against ascomycete and basidiomycete fungi and exhibits good controlling activity against many diseases in the field [33]. Pyraziflumid also contains two different fluorinated moieties (Scheme 7). Trifluoromethyl group is introduced to the pyrazine moiety by cyclization and aromatization of 29 with ethylenediamine. Compound 29 can be synthesized through chlorination of raw material trifluoroacetoacetate 28 [34]. On the other hand, fluorine-containing building block 31 is first converted to boronic acid 32. The Suzuki coupling between 32 and 2-chloroaniline 33 leads to biphenyl amine intermediate 34 in excellent yield [35]. Finally, the amidation reaction between two fluorinated moieties 30 and 34 gives pyraziflumid [36].

|
Download:
|
| Scheme 7. Fluorine introduction of pyraziflumid (F-Ⅴ). | |
2.6. Quinofumelin (F-Ⅵ)
Quinofumelin (F-Ⅵ) was discovered by Mitsui Chemicals Agro and received its ISO name in 2016. It is a novel quinoline fungicide with excellent ability to control Botrytis cinerea, Sclerotinia sclerotiorum, blast disease, and anthracnose. Its mode of action is unknown. The fluorine introduction of quinofumelin is a late-stage fluorination (Scheme 8). The deoxofluorination of ketone intermediate 35 with 2, 2-difluoro-1, 3-dimethylimidazolidine (DFI) 36 [37] affords quinofumelin in excellent yield [38]. Alternatively, the debromofluorination of 1, 1-dibromo-substituted intermediate 37 with triethylamine trihydrofluoride also yields quinofumelin in the same yield [39].
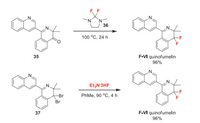
|
Download:
|
| Scheme 8. Fluorine introduction of quinofumelin (F-Ⅵ). | |
2.7. Mefentrifluconazole (F-Ⅶ)
Mefentrifluconazole (F-Ⅶ) was developed by BASF and received the ISO name in 2016. It is the first isopropanol triazole fungicide that belongs to sterol demethylation inhibitor (DMI) group. Mefentrifluconazole is used in several crops for the control of a broad range of important pathogens. It is active against different fungal stages both on the plant surface and in the plant tissue. Very recently, it has been reported to exhibit excellent protective and curative efficacy against Botrytis cinerea on detached leaves of cucumber [40]. The synthesis of mefentrifluconazole starts from fluorine-containing raw material meta-fluorobenzotrifluoride 38 (Scheme 9). First of all, compound 38 is converted to bromobenzene 40 by electrophilic bromination [41]. Then the debromoacetylation of 40 gives acetophenone 41 in excellent yield [42]. The defluoroetherification of 41 leads to key intermediate 43 which can be transferred to mefentrifluconazole eventually.

|
Download:
|
| Scheme 9. Fluorine introduction of mefentrifluconazole (F-Ⅶ). | |
2.8. Isoflucypram (F-Ⅷ)
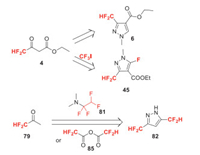
Isoflucypram (F-Ⅷ) was developed by Bayer and received its ISO name in 2017. It belongs to SDHI fungicide, exhibits long-lasting efficacy in controlling leaf spot diseases on a large range of crops [43]. The difluoromethyl group is introduced by using building block 4, 4-difluoroacetoacetate 4 whose synthesis has been discussed in Scheme 2. Compound 4 is first converted to trifluoromethylated intermediate 44 with CF3I. The novel cyclization process of 44 and methyl hydrazine developed by Bayer, can afford 3-difluoromethyl-5-fluoropyrazole carboxylate 45 in moderate yield (Scheme 10). Finally, 45 can be transferred to isoflucypram [44].
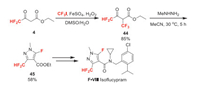
|
Download:
|
| Scheme 10. Fluorine introduction of isoflucypram (F-Ⅷ). | |
2.9. Fluindapyr (F-Ⅸ)
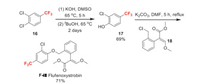
Fluindapyr (F-Ⅸ) was originally discovered by Isagro, now has been jointly developed by FMC and Isagro under a 2012 research and development collaboration agreement. It belongs to the succinate dehydrogenase inhibitor (SDHI) class of compounds. It has high levels of efficacy to a variety of crops, especially for Asian soybean rust (ASR) when used in certain premixed formulations. The key step of the synthesis of fluindapyr is the coupling of two fluorine-containing building blocks, pyrazole carboxylate 6 and aminoindane 49 (Scheme 11). The construction of compound 6 has been discussed in Scheme 2. The preparation of compound 49 starts from cheap raw material 4-fluoroaniline 46. Compound 46 undergoes cyclization with acetone at high temperature to afford 47. Then 47 is reduced by hydrogenation and protected by acetylation to give 48. Finally, the rearrangement of 48 promoted by sulfuric acid produces the key intermediate 49 in excellent yield [45].

|
Download:
|
| Scheme 11. Fluorine introduction of fluindapyr (F-Ⅸ). | |
2.10. Pyridachlometyl (F-Ⅹ)
Pyridachlometyl (F-Ⅹ) is a pyridazine fungicide from Sumitomo Chemical. A recent research showed that pyridachlometyl had a novel anti-tubulin mode of action which could be useful in anti-resistance management [46]. Pyridachlometyl exhibites potent antifungal activity against a broad range of fungal species belonging to the Ascomycota and Basidiomycota. The difluorobenzene moiety of pyridachlometyl comes from difluorobenzaldehyde 24 (Scheme 12). Compound 24 is first converted to cyano 50. Then 51 is obtained through hydrolysis and oxidation of 50. Next, 51 undergoes sequential Knoevenagel reaction with 52 and hydrazine promoted condensation reaction to afford precursor 53, which can be finally transferred to pyridachlometyl by phosphoryl chloride [47, 48].
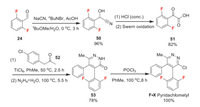
|
Download:
|
| Scheme 12. Fluorine introduction of pyridachlometyl (F-X). | |
2.11. Ipflufenoquin (F-Ⅺ)
Ipflufenoquin (F-Ⅺ) was developed by Nippon-Soda. It has stable efficacy against a wide range of plant diseases, such as gray mold, scab and rice blast. Its mode of action is unknown. Ipflufenoquin is synthesized by coupling of two fluorinated intermediates 60 and 62. The quinoline intermediate 60 can be synthesized from isatin 59 which is made from 2, 3-difluoroaniline 58 via Sandmeyer methodology [49]. The synthesis of compound 58 can be diverse. Here we present one possible manufacturing way (Scheme 13) [50]. The synthesis is based on Halex reaction and Balz-Schiemann reaction to introduce two fluorine atoms respectively. The acetophenone intermediate 62 can be simply synthesized by Grignard reaction of easily available 2, 6-difluorobenzonitrile 61 [51]. Then the defluoroetherification between 60 and 62 affords precursor 63 which can be finally converted to ipflufenoquin (Scheme 14) [52].
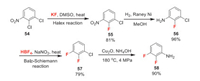
|
Download:
|
| Scheme 13. Fluorine introduction of 2, 3-difluoroaniline 58. | |
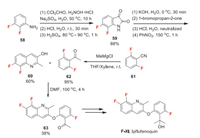
|
Download:
|
| Scheme 14. Fluorine introduction of ipflufenoquin (F-Ⅺ). | |

|
Download:
|
| Scheme 15. Fluorine introduction of fluopimomide (F-Ⅻ). | |
2.12. Fluopimomide (F-Ⅻ)
Fluopimomide (F-Ⅻ) is an innovative nicotinamide fungicide developed by Sino-Agri Union (Shandong Sino-Agri Union Biotechnology). It is a broad-spectrum fungicide used for management of Oomycetes and Rhizoctonia diseases [53]. Its mode of action is under development [54]. Fluopimomide is synthesized by coupling of trifluoromethyl pyridine 67 and tetrafluorobenzoic acid 71. For compound 67, the fluorine introduction is completed by halogen exchange with 64 as starting material [55]. Then 65 undergoes dechlorocyanation [56] and subsequent hydrogenation [57] to give 67. On the other hand, the fluorine introduction of 71 is based on Halex reaction of 68 [58]. The hydrolysis of cyano group of 69 followed by defluorohydroxylation of 70 affords 71 [59, 60]. In the end, the coupling of intermediates 67 and 71 gives fluopimomide in excellent yield [61].
2.13. Fluopimomide (F-ⅩⅢ)Fluopimomide (F-ⅩⅢ) is a neopicolinamide fungicide developed by Corteva Agriscience. Florylpicoxamid is the second member of the quinone outside inhibitors (QiI) class of picolinamide fungicides, joining fenpicoxamid [62]. While fenpicoxamid was obtained through derivatization of the natural product UK-2A, florylpicoxamid was discovered using UK-2A as an inspirational starting point (Scheme 16). Florylpicoxamid controls a wide range of pathogens including Septoria spp., powdery mildew, Botrytis spp., Anthracnose, Alternaria, scab, Monilinia, and others [27]. The fluorobenzene part of fluopimomide is introduced by Grignard reaction using easily available 1-bromo-4-fluorobenzene 72 as starting material [63]. The compound 74 can be easily converted to 76 by reaction with Boc-L-alanine 75. The dehydroxylation of 76 produced 77, which finally reacts with 78 to form fluopimomide (Scheme 17).

|
Download:
|
| Scheme 16. Structures of fenpicoxamid and UK-2A. | |
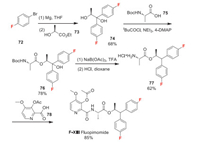
|
Download:
|
| Scheme 17. Fluorine introduction of fluopimomide (F-ⅩⅢ). | |
2.14. Fluoxapiprolin (F-ⅩⅣ)
Fluoxapiprolin (F-ⅩⅣ) is a new piperidinyl thiazole isoxazoline fungicide developed by Bayer Crop Science in 2012. It is effective against Phytophthora rot and downy mildew. Its mode of action has not been reported, but seems to be oxysterol binding protein inhibitors (OSBPI) in chemical structure. Recently, the resistance risk of Phytophthora capsici to fluoxapiprolin was investigated to be moderate [64]. Fluoxapiprolin is synthesized by the alkylation of bis-difluoromethyl pyrazole 82 (Scheme 18) [65]. Compound 82 can be produced by two different ways from the same raw material 1, 1-difluoroacetone 79 [66]. For example, 79 can react with hydrazine to give bis(1, 1-difluoropropan-2-ylidene)hydrazine 80, which then reacts with amine 81 to afford 82 [67]. Alternatively, 79 can also react with hydrazone 83 to get 84, which yields 82 by reaction with difluoroacetic anhydride 85 [68].
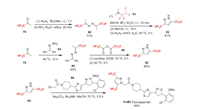
|
Download:
|
| Scheme 18. Fluorine introduction of fluoxapiprolin (F-ⅩⅣ). | |
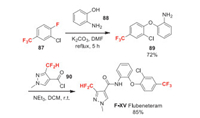
2.15. Flubeneteram (F-XⅤ)
Flubeneteram (F-XⅤ) is a promising fungicide candidate recently discovered in China in 2017. It contains a pyrazole carboxamide diphenyl ether moiety and is considered to be an SDHI based on its chemical structure. Flubeneteram displays excellent protection activity against Rhizoctonia solani and Sphaerotheca fuliginea [69]. Recently, the carbon-silicon switch strategy was used in the design and synthesis of a series of flubeneteram-silyl derivatives and the new silyl compound showed comparable and even higher fungicidal activities compared to flubeneteram lead compound [70]. For the synthesis of flubeneteram, its trifluoromethyl phenol moiety is made through the defluoroetherification of raw material 87 (Scheme 19). Compound 90 can be synthesized from pyrazole carboxylate 6 via simple transformations. The coupling of 89 and 90 affords flubeneteram in good yield [71].
|
Download:
|
| Scheme 19. Fluorine introduction of flubeneteram (F-XⅤ). | |
3. Insecticides
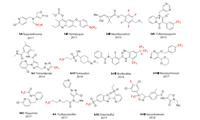
In the insecticide section, fluorine introduction methods of 12 representative insecticides are discussed (Fig. 2).
|
Download:
|
| Fig. 2. Structures of 12 insecticides featured in this review. Ⅰ-Ⅰ means insecticide Ⅰ. | |
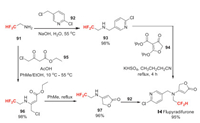
Flupyradifurone (Ⅰ-Ⅰ) was developed by Bayer Crop Science and received its ISO name in 2011. Flupyradifurone is used as an alternative insecticide for controlling sucking pest species, such as aphids, psyllids, stink bugs, and white flies. Although flupyradifurone is chemically classified as a butenolide, many scientists might regard it as a neonicotinoid. Both flupyradifurone and neonicotinoids have the same mode of action involving activation of the nicotinic acetylcholine receptor (i.e., nAChR agonist) and very similar chemical structures [72]. The difluoromethyl group of flupyradifurone is introduced by using difluoroethyl amine 91 as building block (Scheme 20). Bayer reported two different synthetic routes. One route starts with alkylation of 91 with chloromethyl pyridine 92 [73]. The obtained intermediate 93 reacts with 94 to afford the target product in high yield [74]. Another route starts with alkylation of 91 with 4-chloroacetoacetate 95. Then the cyclization of 96 gives 97, which is finally alkylated with 92 to afford flupyradifurone [75].
|
Download:
|
| Scheme 20. Fluorine introduction of flupyradifurone (Ⅰ-Ⅰ). | |
3.2. Flometoquin (Ⅰ-Ⅱ)
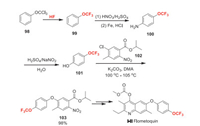
Flometoquin (Ⅰ-Ⅱ) has been presented by Nippon Kayaku as a new insecticide, which is active on controlling lepidoptera, diptera, hemiptera, and mites. It belongs to insect mitochondrial electron transport complex (Ⅲ) inhibitor, acts on complex Ⅲ Qi site [76]. In the synthetic route for flometoquin reported by Nippon Kayaku, 4-(trifluoromethoxy)phenol 101 is used directly. Here, we present a possible manufacturing way for the synthesis of compound 101 (Scheme 21) [77]. The trifluoromethoxy group is introduced by halogen exchange of 98. Then the para-selective nitration of 99 and the subsequent reduction yields 4-(trifluoromethoxy)aniline 100. Finally, the Sandmeyer reaction of 100 produces 101. The etherification reaction between 101 and 102 affords key intermediate 103 which can be converted to flometoquin eventually [78].
|
Download:
|
| Scheme 21. Fluorine introduction of flometoquin (Ⅰ-Ⅱ). | |
3.3. Momfluorothrin (Ⅰ-Ⅲ)
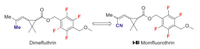
Momfluorothrin (Ⅰ-Ⅲ) was announced by Sumitomo Chemical as a new synthetic pyrethroid and received its ISO name in 2013. It is developed by introducing a cyano group to the acid moiety of the tetrafluorobenzyl ester type pyrethroid dimefluthrin (Scheme 22). Momfluorothrin exhibits excellent knockdown efficacy against the housefly and German cockroach [79]. The synthesis of key fluorine-containing building block 106 is completed in a similar way as compound 71 (Scheme 15). The difference is intermediate 105 is further converted to alcohol 106 through reduction and methylation (Scheme 23) [80]. The esterification of 106 with acid chloride 107 affords cyclopropane carboxylate 108 which can be finally converted to momfluorothrin [81].
|
Download:
|
| Scheme 22. Transformation of dimefluthrin to momfluorothrin. | |

|
Download:
|
| Scheme 23. Fluorine introduction of momfluorothrin (Ⅰ-Ⅲ). | |
3.4. Triflumezopyrim (Ⅰ-Ⅳ)
Triflumezopyrim (Ⅰ-Ⅳ) was discovered by DuPont as a novel and highly potent insecticide to control hoppers in rice. This compound has been found to have a very favorable environmental profile with minimal effects on fish, aquatic invertebrates, avian species, earthworms, beneficial arthropods, or honeybees. Triflumezopyrim belongs to the novel class of mesoionic insecticides, and binds to the orthosteric site of the nicotinic acetylcholine (nAChR) receptor and acts primarily via inhibition of the binding site [82]. Trifluoromethyl group is introduced through the coupling between 1-iodo-3-(trifluoromethyl)benzene 109 and dimethyl malonate 110 (Scheme 24). The obtained compound 111 is then hydrolyzed to give malonic acid derivative 112. Finally, the condensation of 112 and 113 produces triflumezopyrim [82].

|
Download:
|
| Scheme 24. Fluorine introduction of triflumezopyrim (Ⅰ-Ⅳ). | |
3.5. Tetraniliprole (Ⅰ-Ⅴ)
Tetraniliprole (Ⅰ-Ⅴ) was announced by Bayer Crop Science as a new diamide insecticide. It exhibits excellent control performance on lepidopteran pests. Tetraniliprole has a novel mode of action involving activation ryanodine receptor, which plays an important role in the muscle function of insects [83]. The trifluoromethyl tetrazole moiety is introduced through alkylation of 115 with 116 (Scheme 25). Compound 115 can be generally made from easily available 2, 2, 2-trifluoroacetamide 114 [84]. This transformation involves dehydration of 114 and subsequent 1, 3-dipolar cyclization with sodium azide [85].

|
Download:
|
| Scheme 25. Fluorine introduction of tetraniliprole (Ⅰ-Ⅴ). | |
3.6. Fluhexafon (Ⅰ-Ⅵ)
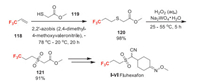
Fluhexafon (Ⅰ-Ⅵ) was developed by Sumitomo Chemical for controlling aphids and hoppers [86]. It has also been reported to be able to control cockroaches, house flies, and mosquitoes [86]. The mode of action has not yet been reported. The trifluoromethyl group is introduced through a novel radical addition reaction of methyl thioglycolate 119 to trifluoropropene 118 [87]. The obtained compound 120 is oxidized to sulfonyl acetate 121, which can be further converted to fluhexafon by a couple of steps [88].
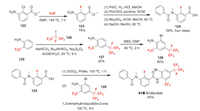
3.7. Broflanilide (Ⅰ-Ⅶ)Broflanilide (Ⅰ-Ⅶ) was announced by Mitsui Chemicals Agroin and received the ISO name in 2014. It has a unique meta-diamide chemical structure and exhibits high activity against various pests, including lepidopteran, coleopteran, and thysanopteran pests. Because broflanilide has a novel mode of action, the Insecticide Resistance Action Committee (IRAC) classified it as a member of a new group: Group 30 [89]. Broflanilide is classified as a negative allosteric modulator of insect γ-aminobutyric acid receptors (GABARs) as desmethyl-broflanilide allosterically inhibits the GABA-induced responses [90, 91]. The synthesis of broflanilide is completed by the coupling of two fluorine-containing building blocks 124 and 128 (Scheme 27) [92]. Compound 124 is synthesized from 122, which is first fluorinated through Halex reaction [92]. Compound 123 undergoes sequential reduction, benzoylation, methylation and hydrolysis to obtain 124 in good yield [92]. In the other hand, compound 128 is prepared by sequential radical alkylation of easily available 2-trifluoromethyl aniline 125 with heptafluoro-2-iodopropane 126 and bromination of 127 with NBS [93].
|
Download:
|
| Scheme 26. Fluorine introduction of fluhexafon (Ⅰ-Ⅵ). | |
|
Download:
|
| Scheme 27. Fluorine introduction of broflanilide (Ⅰ-Ⅶ). | |
3.8. Benzpyrimoxan (Ⅰ-Ⅷ)
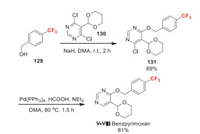
Benzpyrimoxan (Ⅰ-Ⅷ) was developed by Nihon Nohyaku as a novel insecticide structurally characterized by a pyrimidine derivative substituted with 1, 3-dioxanyl and 4-trifluoromethylbenzyloxy groups [94]. Benzpyrimoxan exhibits excellent activity against nymphal stages of rice plant hoppers, including strains resistant to existing insecticides [95]. Furthermore, benzpyrimoxan has low adverse effects on pollinators and beneficial arthropods. The study on its mode of action is undergoing. For the synthesis of benzpyrimoxan, 4-trifluoromethyl benzyl alcohol 129 is employed as raw material to introduce trifluoromethyl group (Scheme 28). The dechloroetherification of 130 with 129 leads to 131, which then undergoes palladium-catalyzed dechlorohydrogenation to afford benzpyrimoxan [94].
|
Download:
|
| Scheme 28. Fluorine introduction of benzpyrimoxan (Ⅰ-Ⅷ). | |
3.9. Flupyrimin (Ⅰ-Ⅸ)
Flupyrimin (Ⅰ-Ⅸ) was developed by Meiji Seika Pharma as a novel neonicotinoid insecticide. It exhibits remarkable biological properties featuring outstanding potency to neonicotinoid-insensitive rice insect pests and superior safety toward pollinators [96]. Intriguingly, flupyrimin acts on the insect nAChRs as an antagonist via a recognition manner different from those of the other nicotinic insecticides [97]. Flupyrimin is synthesized through trifluoroacetylation of 132 with trifluoroacetoacetate 28 and subsequent alkylation with 2-chloro-5-(chloromethyl)pyridine 92 in one-pot (Scheme 29) [98].
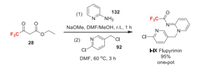
|
Download:
|
| Scheme 29. Fluorine introduction of flupyrimin (Ⅰ-Ⅸ). | |
3.10. Tyclopyrazoflor (Ⅰ-Ⅹ)
Tyclopyrazoflor (Ⅰ-Ⅹ) was developed by Dow (Corteva Agriscience) and received its ISO name in 2017. It is a pyrazole-containing insecticidal candidate with excellent activities against sap-feeding pests [99]. The synthesis of tyclopyrazoflor is based on the amidation of advanced pyridinylpyrazole derivative 136 with acid chloride 135. A radical strategy is applied for the synthesis of intermediate 134 with trifluoropropene 118 as raw material. Its synthesis is similar as the preparation of 120 in Scheme 26. The major challenge of this radical thiol−ene chemistry is the regioselectivity between the linear and branched products. Recently, Gray and coworkers reoptimized this transformation and developed a two-component initiator system consisting of benzoyl peroxide and N, N-dimethylaniline, which improved the overall yield and selectivity of the radical thiol−ene reaction from 78% yield and 11:1 selectivity with azobis(isobutyronitrile) to 91% yield and 50:1 selectivity (Scheme 30) [100].

|
Download:
|
| Scheme 30. Fluorine introduction of tyclopyrazoflor (Ⅰ-Ⅹ). | |
3.11. Oxazosulfyl (Ⅰ-Ⅺ)
Oxazosulfyl (Ⅰ-Ⅺ) was developed by Sumitomo Chemical as the first representative of a novel sulfyl class of insecticides [101]. It exhibits broad-spectrum control of insect pests, such as hemiptera, lepidoptera and coleoptera. Especially, this compound is effective in controlling major rice insects, including the three planthopper species, Nephotettix cincticeps, Cnaphalocrocis medinalis and Oulema oryzae [89]. Even though, its mode of action is unclear, Suzuki and Yamato recently discovered that the mechanism of oxazosulfyl involved the state-dependent blockage of voltage-gated sodium channels [102]. Oxazosulfyl can be synthesized as described in Scheme 31 [101]. The trifluoromethylthio group is introduced by halogen exchange of 137. Then the obtained compound 138 undergoes reduction and Sandmeyer reaction to afford 139. Compound 139 can produce key intermediate 140 through nitration and reduction processes [103]. Next, the condensation between aminophenol 140 and carboxylic acid 141 generates benzoxazole intermediate 142. In the end, cyclization of 142 and subsequent oxidation of trifluoromethylthio group and ethylthio group give oxazosulfyl.

|
Download:
|
| Scheme 31. Fluorine introduction of oxazosulfyl (Ⅰ-Ⅺ). DMEAD = bis(2-methoxyethyl)azodicarboxylate. | |
3.12. Isocycloseram (Ⅰ-Ⅻ)
Isocycloseram (Ⅰ-ⅩⅡ) was announced by Syngenta Crop Protection as a new member of the isoxazoline class of insecticides [103]. It exhibits broad control of a range of crop-damaging pests, in particular order lepidoptera, hemiptera, coleoptera, thysanoptera, diptera, and acari [103]. There is now a consensus that isoxazoline insecticides act as unique modulators of the invertebrate GABA-gated chloride channels mainly present in the insect central nervous system. The key step of the synthesis of isocycloseram is the construction of trifluoromethyl substituted isoxazoline ring which can be completed in two different routes. In Scheme 32, a [3+2] dipolar cyclization approach is shown. First of all, the para-selective borylation of fluorobenzene 143 affords 145. The Suzuki coupling of 145 and bromotrifluoropropene 146 produces key building block 147 [104]. Then the [3+2] dipolar cyclization of 147 and 148 affords trifluoromethyl isoxazoline 149, which can be finally converted to isocycloseram [105]. Alternatively, the isoxazoline core structure can also be assembled by the condensation of chalcone derivative 154 and hydroxylamine catalyzed by chiral phase transfer catalyst [103]. This synthetic breakthrough enabled an efficient access to highly enantioenriched mixtures of the desired compound [106]. The chalcone precursor 152 is made through Grignard reaction between 150 and trifluoroacetate 151 (Scheme 33).
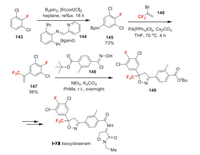
|
Download:
|
| Scheme 32. Fluorine introduction of isocycloseram (Ⅰ-ⅩⅡ). | |

|
Download:
|
| Scheme 33. Alternative fluorine introduction method of isocycloseram (Ⅰ-ⅩⅡ). PTC means phase transfer catalyst. | |
4. Herbicides
In the herbicide section, fluorine introduction methods of 7 representative herbicides are discussed (Fig. 3).
|
Download:
|
| Fig. 3. Structure of 7 herbicides featured in this review. a H-Ⅰ means herbicide Ⅰ. | |
Tiafenacil was developed by Farm Hannong as a new pyrimidinedione herbicide. It is a new protoporphyrinogen Ⅸ oxidase (PPO)-inhibiting herbicide, with IC50 values of 22-28 nmol/L for various plant species, including amaranth (Amaranthus tuberculatus), soybean (Glycine max), arabidopsis (Arabidopsis thaliana), and rapeseed (Brassica napus) [107]. The key step for preparing tiafenacil is the condensation between 2-fluorophenyl carbamate 156 and ethyl 3-amino-4, 4, 4-trifluorocrotonate 157 to afford the pyrimidinedione core structure 158 (Scheme 34) [108]. The precursor 156 can be made from easily available 4-chloro-2-fluoroaniline 155. It is worth noting that compound 157 is a useful raw material, which is generally made from the amidation of trifluoroacetylacetate 28.
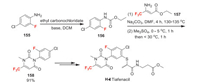
|
Download:
|
| Scheme 34. Fluorine introduction of tiafenacil (H-Ⅰ). | |
4.2. Trifludimoxazin (H-Ⅱ)
Trifludimoxazin was developed by BASF as a new 1, 3, 5-triazinane herbicide. It is intended for the non-selective pre-plant knockdown and selective pre-emergence residual control of a range of broadleaf weeds and suppression of key grass weeds prior to planting of cereal crops [109]. Trifludimoxazin inhibits the protoporphyrinogen oxidase (PPO) enzyme, ultimately interfering with the chlorophyll biosynthetic pathway [109]. The fluorine introduction of trifludimoxazin is achieved by coupling of 3-fluorophenol 159 and 2-bromo-2, 2-difluoroacetamide 160 (Scheme 35). The obtained intermediate 161 undergoes double-nitration to yield compound 162. Then 162 occurs reduction and intramolecular cyclization to obtain gem-difluoro-benzoxazinone intermediate 163, which can be finally converted to trifludimoxazin by a couple of transformations [110].
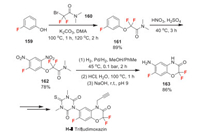
|
Download:
|
| Scheme 35. Fluorine introduction of trifludimoxazin (H-Ⅱ). | |
4.3. Florpyrauxifen (H-Ⅲ)
Florpyrauxifen was developed by Corteva Agriscience as an auxin-like herbicide, which structurally characterized by arylpicolinate. It is usually used as a benzyl derivative with various applications including controlling of grass, sedge and broadleaf weed in rice, as well as managing freshwater aquatic vegetation and invasive freshwater aquatic vegetation [111]. The specific mode of action of synthetic auxins is actually not fully known, but the possible effects on plants include alterations in cell wall elasticity and gene expression [111]. For the synthesis of florpyrauxifen, compound 165 is applied as a fluorine-containing building block. It can be made through the electrophilic fluorination of 164 with selectfluor [112] and subsequent methylation process. Another fluorine-containing building block 167 is prepared by borylation of raw material 166 [113]. In the end, the Suzuki coupling of 165 and 167 affords florpyrauxifen in good yield (Scheme 36) [114].
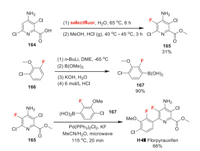
|
Download:
|
| Scheme 36. Fluorine introduction of florpyrauxifen (H-Ⅲ). | |
4.4. Beflubutamid-M (H-Ⅳ)
Beflubutamid was developed in the nineties by Ube Industries as a phenoxybutanamide herbicide. It is a soil herbicide for pre- and early post-emergence control of dicotyledonous weeds in cereals [115]. Beflubutamid consists of a pair of enantiomers and is marketed as racemates. However, the biotests have shown that (-)-beflubutamid has at least 1000-fold higher activity than (+)-beflubutamid [116]. So, the (-)-beflubutamid has received an ISO name as beflubutamid-M in 2018. Beflubutamid inhibits the enzyme phytoene-desaturase, which is involved in the biosynthesis of carotenoids. The chiral beflubutamid-M was isolated by high-performance liquid chromatography (HPLC) [116]. The synthesis of the racemic beflubutamid is shown in Scheme 37. First of all, fluorine is introducted by using 1-fluoro-2-(trifluoromethyl)benzene 168 as raw material. It undergoes nitration, reduction and Sandmeyer reaction to afford compound 169 [117]. Then the coupling of 169 and 170 gives ester intermediate 171 [118]. Finally, the amidation of 172 produces the racemic beflubutamid [119].
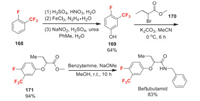
|
Download:
|
| Scheme 37. Fluorine introduction of beflubutamid. | |
4.5. Tetflupyrolimet (H-Ⅴ)
Tetflupyrolimet was developed by FMC Corporation as an aryl pyrrolidinone anilide herbicide. It provides season-long control of important grass weeds in the rice market, as well as key hard-to-control broadleaf weeds and sedges. Tetflupyrolimet is a new mode of action herbicide for the first time in last three decades, it interferes with de novo pyrimidine biosynthesis via the inhibition of dihydroorotate dehydrogenase (DHODH) [120]. The FMC Corporation reported a synthetic route for tetflupyrolimet (Scheme 38) [121]. The synthesis starts from the coupling of 2-bromoacetophenone 173 and amine 174, in which 173 can be usually made by bromination of raw material 172. The obtained amine hydrochloric acid salt 175 undergoes asymmetric hydrogenation to produce alcohol 176 with R configuration. Compound 176 is then converted to 177 by sequential mesylation and alkylation. Next, the debenzylation followed by intramolecular cyclization affords lactam 178. Finally, the 2-fluoroaniline moiety is introduced by amidation of 178 to obtain tetflupyrolimet in high ee value.
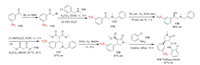
|
Download:
|
| Scheme 38. Fluorine introduction of tetflupyrolimet (H-Ⅴ). Ru cat. = dichloro[(5)-(-)-2, 2′, 6, 6′-tetramethoxy-4, 4′-bis(di(3, 5-xylyl)phosphino)-3, 3′-bipyridine][(15, 2S)-(-)-l, 2-diphenylethylenediamine]ruthenium(Ⅱ) (CAS Registry No. 821793-37-7). | |
4.6. Dimesulfazet (H-Ⅵ)
Dimesulfazet was developed by Nissan Chemical as a haloalkylsulfonanilide herbicide used in paddy fields. Its mode of action is presumed to inhibit a very-long-chain fatty acid synthesis (VLCFAS) [122]. The fluorine introduction is achived by trifluoromethanesulfonylation of 181 with trifluoromethanesulfonic anhydride 180 (Scheme 39). Then the obtained compound 182 is reduced to 183. The amidation of 183 and 184 gives 185. Finally, the intramolecular cyclization of 185 promoted by base yields dimesulfazet [123].
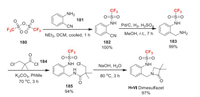
|
Download:
|
| Scheme 39. Fluorine introduction of dimesulfazet (H-Ⅵ). | |
4.7. Epyrifenacil (H-ⅥⅠ)
Epyrifenacil was developed by Sumitomo Chemical as a phenyluracil herbicide with excellent activity [124]. It is a protoporphyrinogen oxidase (PPOX) inhibitor. The key intermediate of the synthesis of epyrifenacil is phenylether 193, which can be made in different routes. Here, we select one route that has been reported by Syngenta [125]. The synthesis starts with the alkylation of fluoring-containing raw material 186 with 187. The obtained 188 undergoes cyclization with 1, 1, 3, 3-tetramethoxypropane 189 to produce phenylether 190. Then the O-alkylation of 190 with diazo compound 191 affords 192. The nitration and subsequent reduction of 192 yields 193. Next, the trifluoromethyl group is introduced by amidation between 193 and trifluoroacetoacetate 28 to obtain 194 [126]. In the end, the uracil moiety is assembled by the condensation of 194 and potassium cyanate (Scheme 40).
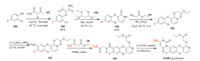
|
Download:
|
| Scheme 40. Fluorine introduction of epyrifenacil (H-Ⅶ). | |
5. Acaricides and nematicides
In this section, fluorine introduction methods of 4 acaricides and 2 nematicides are discussed (Fig. 4).
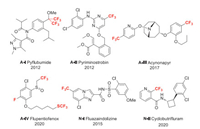
|
Download:
|
| Fig. 4. Structures of 4 acaricides and 2 nematicides featured in this review. A-Ⅰ means acaricide Ⅰ. B-Ⅰ means nematicide Ⅰ. | |
Pyflubumide was developed by Nihon Nohyaku as a novel carboxanilide acaricide which has a unique methoxyhexafluoroisopropyl substituent on the aniline [127]. It exhibits high activity against phytophagous mites, including populations of spider mites that have developed resistance to conventional acaricides. Pyflubumide is a novel inhibitor of mitochondrial complex Ⅱ on the respiratory chain [127]. The fluorine moiety is introduced by employing heptafluoro-2-iodopropane 126 as raw material (Scheme 41). It undergoes a radical reaction with aniline 195 to obtain 196. Then the defluoromethoxylation of 196 with sodium methoxide affords 197 [128]. Finally, 197 undergoes twice amidation reactions with acid chloride 198 and 199 to yield pyflubumide [129].
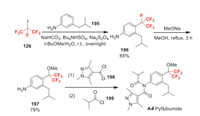
|
Download:
|
| Scheme 41. Fluorine introduction of pyflubumide (A-Ⅰ). | |
5.2. Pyriminostrobin (A-Ⅱ)
Pyriminostrobin was announced by Shenyang Research Institute of Chemical Industry (SYRICI) as a novel methoxyacrylate strobilurin acaricide. It exhibits notable control of Tetranychus cinnabarinus [130]. The acaricidal potency of pyriminostrobin is higher than that of fluacrypyrim in greenhouse application, and is comparable with those of commercial acaricides such as spirodiclofen and propargite in field trials [130]. For the synthesis of pyriminostrobin, trifluoroacetoacetate 28 is used for the construction of pyrimidine moiety (Scheme 42). The condensation of 28 and phenyl guanidine 200 affords intermediate 201 in excellent yield [131]. Then the O-alkylation of compound 201 with 18 produces pyriminostrobin.

|
Download:
|
| Scheme 42. Fluorine introduction of pyriminostrobin (A-Ⅱ). | |
5.3. Acynonapyr (A-Ⅲ)
Acynonapyr was developed by Nippon Soda as a new acaricide, which structurally characterized by an azabicyclo ring. It exhibits a selective effect on spider mites of tetranychus and panonychus. Its application is currently being expanded to fruit, tea, vegetables, and flowering fields. Acynonapyr has been reported to act on glutamate receptors and interfere neurotransmission [27]. The synthesis of acynonapyr starts with the alkylation of raw material 202 to obtain compound 203 (Scheme 43). The defluoroetherification of 203 with azabicycle 204 and subsequent debenzylation process produces intermediate 205. Then 205 is oxidized to hydroxylamine 206 [132]. The final O-arylation of 206 affords acynonapyr with trifluoromethyl pyridine 207 as aromatic source [133]. Compound 207 can be easily prepared in the same way as compound 65 (Scheme 15) by halogen exchange.
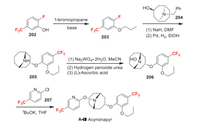
|
Download:
|
| Scheme 43. Fluorine introduction of acynonapyr (A-Ⅲ). | |
5.4. Flupentiofenox (A-Ⅳ)
Flupentiofenox was developed by Kumiai Chemical Industry as an alkylphenylsulphide acaricide candidate [134]. It has three different fluorine-containing functional groups. The fluorobenzene moiety is introduced by using 4-chloro-2-fluorophenol 208 as starting material (Scheme 44). It can be transferred to fluorobenzenethiol 212 by sequential O-protection, Friedel-Crafts-type thiolation, radical reduction and O-deprotection [135]. Then compound 212 is converted to 214 by alkylation with 213 which comes from trifluoroethanol 12. In this step, the trifluoromethyl group is introduced. On the other hand, the trifluoromethylthiolation of 215 produces 216 by in situ generated trifluoromethylthio anion [136]. Next, the coupling between 214 and 216 yields compound 217, in which all the three fluorine-containing functional groups are assembled [137]. In the end, 217 is converted to flupentiofenox by oxidation with mCPBA [138].
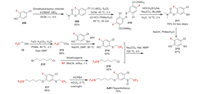
|
Download:
|
| Scheme 44. Fluorine introduction of flupentiofenox (A-Ⅳ). | |
5.5. Fluazaindolizine (N-Ⅰ)
Fluazaindolizine was developed by DuPont Company as a new member of the class of imidazopyridine nematicides [139]. Fluazaindolizine exhibits excellent control of plant parasitic nematodes and the damage they cause to plant roots. Specificity for nematodes coupled with absence of activity against the target sites of commercial nematicides suggests that fluazaindolizine has a novel mode of action. The core trifluoromethyl imidazopyridine structure (220) of fluazaindolizine is constructed by condensation of 2-aminopyridine 218 and ketoester 219 (Scheme 45) [140]. Compound 218 is obtained via amination of 65 [141], the synthesis of which has been shown in Scheme 15. The coupling of 220 and 221 produces imidazopyridine in good yield.
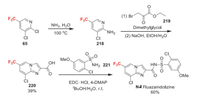
|
Download:
|
| Scheme 45. Fluorine introduction of fluazaindolizine (N-Ⅰ). | |
5.6. Cyclobutrifluram (N-Ⅱ)
Cyclobutrifluram was announced by Syngenta as a novel nematicides. It contains 80%–100% of the (1S, 2S)-enantiomer and 20%–0% of the (1R, 2R)-enantiomer. It is a novel multi-use pesticide that offers control of a broad spectrum of nematode pests and diseases across all major crops. Cyclobutrifluram is presumed to be an inhibitor of the mitochondrial electron transport chain complex Ⅱ based on its similarity in chemical structure to fluopyram [27]. The Syngenta Company reported a route for the synthesis of (1S, 2S) enantiomer of cyclobutrifluram [142]. In this synthesis, the key step is the coupling of chiral cyclobutylamine 227 and 2-trifluoromethyl nicotinic acid 226 (Scheme 46). Here, we present a general synthetic approach for preparing compound 226 [143]. First of all, trifluoroacetic anhydride 222 reacts with ethyl vinyl ether to obtain compound 223. Then the addition of 224 to 223 followed by cyclization affords 2-trifluoromethyl nicotinic acid ethyl ester 225. Finally, 225 is hydrolyzed to produce acid 226.
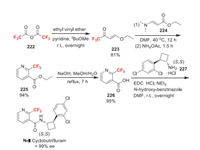
|
Download:
|
| Scheme 46. Fluorine introduction of cyclobutrifluram (N-Ⅱ). | |
Here we summarize all the fluorine-containing building blocks that have been used in this review (Figs. 5-9). There are 22 fluorobenzene and trifluoromethyl benzene building blocks that have been used directly (Fig. 5). Their colorful reaction pathways for being introduced into the final agrochemicals are described in the main text.
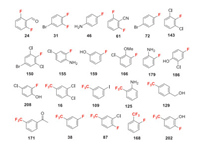
|
Download:
|
| Fig. 5. Fluoroarene and trifluoromethylarene building blocks used directly. | |

|
Download:
|
| Fig. 6. Fluoroarene building blocks synthesized. | |
|
Download:
|
| Fig. 7. Difluoromethylarene building blocks synthesized. | |
|
Download:
|
| Fig. 8. Trifluoromethyl cyclic building blocks synthesized. | |

|
Download:
|
| Fig. 9. Other fluorine-containing building blocks. | |
The fluorine introduction method of five representative and not easily available fluoroarene building blocks are discussed in this review (Fig. 6). These methods include Halex reaction, Balz-Schiemann reaction and electrophilic fluorination with selectfluor.
Difluoromethyl pyrazoles are usually used for the synthesis of fungicides. They are made by condensation of fluorine-containing ketones with hydrazines or hydrazones (Fig. 7).
Trifluoromethyl cyclic building blocks include aromatic and non-aromatic structures. They are usually made through cyclization of small fluorine-containing molecules, except trifluoromethyl pyridine 65, which is obtained by halogen exchange (Fig. 8). It is worth noting that imidazopyridine 220 is made from 65.
There are also some other fluorine-containing building blocks that have been used in this review (Fig. 9). Some of them are used directly, like alcohol 12, amine 91, olefin 118, alkyl iodide 126, amide 160 and sulfonic anhydride 180. The trifluoromethyl ether 99 and trifluoromethyl thioether 138 are made by halogen exchange, and alkyl chloride 216 is made by nucleophilic substitution via in situ generated trifluoromethylthio anion.
It is worth noting that there is only one example of late-stage fluorination. The final fluorine introduction of fungicide quinofumelin can be completed either by deoxofluorination with DFI or debromofluorination with triethylamine trihydrofluoride (Scheme 8).
6. ConclusionThis review has showcased the fluorine introduction methods of 40 agrochemicals in the last decade. It can be seen throughout this review that fluoroarenes, difluomethylarenes and trifluomethylarenes are most widely used building blocks for the synthesis of agrochemicals. Fluorine-containing small molecules, such as alcohol, amine, ketoester, olefin, etc., are also widely used, either for the synthesis of more complex cyclic fluorine-containing building blocks or used directly in the final agrochemicals. Here we have not only shown the introduction of fluorine and fluorine-containing functional groups into representative building blocks, but also have revealed how exactly these building blocks were assembled into the final agrochemicals. We think the chemistries showed here can spark ideas for the development of new and economical pesticide synthesis routes, and stimulate researchers to develop new fluorine incorporation methods and creat new pesticides.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (Nos. 21732002, 22077071) and Frontiers Science Center for New Organic Matter, Nankai University (No. 63181206) for generous financial support for our programs. Dedicated to the 100th anniversary of Chemistry at Nankai University.
| [1] |
R. Berger, G. Resnati, P. Metrangolo, et al., Chem. Soc. Rev. 40 (2011) 3496-3508. DOI:10.1039/c0cs00221f |
| [2] |
K. Müller, C. Faeh, F. Diederich, Science 317 (2007) 1881-1886. DOI:10.1126/science.1131943 |
| [3] |
J. Nie, H.C. Guo, D. Cahard, J.A. Ma, Chem. Rev. 111 (2011) 455-529. DOI:10.1021/cr100166a |
| [4] |
E.P. Gillis, K.J. Eastman, M.D. Hill, et al., J. Med. Chem. 58 (2015) 8315-8359. DOI:10.1021/acs.jmedchem.5b00258 |
| [5] |
J.A. Ma, D. Cahard, Emerging Fluorinated Motifs, Wiley-VCH, Weinheim, 2020.
|
| [6] |
G. Theodoridis, Fluorine-containing agrochemicals: An overview of recent developments, in: A. Tressaud (Ed.), Fluorine and the Enviroment, Elsevier, New York, 2006, pp. 121-175.
|
| [7] |
Y.Y. Huang, X. Yang, Z. Chen, et al., Chem. Eur. J. 21 (2015) 8664-8684. DOI:10.1002/chem.201500361 |
| [8] |
Y. Zhu, J.L. Han, J.D. Wang, et al., Chem. Rev. 118 (2018) 3887-3964. DOI:10.1021/acs.chemrev.7b00778 |
| [9] |
P. Jeschke, ChemBioChem 5 (2004) 570-589. DOI:10.1002/cbic.200300833 |
| [10] |
P. Jeschke, Pest Manag. Sci. 73 (2017) 1053-1066. DOI:10.1002/ps.4540 |
| [11] |
Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai, N. Shibata, iScience 23 (2020) 101467-101520. DOI:10.1016/j.isci.2020.101467 |
| [12] |
The full list of ISO common names and some details on the molecules can be found at the following website: Compendium of Pesticide Common Names; Index of new ISO common names. http://www.alanwood.net/pesticides/index_new_frame.html.
|
| [13] |
D.T. Flood, Org. Synth. 13 (1933) 46. DOI:10.15227/orgsyn.013.0046 |
| [14] |
G.C. Finger, C.W. Kruse, J. Am. Chem. Soc. 78 (1956) 6034-6037. DOI:10.1021/ja01604a022 |
| [15] |
H. Walter, H. Tobler, D. Gribkov, C. Corsi, Chimia 69 (2015) 425-434. DOI:10.2533/chimia.2015.425 |
| [16] |
D. Gribkov, A. Müller, M. Lagger, F. Giordano, WO 2011/015416.
|
| [17] |
H. Walter, C. Corsi, J. Ehrenfreund, C. Lamberth, H. Tobler, WO 2006/005612.
|
| [18] |
J. Iwata, F. Nishio, T. Iwasa, et al., JP 2019/085371.
|
| [19] |
H. Nobeshima, N. Koyama, M. Harada, JP 2014/144930.
|
| [20] |
K. Patzer, J. Kratchmer, D. Blatta, WO 2021/074312.
|
| [21] |
S. Watanabe, JP 2020/109133.
|
| [22] |
T. Shigyo, N. Hasunuma, Y. Fukami, JP 2021/066685.
|
| [23] |
S. Banba, T. Hamada, N. Araki, et al., J. Pestic. Sci. 42 (2017) 25-31. DOI:10.1584/jpestics.D16-100 |
| [24] |
T. Hamada, M. Asanagi, T. Satozawa, et al., J. Pestic. Sci. 39 (2014) 152-158. |
| [25] |
N. Kamegawa, K. Morizane, JP 2012/067030.
|
| [26] |
H. Umetani, T. Kohno, H. Kamekawa, WO 2012/039132.
|
| [27] |
N. Umetsu1, Y. Shirai, J. Pestic. Sci. 45 (2020) 54-74. DOI:10.1584/jpestics.D20-201 |
| [28] |
L. Li, M. Li, H.W. Chi, et al., J. Fluor. Chem. 185 (2016) 173-180. DOI:10.1016/j.jfluchem.2016.03.013 |
| [29] |
C.L. Liu, H.W. Chi, D.L. Cui, et al., WO 2007/000098.
|
| [30] |
W.O. Johnson, EP 0019388, 1980.
|
| [31] |
M.A. Hanagan, A.J. Liepa, E.A. Marshall, et al., WO 2011/146182.
|
| [32] |
A.J. Liepa, R.J. Pasteris, T.M. Stevenson, WO 2011/085170.
|
| [33] |
K. Kikutake, T. Furuya, M. Hasebe, et al., J. Pestic. Sci. 45 (2020) 184-190. DOI:10.1584/jpestics.J20-02 |
| [34] |
M. Oda, Y. Morishita, WO 2010/122794.
|
| [35] |
M. Kawaguchi, JP 2013/023466.
|
| [36] |
M. Oda, Y. Morishita, WO 2010/122794.
|
| [37] |
H. Hayashi, H. Sonoda, K. Fukumura, et al., Chem. Commun. 15 (2002) 1618-1619. |
| [38] |
H. Ito, H. Komai, F. Kajino, JP 2013/124248.
|
| [39] |
H. Umetani, N. Kondo, F. Kajino, WO 2013/047749.
|
| [40] |
T.T. Li, H.L. Li, T.T. Liu, et al., Pest. Biochem. Physiol. 173 (2021) 104784-104791. DOI:10.1016/j.pestbp.2021.104784 |
| [41] |
G.Y. Li, Y.Z. Jun, CN 101759597, 2013.
|
| [42] |
U.J. Vogelbacher, J. Gebhardt, M. Rack, et al., WO 2015/091045.
|
| [43] |
P. Desbordes, B. Essigmann, S. Gary, et al., Pest Manag. Sci. 76 (2020) 3340-3347. DOI:10.1002/ps.5951 |
| [44] |
S. Pazenok, W. Etzel, N. Lui, A. Neeff, WO 2014/023667.
|
| [45] |
P. Bellandi, G. Zanardi, R.V. Datar, et al., WO 2017/178868.
|
| [46] |
Y. Matsuzaki, S. Watanabe, T. Harada, F. Iwahashi, Pest Manag. Sci. 76 (2020) 1393-1401. DOI:10.1002/ps.5652 |
| [47] |
Y. Nakae, A. Manabe, T. Miyamoto, WO 2014/129612.
|
| [48] |
T. Toriumi, JP 2015/214503.
|
| [49] |
T. Sandmeyer, Helv. Chim. Acta 2 (1919) 234-242. DOI:10.1002/hlca.19190020125 |
| [50] |
R.B. He, J.X. Zhong, Y.M. Wang, et al., CN 101245020, 2011.
|
| [51] |
T. Ito, K. Hirakuri, JP 2016/169165.
|
| [52] |
K. Shibayama, J. Inagaki, Y. Saiki, et al., WO 2011/081174.
|
| [53] |
R. Zhang, H.Y. Wang, H. Xv, et al., J. Pestic. Sci. 39 (2014) 43-47. DOI:10.1584/jpestics.D13-017 |
| [54] |
H. Zhang, M. Zhai, K. Wang, et al., Chin. J. Pesticide Sci. 15 (2013) 405-411. |
| [55] |
B Wang, CN 101062914, 2007.
|
| [56] |
P.D. Riordan, M.R. Amin, T.H. Jackson, WO 2001/017970.
|
| [57] |
N. Dann, P.D. Riordan, M.R. Amin, M. Mellor, WO 2002/016322.
|
| [58] |
K. Hirota, JP 2004/075556.
|
| [59] |
Z.H. Guo, J.C. Gu, X.J. Lin, X.B. Fan, CN 106673964, 2017.
|
| [60] |
Z. Zhang, D.P. Jiang, H.X. Zhang, Y.X. Li, CN 108069832, 2018.
|
| [61] |
J.F. Tang, A.L. Wang, CN 102086173, 2011.
|
| [62] |
K.G. Meyer, C.L. Yao, Y. Lu, et al., The discovery of florylpicoxamid, a new picolinamide for disease control, in: P. Maienfisch, S. Mangelinckx (Eds.), Recent Highlights in the Discovery and Optimization of Crop Protection Products, Academic Press, Cambridge, Massachusetts, 2021, pp. 433-442.
|
| [63] |
G.T. Whiteker, N. Choy, P. Borromeo, et al., WO 2018/009618.
|
| [64] |
J.Q. Miao, C.C. Li, X.F. Liu, et al., J. Agric. Food Chem. 69 (2021) 3827-3835. DOI:10.1021/acs.jafc.0c05119 |
| [65] |
G. Hoemberger, M.J. Ford, WO 2015/181097.
|
| [66] |
E. Schmitt, A. Panossian, J.P. Vors, et al., Chem. Eur. J. 22 (2016) 11239-11244. DOI:10.1002/chem.201601621 |
| [67] |
S. Pazenok, M.J. Ford, A. Neeff, WO 2015/067802.
|
| [68] |
S. Pazenok, N. Liu, WO 2015/189141.
|
| [69] |
L. Xiong, H. Li, L.N. Jiang, et al., J. Agric. Food Chem. 65 (2017) 1021-1029. DOI:10.1021/acs.jafc.6b05134 |
| [70] |
G. Wei, M.W. Huang, W.J. Wang, et al., J. Agric. Food Chem. 69 (2021) 3965-3971. DOI:10.1021/acs.jafc.0c07322 |
| [71] |
G.F. Yang, L. Xiong, Q. Chen, CN 104557709, 2017.
|
| [72] |
R. Nauen, P. Jeschke, R. Velten, et al., Pest Manag Sci 71 (2015) 850-862. DOI:10.1002/ps.3932 |
| [73] |
W.A. Moradi, G. Schlegel, A. Schnatterer, WO 2015/140198.
|
| [74] |
N. Lui, J.D. Heinrich, WO 2010/105772.
|
| [75] |
N. Lui, J.D. Heinrich, WO 2009/036898.
|
| [76] |
X.L. He, Pesticide News 19 (2018) 36-37. |
| [77] |
C.S.R. Kokatam, A.N. Chimmiri, P. Kumar, et al., WO 2016/125185.
|
| [78] |
Y. Kato, S. Shimano, A. Morikawa, et al., WO 2010/007964.
|
| [79] |
T. Mori, Y. Tanaka, T. Uekawa, et al., Jpn. J. Environ. Entomol. Zool. 28 (2017) 87-90. |
| [80] |
K. Hagiya, WO 2008/099966.
|
| [81] |
T. Mori, US 2003/0195119.
|
| [82] |
W.M. Zhang, C.W. Holyoke Jr, T.F. Pahutski, et al., Bioorg. Med. Chem. Lett. 27 (2017) 16-20. DOI:10.1016/j.bmcl.2016.11.042 |
| [83] |
D.N. Kambrekar, S. Jahagirdar, J. Aruna, Biochem. Cell. Arch. 17 (2017) 801-804. |
| [84] |
S. Pazenok, N. Lui, F. Volz, et al., WO 2011/157664.
|
| [85] |
C.C. Haeselhoff, A. Schnatterer, J. Vermehren, WO 2018/019693.
|
| [86] |
H. Mitsudera, WO 2009/005110.
|
| [87] |
M. Hirota, H. Miyazaki, T. Itoh, WO 2011/019076.
|
| [88] |
M. Kawamura, DE 102014004684, 2014.
|
| [89] |
IRAC Mode of action classification, version 9.4, 2020. https: //irac-online.org/mode-of-action/.
|
| [90] |
T. Nakao, S. Banba, Bioorg. Med, Chem. 24 (2016) 372-377. DOI:10.1016/j.bmc.2015.08.008 |
| [91] |
Y. Gao, Y.C. Zhang, F.S. Wu, et al., J. Agric. Food Chem. 68 (2020) 14768-14780. DOI:10.1021/acs.jafc.0c05728 |
| [92] |
Y. Aoki, Y. Kobayashi, H. Daido, et al., WO 2010/018857.
|
| [93] |
Y. Kobayashi, H. Katsuta, M. Nomura, et al., WO 2010/013567.
|
| [94] |
E. Satoh, R. Kasahara, K. Fukatsu, et al., J. Pestic. Sci. 46 (2021) 109-114. DOI:10.1584/jpestics.D20-069 |
| [95] |
E. Satoh, R. Kasahara, T. Aoki, et al., International Scholarly and Scientific Research & Innovation 11 (2017) 725-728. |
| [96] |
S. Kagabu, M. Mitomi, S. Kitsuda, et al., WO 2012/029672.
|
| [97] |
Y. Onozaki, R. Horikoshi, I. Ohno, et al., J. Agric. Food Chem. 65 (2017) 7865-7873. DOI:10.1021/acs.jafc.7b02924 |
| [98] |
S. Kitsuda, N. Nakanishi, S. Sumi, WO 2018/052115.
|
| [99] |
A.M. Buysse, N.M. Niyaz, Y. Zhang, et al., WO 2013/162715.
|
| [100] |
K.C. Gray, P. Heider, P. McGough, et al., Org. Process Res. Dev. 23 (2019) 2142-2147. DOI:10.1021/acs.oprd.9b00129 |
| [101] |
M. Ito, Y. Nokura, M. Takahashi, et al., Discovery of oxazosulfyl: A novel broad-spectrum insecticide, in: P. Maienfisch, S. Mangelinckx (Eds.), Recent Highlights in the Discovery and Optimization of Crop Protection Products, Academic Press, Cambridge, Massachusetts, 2021, pp. 261-267.
|
| [102] |
T. Suzuki, S. Yamato, J. Agric. Food Chem. 69 (2021) 4048-4055. DOI:10.1021/acs.jafc.0c04617 |
| [103] |
J. Cassayre, T. Smejkal, J. Blythe, et al., The discovery of isocycloseram: A novel isoxazoline insecticide, in: P. Maienfisch, S. Mangelinckx (Eds.), Recent Highlights in the Discovery and Optimization of Crop Protection Products, Academic Press, Cambridge, Massachusetts, 2021, pp. 165-212.
|
| [104] |
T. Akama, T.W. Balko, J.M. Defauw, et al., US 2013/0131016.
|
| [105] |
M. El Qacemi, J.Y. Cassayre, WO 2013/050302.
|
| [106] |
K. Matoba, H. Kawai, T. Furukawa, et al., Angew. Chem. Int. Ed. 49 (2010) 5762-5766. DOI:10.1002/anie.201002065 |
| [107] |
J. Park, Y.O. Ahn, J.W. Nam, et al., Pestic. Biochem. Physiol. 152 (2018) 38-44. DOI:10.1016/j.pestbp.2018.08.010 |
| [108] |
K. Lee, J. Kuk, Y. C, Ahn, J. Lee, WO 2018/128387.
|
| [109] |
Public release summary on the evaluation of the new active trifludimoxazin in the product voraxor herbicide, 2020. https://apvma.gov.au/node/65676.
|
| [110] |
M. Dochnahl, R. Goetz, F. Gebhardt, et al., WO 2014/026893.
|
| [111] |
Review of florpyrauxifen-benzyl for application to massachusetts lakes and ponds, 2019. https://www.mass.gov/doc/florpyrauxifen-benzyl/download.
|
| [112] |
S.C. Fields, W.C. Lo, W.K. Brewster, et al., Tetrahedron Lett. 51 (2010) 79-81. DOI:10.1016/j.tetlet.2009.10.089 |
| [113] |
J. Oppenheimer, M.V.M. Emonds, C.W. Derstine, R.C. Clouse, WO 2013/102078.
|
| [114] |
J.B. Epp, A.L. Alexander, T.W. Balko, et al., Bioorg. Med. Chem. 24 (2016) 362-371. DOI:10.1016/j.bmc.2015.08.011 |
| [115] |
S. Takamura, T. Okada, S. Fukuda, et al., Proc. Br. Crop Prot. Conf. Weeds 1 (1999) 41-46. |
| [116] |
I.J. Buerge, A. Bächli, J.P.D. Joffrey, et al., Environ. Sci. Technol. 47 (2013) 6806-6811. DOI:10.1021/es301876d |
| [117] |
W.D. Zhang, CN 102766067, 2012.
|
| [118] |
Y. Kiyoshi, JP 10130204 A, 1998.
|
| [119] |
T. Takematsu, Y. Takeuchi, M. Takenaka, et al., EP 239414, 2016.
|
| [120] |
Dayan F.E, Plants 8 (2019) 341-358. DOI:10.3390/plants8090341 |
| [121] |
Y.Z. Chen, WO 2018/175226.
|
| [122] |
S. Tresch, M. Heilmann, N. Christiansen, et al., Phytochemistry 76 (2012) 162-171. DOI:10.1016/j.phytochem.2011.12.023 |
| [123] |
Y. Kusuoka, T. Akeboshi, T. Io, D. Nakamura, WO 2015/060402.
|
| [124] |
Y. Tohyama, Y. Sanemitsu, EP 1122244, 2001.
|
| [125] |
R. Beaudegnies, J. Cassayre, B.D. Gott, et al., WO 2007/083090.
|
| [126] |
Y. Sato, JP 2018/123060.
|
| [127] |
T. Furuya, K. Machiya, S. Fujioka, M. Nakano, K. Inagaki, J. Pestic. Sci. 42 (2017) 132-136. DOI:10.1584/jpestics.J17-02 |
| [128] |
N. Abe, O. Sanbe, JP 2010/275207.
|
| [129] |
S. Cao, C. Liu, J.J. He, et al., CN 111825616, 2020.
|
| [130] |
B.S. Chai, C.L. Liu, H.C. Li, et al., Pest. Manag. Sci. 67 (2011) 1141-1146. |
| [131] |
Y.P. Zhou, G.X. Wu, C.L. Liu, et al., CN 103387546, 2013.
|
| [132] |
I. Hamamoto, JP 2013/170128.
|
| [133] |
I. Hamamoto, K. Koizumi, M. Kawaguchi, et al., WO 2011/105506.
|
| [134] |
K. Domon, K. Toriyabe, Y. Ogawa, et al., WO 2013/157229.
|
| [135] |
K. Sambai, WO 2019/167814.
|
| [136] |
S. Yasumura, WO 2019/208355.
|
| [137] |
S. Yasumura, WO 2019/167733.
|
| [138] |
S. Ito, T. Matsuda, S. Mukawa, JP 2015/160813.
|
| [139] |
G.P. Lahm, J. Desaeger, B.K. Smith, et al., Bioorg. Med. Chem. Lett. 27 (2017) 1572-1575. DOI:10.1016/j.bmcl.2017.02.029 |
| [140] |
G.P. Lahm, R.M. Lett, B.T. Smith, et al., WO 2010/129500.
|
| [141] |
J. Jiricek, S.P. Ng, S.P.S. Rao, WO 2019/244049.
|
| [142] |
A.C. O'Sullivan, R.J.G. Mondiere, O. Loiseleur, et al., WO 2015/003951.
|
| [143] |
P. Mueller, EP 1340747, 2003.
|
 2022, Vol. 33
2022, Vol. 33 

