b CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Fluorescence plays an important role in biological recognition, sensing and imaging, and the sensitivity of single-molecule detection and nano-level spatial resolution are achieved through molecular labelling and fluorescent switches [1-9]. Triphenylamine (TPA) and derivatives exhibit outstanding properties of high quantum yield and photo-stability due to the aggregation-induced emission, which are now widely applied in the construction of solar cells, fluorescent probes, biological imaging materials and organic light-emitting devices [10-13].
In the recent paper [14], Xu et al. reported a series of TPA derivatives with various substituent groups, connecting to two pyridine units. These compounds created a range of internal charge transfer (ICT) systems in consequence of the combination of electron-withdrawing group pyridine moieties and electron donors TPA derivatives. It led to the formation of the ability in acid-induced multicolor fluorescence regulation, especially including white light emission. With the addition of trifluoroacetic acid (TFA), pyridines were protonated and the enhanced electron-withdrawing ability resulted in the red-shifts of absorption and emission wavelengths, along with the decrease of fluorescent intensity. All the processes were reversible upon the addition of trimethylamine. Besides, it is also noteworthy that most white light emission systems are composed of multiple chromophores [15-17], only a few examples benefit from the single fluorophore [18, 19]. In the Communication, the simple strategy was to regulate the ratio of free pyridine units and protonated pyridine units by adding TFA, giving rise to various degrees of ICT effects. Interestingly, the photo-induced electron transfer process was observed when −CH=C(CN)2 group exists, leading to different absorption and fluorescence characteristics in comparison with other groups, which illustrates the significance of the substituent effect. The mechanism of the acid-induced fluorescence regulation was revealed intuitively by DFT calculation, which confirmed the difference of LUMO's electron densities of TPA derivatives in two states. Inkjet printing was further achieved by doping the compounds into polyvinylidene fluoride films. These TPA derivatives exhibit a promising potential for the fabrication of simple but smart tunable multicolor fluorescent materials.
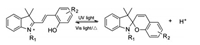
In the Communication, the acid-induced switchable process, which may be achieved by introducing the photoacid into the systems. A reversible radiation-responsive transducer can be established to switch the environmental pH [20, 21]. The photoacid could release a proton during the excited state and recombined a proton in a different environment (Fig. 1). Moreover, pyridine is a useful ligand in coordination complexes owing to the existence of its lone pair of nitrogen. Many pyridine coordination complexes have grown over the years, but always with fluorescence quenching phenomenon due to the heavy-atom effect [22]. Considering their high fluorescence quantum yield and efficient tunable fluorescence emission, the TPA derivatives mentioned in the Communication can be regarded as favorable ligands to construct powerful supramolecular coordination complexes (SCCs) including metallacycles and metallacages (Fig. 2). A series of good instances were presented by Yang, Xu and co-workers [23-25]. Because of the distinguished property of TPA derivatives, the self-assembled organoplatinum metallacycles can exhibit high fluorescence quantum yields and tunable fluorescence wavelengths, which provides an available efficient approach to overcome the heavy-atom effect of Pt(Ⅱ) to Pt-based SCCs.
|
Download:
|
| Fig. 1. An example of photoacid. | |

|
Download:
|
| Fig. 2. Possible metallacycles and metallacages by using the TPA derivatives 1-6 as ligands. The chemical structures of TPA derivatives were shown in Ref. [14]. | |
Near-infrared fluorophores are particularly fascinated and have attracted increasing attention owing to their biocompatibility in terms of good tissue penetration and low autofluorescence from adjacent tissues [26]. TPA derivatives are popular as electron donors to construct donor-acceptor type fluorophores, which can exhibit deep red or near-infrared (NIR) emission [27, 28]. With the help of the substituent effect, the TPA derivatives presented in the Communication can produce various emissions at different wavelengths, especially at 636 nm of wavelength with electron pull unit −CH=C(CN)2 group. Thus, these systems may be easy to emit NIR emission when connecting to enhanced electron pull units. Compared with the TPA derivatives reported in former literature [29], the TPA derivatives connecting to two pyridine units were easy to be synthesized. In addition, the exploration of the construction of white light emissive organic materials is of great importance because of their application in lighting sources [30]. Meanwhile, white light emission from single-molecule provides several merits including enhanced stability, good reproducibility [31]. Although the systems reported in the Communication are able to produce white light emission, this function was realized by the combination of two states. The TPA derivatives have a promising potential as a component to construct a white light emissive single-molecule in terms of their excellent tunable multicolor fluorescent characteristic [32].
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank Prof. Hai-Bo Yang (ECNU) for valuable discussions. This work is supported by the National Natural Science Foundation of China (Nos. 22078314, 21878286) and Dalian Institute of Chemical Physics (Nos. DICPI202142, DICPI201938, DICPZZBS201805).
| [1] |
J. Zhu, X. Liu, J. Huang, L. Xu, Chin. Chem. Lett. 30 (2019) 1767-1774. DOI:10.1016/j.cclet.2019.08.027 |
| [2] |
Q. Ling, T. Cheng, S. Tan, et al., Chin. Chem. Lett. 31 (2020) 2884-2890. DOI:10.1016/j.cclet.2020.08.020 |
| [3] |
L.J. Chen, S. Chen, Y. Qin, et al., J. Am. Chem. Soc. 140 (2018) 5049-5052. DOI:10.1021/jacs.8b02386 |
| [4] |
A.P. de Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, et al., Chem. Rev. 97 (1997) 1515-1566. DOI:10.1021/cr960386p |
| [5] |
C.Y. Yao, H. Y. Lin, H.S.N. Crory, A.P. de Silva, Mol. Syst. Des. Eng. 5 (2020) 1325-1353. DOI:10.1039/D0ME00082E |
| [6] |
Z. Li, X. Xia, Y. You, et al., Chin. Chem. Lett. 32 (2021) 1785-1789. DOI:10.1016/j.cclet.2020.12.053 |
| [7] |
Q. Qi, S. Jiang, Q. Qiao, et al., Chin. Chem. Lett. 31 (2020) 2985-2987. DOI:10.1016/j.cclet.2020.05.044 |
| [8] |
C. Li, P.P. Jia, Y.L. Xu, et al., Sci. China Chem. 64 (2021) 134-142. DOI:10.1007/s11426-020-9856-7 |
| [9] |
Y. Qin, X. Liu, P. P.-Jia, et al., Chem. Soc. Rev. 49 (2020) 5678-5700. DOI:10.1039/C9CS00797K |
| [10] |
C. Shen, Y. Wu, H. Zhang, et al., Angew. Chem. Int. Ed. 58 (2019) 3784-3789. DOI:10.1002/anie.201811593 |
| [11] |
P. Jia, L. Xu, Y. Hu, et al., J. Am. Chem. Soc. 143 (2021) 399-408. DOI:10.1021/jacs.0c11370 |
| [12] |
W. Yin, Y. Li, N. Li, et al., Adv. Optical Mater. 8 (2020) 1902027. DOI:10.1002/adom.201902027 |
| [13] |
S. Izumi, H.F. Higginbotham, A. Nyga, et al., J. Am. Chem. Soc. 142 (2020) 1482-1491. DOI:10.1021/jacs.9b11578 |
| [14] |
X. Liu, Y. Qin, J. Zhu, et al., Chin. Chem. Lett. 32 (2021) 1537-1540. DOI:10.1016/j.cclet.2020.10.012 |
| [15] |
X.Y. Lou, N. Song, Y.W. Yang, Chem. Eur. J. 25 (2019) 11975-11982. DOI:10.1002/chem.201902700 |
| [16] |
Z. Chen, C.L. Ho, L. Wang, W.Y. Wong, Adv. Mater. 32 (2020) 1903269. DOI:10.1002/adma.201903269 |
| [17] |
J. Wang, Y. Zhang, Y. Yu, et al., Opt. Mater. 89 (2019) 209-213. DOI:10.1016/j.optmat.2019.01.019 |
| [18] |
X.L. Ni, S. Chen, Y. Yang, Z. Tao, J. Am. Chem. Soc. 138 (2016) 6177-6183. DOI:10.1021/jacs.6b01223 |
| [19] |
Q. Wang, Q. Zhang, Q.W. Zhang, et al., Nat. Commun. (2020) 11. |
| [20] |
S. Silvi, A. Arduini, A. Pochini, et al., J. Am. Chem. Soc. 129 (2007) 13378-13379. DOI:10.1021/ja0753851 |
| [21] |
H. Kagel, F.F. Bier, M. Frohme, J.F. Glökler, Sci. Rep. 9 (2019) 14372. DOI:10.1038/s41598-019-50867-w |
| [22] |
Y.X. Hu, X. Hao, L. Xu, et al., J. Am. Chem. Soc. 142 (2020) 6285-6294. DOI:10.1021/jacs.0c00698 |
| [23] |
J.L. Zhu, L. Xu, Y.Y. Ren, et al., Nat. Commun. (2019) 10. |
| [24] |
B. Huang, X. Liu, G. Yang, et al., CCS Chem. (2021) 3. |
| [25] |
C.B. Huang, L. Xu, J.L. Zhu, et al., J. Am. Chem. Soc. 139 (2017) 9459-9462. DOI:10.1021/jacs.7b04659 |
| [26] |
S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol. 14 (2010) 71-79. DOI:10.1016/j.cbpa.2009.09.029 |
| [27] |
S. Wang, X. Yan, Z. Cheng, et al., Angew. Chem. Int. Ed. 54 (2015) 13068-13072. DOI:10.1002/anie.201506687 |
| [28] |
Y. Liu, Y. Yi, Y. Wang, Angew. Chem. Int. Ed. 56 (2017) 11525-11529. DOI:10.1002/anie.201706464 |
| [29] |
H.J. Yen, G.S. Liou, Polym. Chem. 9 (2018) 3001-3018. DOI:10.1039/C8PY00367J |
| [30] |
S. Reinike, F. Lindner, G. Schwartz, et al., Nature 459 (2009) 234-238. DOI:10.1038/nature08003 |
| [31] |
D. Li, W. Hu, J. Wang, et al., Chem. Sci. 9 (2018) 5709-5715. DOI:10.1039/C8SC01915K |
| [32] |
J. Cheng, D. Liu, L. Bao, et al., Chem. Asian J. 9 (2014) 3215-3220. DOI:10.1002/asia.201402779 |
 2022, Vol. 33
2022, Vol. 33 

