b College of Arts and Sciences, Shanxi Agricultural University, Taigu 030801, China;
c State Key Laboratory of Medicinal Chemical Biology, Research Center for Analytical Sciences, and Tianjin Key Laboratory of Biosensing and Molecular Recognition, College of Chemistry, Nankai University, Tianjin 300071, China
With the rapid development of modern agriculture, pesticides serve as the most potent weapon in fighting against pests and weeds. However, pesticides are generally harmful to human health and the environment. Nitrile (C≡N) is a bioisostere of carbonyl, halogen, and other functional groups. The introduction of nitrile into pesticides could enhance the interactions between pesticides and target proteins, thus improving the insecticidal efficacy [1]. Therefore, nitrile-bearing pesticides are widely used in agriculture. When spraying pesticides on crops, some pesticides are weakly adsorbed on the crop surfaces, most of which can be washed away by rainwater. However, some adsorbed pesticides tend to penetrate into the interior of crops, causing the formation of pesticide residues in agricultural products to harm our health [2]. Thus, it is imperative to monitor the pesticide residues in crop growth and related products in a non-destructive manner.
Several methods have been developed to detect pesticide residues, such as gas chromatography (GC) [3], high-performance liquid chromatography (HPLC) [4], liquid chromatography-mass spectrometry (LC-MS) [5], and photocatalytic sensing platform [6]. Although sensitive and accurate, these methods require complex sample preparation, time-consuming procedures, and expensive instruments. Besides, these methods rely on the extraction of pesticides from the crop samples before analysis. Therefore, they cannot provide the in-situ spatial-temporal information of pesticide residues in crops.
In recent years, surface-enhanced Raman spectroscopy (SERS) has become a powerful chemical analysis and imaging technique [7], which is usually used to detect trace substances. SERS has already shined high analytical performance in various fields because of its extremely high sensitivity (single-molecule level), insusceptibility to photobleaching, and non-destructivity to samples [8-11]. Over the past decades, SERS has been developed to detect pesticide residues such as thiabendazole [12], thiram [13] and methamidophos [14] in various kinds of crops. However, the SERS effect could enhance all the signals of the pesticide residues, unknown contaminates, and the endogenous components of crops simultaneously within the fingerprint region (< 1800 cm‒1), most likely causing spectral overlapping [15]. The spectral overlapping makes it difficult to accurately quantify and track the pesticide residues in crops and related products.
Recently, it has been reported that certain exogenous moieties such as alkyne, nitrile, azide, and deuterium show distinct and sharp peaks in the cellular Raman-silent spectral window (1800–2800 cm‒1) [16-18], where no signals can be detected for endogenous biomolecules. Based on the principle, exogenous moieties (typically alkyne and nitrile) were conjugated with target molecules to profile their physiological events in living cells without the background interference. In this study, we, for the first time, propose a background-free SERS (bf-SERS) strategy to non-destructively monitor the dynamic accumulation of nitrile-bearing pesticides in soybean leaves. We further investigated the effects of various rinsing conditions on the pesticide residues, providing a simple and effective means to control the use of pesticides during crop growth.
Acetamiprid and chlorothalonil were taken as examples to evaluate the efficacy of this bf-SERS method owing to their wide applications in agriculture. Acetamiprid is a neonicotinoid systemic insecticide, which mainly acts on the nicotinic acetylcholine receptors in the synapses of the insect nervous system [19]. It can be absorbed by the body to damage the human endocrine system [20]. Chlorothalonil, a broad-spectrum non-systemic pesticide, is highly toxic to amphibians and was listed as a 2B carcinogen by the World Health Organization (WHO) in 2017 [21, 22]. Recent studies have shown that chlorothalonil and its metabolites can inhibit the development of the mouse reproductive system [23].
We have started this study by determining the Raman spectra of acetamiprid and chlorothalonil powders (Figs. 1a and b). The assignments of the Raman peaks are provided in (Table S1 in Supporting information). Clearly, a single strong and sharp peak that is assigned to the C≡N of the pesticides can be detected in the Raman-silent window (1800–2800 cm‒1). To detect the pesticides in plants, 10 µL of acetamiprid and chlorothalonil solutions at a concentration of 200 mg/L were sprayed onto the soybean leaves. After drying, their Raman spectra were recorded under the same conditions. The results showed that the Raman signals of both acetamiprid and chlorothalonil were rather weak, which were masked by the background signals of the leaf themselves (Fig. S1 in Supporting information). Therefore, it is essential to employ SERS to track the pesticide residues.

|
Download:
|
| Fig. 1. Raman spectra of acetamiprid and chlorothalonil and their SERS effects. Raman spectra of the (a) acetamiprid and (b) chlorothalonil powders. SERS spectra of the 50 nm AuNPs towards different concentrations of (c) acetamiprid and (d) chlorothalonil sprayed on the soybean leaves. (e, f) The concentration-dependent Raman intensities of the bands at 2175 cm−1 as shown in (c) and (d) respectively. | |
Gold nanoparticles (AuNPs, 50 nm) were prepared as typical SERS substrates to amplify the Raman signals of the pesticide residues. The particles' plasmonic properties, morphology, and SERS effects towards the two pesticides were provided in Fig. S2 (Supporting information). Different concentrations of acetamiprid and chlorothalonil were sprayed onto the soybean leaves and then incubated with the freshly-prepared AuNPs (2 nmol/L). As a blank, a soybean leaf without the treatment of pesticides was only loaded with the same concentration of AuNPs. The results showed that the molecular vibrations of both the pesticides and the leaf could be dramatically amplified by the AuNP substrates (Fig. S2c). It should be noted that, in the fingerprint region (< 1800 cm‒1), the Raman signals derived from the endogenous molecules and the pesticides were overlapped each other, making them difficult to be separated. However, a single peak at 2160–2190 cm‒1 that is assigned to the exogenous C≡N in the two pesticides was observed in the Raman-silent window, where no background signals from the leaves can be detectable. It is also worth noting that a peak at 2130 cm‒1 was observed in the spectra, which could be attributed to the CO stretching bond [24, 25] that is present in CO2. The peak intensity can be enhanced particularly when the CO2 molecules are present in the nanogaps of the AuNP aggregates. With the increase of pesticide concentrations, the peak intensities of C≡N were enhanced accordingly. The lowest detectable concentrations of acetamiprid and chlorothalonil in the leaves were estimated to be 0.1 (Figs. 1c and e) and 1 mg/L (Figs. 1d and f), respectively, which are below the maximal values in agricultural products guided by the U.S. Environmental Protection Agency [26, 27]. Then, we tested the reproducibility of this bf-SERS assay by spraying acetamiprid and chlorothalonil on six different leaves respectively, and their spectra were collected with the same procedures. As shown in Fig. S3 (Supporting information), both acetamiprid and chlorothalonil displayed high reproducibility between the six leaves, particularly for the nitrile band in the Raman-silent window.
We then investigated the necessity of using the C≡N signal to track the pesticide residues in the leaves. Both acetamiprid and chlorothalonil exhibit two Raman scattering bands at 1520–1540 and 2160–2190 cm‒1 (Fig. 2a), which correspond to the C=C and C≡N, respectively. The two bands were utilized as mapping channels to explore the distribution of the pesticides in the leaves on a confocal Raman microscope. In the absence of pesticides, only the signals in the 1520–1540 cm‒1 channel (green) were detected across the leaves (Fig. 2b, i). In the presence of pesticides, the signals in the two channels were successfully detected (Fig. 2b, ii and iii). However, the signal spots in the 2160–2190 cm‒1 channel (blue) are less distributed than those in the 1520–1540 cm‒1 channel. We reasoned that the green signals could be attributed to both the pesticides and the endogenous molecules surrounding the AuNPs, which are hard to be resolved. In terms of the mapping images in the blue channel, the signals were exclusively recorded from the exogenous C≡N of the pesticides, eliminating spectral interference derived from the endogenous plant species.

|
Download:
|
| Fig. 2. SERS detection and imaging of nitrile-bearing pesticides in soybean leaves. (a) SERS spectra of soybean leaves and those previously sprayed with acetamiprid and chlorothalonil (10 µL, 200 mg/L). (b) Bright-field (BF), Raman mapping at 1520–1540 cm‒1 channel (green) and 2160–2190 cm‒1 channel (blue), and merged images for the AuNP-dropped soybean leaves (i) and those previously sprayed with acetamiprid (ii) and chlorothalonil (iii). | |
The pesticide residues in crops will be transferred to agricultural products, thus negatively affecting our health. In-situ monitoring of the pesticide residues in crops is crucial for selecting appropriate rinsing conditions to remove pesticide residues. In this study, we used three different rinsing solutions, including distilled water, NaHCO3 (5%), and detergents, to remove the residues of acetamiprid and chlorothalonil in the leaves. This result can be reasoned that NaHCO3 can decompose pesticides to remove the residues effectively [28]. The main components of detergent are surfactants, which can increase the solubility of pesticides, thus reducing adhesion on the leaf surfaces.
We first studied the residues of acetamiprid in the soybean leaves and those rinsed with different solutions. After dropping with AuNPs, the Raman signals of the sample-treated regions were imaged (mapping area = 200 × 200 µm2) by a confocal Raman microscope. Fig. 3a indicates that the mapping signals of acetamiprid in the 2160–2190 cm‒1 channel became weak after washing with different methods (Fig. 3a, 2–4). We collected the nitrile signals in ten mapping regions that were selected randomly on the same leaves. The average Raman intensities for each case are provided in Fig. 3b. obviously, detergents possess the highest efficacy to remove the acetamiprid residues from the leaves.
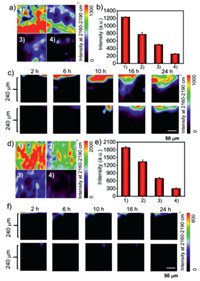
|
Download:
|
| Fig. 3. Monitoring of nitrile-bearing pesticide residues on soybean leaves under different rinsing conditions. (a) SERS imaging of the acetamiprid residues at the 2160–2190 cm‒1 channel after rinsing with different solutions: (1) No-rinsing (control), (2) distilled water, (3) 5% NaHCO3 and (4) commercially available detergents. (b) Average Raman intensities of acetamiprid in ten mapping regions that were selected randomly on the same leaves with the solutions in (a). (c) SERS depth mapping of acetamiprid penetration in the leaves with (bottom) or without (top) detergent rinsing within different exposure periods. (d) SERS imaging of the chlorothalonil residues with different rinsing solutions (1–4). (e) Average Raman intensities of chlorothalonil in ten mapping regions that were selected randomly on the same leaves with the solutions in (d). (f) SERS depth mapping of chlorothalonil penetration in the leaves with (bottom) or without (top) detergent rinsing within different exposure periods. Error bars represent standard deviations of ten mapping regions selected randomly on the same leaves. | |
We further measured the penetration depth of the residues over different times (2–24 h). For the no-rinsing samples (top), we observed that the acetamiprid molecules adsorbed on the leaf surfaces and gradually penetrated into the interior of the leaves. The penetration depth is proportional to the staining time (Fig. 3c). After 24 h, the penetration depth can reach 200 µm. When the acetamiprid-treated leaves were rinsed with detergents (bottom), the residues can be removed effectively especially in the early stages. The penetration depth can be reduced by ~50% in the late stages. It remains challenging to completely remove the acetamiprid residues in the leaves, due to the fact that acetamiprid is a systemic pesticide that could interact with the plant tissues tightly [29].
In parallel, we studied the residues of chlorothalonil, a typical non-systemic pesticide that is widely used in agriculture. The chlorothalonil-treated soybean leaves were also rinsed with different solutions and then incubated with AuNPs. Similar results were achieved that detergents showed the highest efficacy to remove the chlorothalonil residues from the leaves (Figs. 3d and e). Concerning the residue penetration over time, we observed that chlorothalonil shows a much lower ability than acetamiprid to penetrate the leaves (Fig. 3f). The chlorothalonil residues can be almost entirely removed from the leaves by detergents. The results suggest that the bf-SERS assay is highly suitable for monitoring nitrile-bearing pesticide residues in complex samples.
Crop growth is a long-term process, during which monitoring of pesticides could be interfered with by many molecular factors in the real world. We employed three other commonly-used pesticides, including chlorpyrifos, dichlorvos, and dimethoate, to simulate the possible interfering factors in crop growth. Melamine, tartrazine, malachite, and oil-soluble yellow were utilized as possible pollutes processed in the production stage. Fig. S4 (Supporting information) shows the SERS spectra of various additives on the surfaces of soybean leaves. Multiple peaks can be detected in the fingerprint region (< 1800 cm‒1), where the peaks cannot be separated. In the Raman-silent region, only the C≡N-bearing pesticides show a distinct peak at 2160–2190 cm‒1, which can be clearly resolved from the spectra derived from other components as well as CO stretching bonds in CO2. Meanwhile, We tested whether the heavy metallic ions may interfere with the spectra of nitrile-bearing pesticides on the leaves. Acetamiprid and chlorothalonil were independently incubated with different common heavy metallic ions (Cu2+, Zn2+, Pb2+, Fe2+ and Cd2+). Then, the mixtures were sprayed on soybean leaves for Raman recording. The results show that the metallic ions had negligible influence on both the mapping and spectra of the nitrile-bearing pesticides (Fig. S5 in Supporting information). These investigations show that the bf-SERS strategy can report the nitrile-bearing pesticides with high accuracy in complex conditions, avoiding the interference from both common exogenous factors and endogenous species.
In summary, we have developed a bf-SERS method for in-situ tracking of nitrile-bearing pesticide residues in soybean leaves. This method provides at least two distinct features. First, the distribution of pesticides in crop surfaces can be recorded in a non-destructive manner, without the need for tedious sample pretreatment. Second, a single Raman band assigned to the C≡N from the pesticide appears in the Raman-silent region, which was used to monitor the pesticide residues with extremely high fidelity. This bf-SERS method was further employed to evaluate the removal efficacy of different rinsing conditions. We found that (i) the commercial detergents showed the highest cleaning efficacy to remove the residues from the leaves and (ii) the adsorbed acetamiprid (a systemic pesticide) showed much stronger binding and anti-scouring ability than chlorothalonil (a non-systemic pesticide) in the leaves. When exogenous additives exist, the single bands of the nitrile-bearing pesticide in the Raman-silent region make this method particularly useful for tracking target residues in diverse environments, thus providing valuable guidance for precision agriculture.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsWe acknowledge the support from the Shanxi Province Key R & D Plans (Nos. 201903D211006-1 and 201803D221020-2), the Natural Science Foundation of Shanxi Province (No. 201901D111225), the National Natural Science Foundation of China (Nos. 21775075 and 21977053), and the Fundamental Research Funds for the Central Universities, Nankai University (No. 2122018165).
Appendix A. Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.051.
| [1] |
J. Wang, H. Liu, Chin. J. Org. Chem. 32 (2012) 1643-1652. DOI:10.6023/cjoc1202132 |
| [2] |
Z. Wei, H. Li, C. Ren, Chin. Chem. Lett. 31 (2020) 177-180. DOI:10.1016/j.cclet.2019.05.031 |
| [3] |
R. Boussahel, S. Bouland, K.M. Moussaoui, M. Baudu, A. Montiel, Water Res. 36 (2002) 1909-1911. DOI:10.1016/S0043-1354(01)00372-4 |
| [4] |
D. Harshit, K. Charmy, P. Nrupesh, Food Chem. 230 (2017) 448-453. DOI:10.1016/j.foodchem.2017.03.083 |
| [5] |
E.M. Thurman, I. Ferrer, D. Barceló, Anal. Chem. 73 (2001) 5441-5449. DOI:10.1021/ac010506f |
| [6] |
X. Ge, P. Zhou, Q. Zhang, et al., Angew. Chem. Int. Ed. 59 (2020) 232-236. DOI:10.1002/anie.201911516 |
| [7] |
S. Li, J. Xu, S. Wang, X. Xia, Z. Chen, Chin. Chem. Lett. 30 (2019) 1581-1592. DOI:10.1016/j.cclet.2019.05.049 |
| [8] |
W. Zhou, X. Gao, D. Liu, X. Chen, Chem. Rev. 115 (2015) 10575-10636. DOI:10.1021/acs.chemrev.5b00100 |
| [9] |
S. Sinha, S. Jones, A. Pramanik, P. Ray, Acc. Chem. Res. 49 (2016) 2725-2735. DOI:10.1021/acs.accounts.6b00384 |
| [10] |
Y. Lai, L. Dong, J. Liu, Chin. Chem. Lett. 31 (2020) 2437-2441. DOI:10.1016/j.cclet.2020.04.050 |
| [11] |
S. Liu, Y. Ying, Y. Long, Chin. Chem. Lett. 31 (2020) 473-475. DOI:10.1016/j.cclet.2019.07.057 |
| [12] |
T. Yang, Z. Zhang, B. Zhao, et al., Anal. Chem. 88 (2016) 5243-5250. DOI:10.1021/acs.analchem.6b00320 |
| [13] |
H. Zheng, L. Chen, Y. Wang, X. Zhang, S. Zhou, CrystEngComm 17 (2015) 6393-6398. DOI:10.1039/C5CE01017A |
| [14] |
Y. Zhang, Z. Wang, L. Wu, et al., Analyst 139 (2014) 5148-5154. DOI:10.1039/C4AN00771A |
| [15] |
W. Zhou, Q. Li, H. Liu, J. Yang, D. Liu, ACS Nano 11 (2017) 3532-3541. DOI:10.1021/acsnano.7b00531 |
| [16] |
H. Di, H. Liu, M. Li, J. Li, D. Liu, Anal. Chem. 89 (2017) 5874-5881. DOI:10.1021/acs.analchem.7b00199 |
| [17] |
Y. Yin, Q. Li, S. Ma, et al., Anal. Chem. 89 (2017) 1551-1557. DOI:10.1021/acs.analchem.6b03521 |
| [18] |
X.R. Bai, Y. Zeng, X.D. Zhou, et al., Anal. Chem. 89 (2017) 10335-10342. DOI:10.1021/acs.analchem.7b02172 |
| [19] |
D. Sanyal, D. Chakma, S. Alam, Bull. Environ. Contam. Toxicol. 81 (2008) 365-368. DOI:10.1007/s00128-008-9479-5 |
| [20] |
A. Lidia, L. Rosamaria, M. Antonio, et al., Environ. Health Perspect. 116 (2008) 1648-1655. DOI:10.1289/ehp.11297 |
| [21] |
Q. Zhang, C. Ji, L. Yan, Environ. Pollut. 218 (2016) 8-15. DOI:10.1016/j.envpol.2016.08.026 |
| [22] |
A. Guerreiro, F. Abreu, G. Fillmann, Ecotoxicol. Environ. Saf. 190 (2020) 110119. DOI:10.1016/j.ecoenv.2019.110119 |
| [23] |
Y. Hao, H. Zhang, P. Shuai, Toxicol. Lett. 303 (2019) 38-47. DOI:10.1016/j.toxlet.2018.12.011 |
| [24] |
X. Qin, Y. Si, Z. Wu, et al., Anal. Chem. 92 (2020) 924-931. DOI:10.1021/acs.analchem.9b03769 |
| [25] |
P. Claire, D. Françoise, T. David, D. Arnaud, J. Phys. Chem. C 121 (2017) 13798-13802. DOI:10.1021/acs.jpcc.7b04947 |
| [26] | |
| [27] | |
| [28] |
T. Yan, J. Doherty, B. Zhao, J. Agric. Food Chem. 65 (2017) 9744-9752. DOI:10.1021/acs.jafc.7b03118 |
| [29] |
A.R. Morgan, Asp. Appl. Biol. 83 (2007) 47-49. |
 2022, Vol. 33
2022, Vol. 33 

