b Chemistry and Biomedicine Innovation Center (ChemBIC), Nanjing University, Nanjing 210023, China
Infections caused by pathogenic bacteria are becoming serious global public health crisis [1-3]. In particular, this crisis is even complicated due to the rapid emergence of multiple drug resistant strains that are no longer susceptible to clinical antibiotics, such as methicillin and vancomycin [4, 5]. It is estimated that antibiotic-resistant infections will cause 10 million deaths and 100 trillion United States dollar in productivity lost each year by 2050 [6]. Therefore, it is becoming urgent to discover novel antibacterial compounds against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) and other clinically important Gram-positive or -negative resistant pathogens [1].
Lanthipeptides are a rapidly growing class of ribosomally synthesized and post-translationally modified peptides (RiPPs) mainly produced by Gram-positive bacteria [7, 8]. Lanthipeptides feature noncanonical thioether cross-linked amino acids lanthionine (Lan) and/or methyllanthionine (MeLan), in addition to unsaturated amino acids, 2, 3-didehydroalanine (Dha) and/or 2, 3-didehydrobutyrines (Dhb) [9, 10]. Moreover, several lanthipeptides contain an additional C-terminal 2-aminovinyl-cysteine (AviCys) motif as exemplified by NAI-107 [11], gallidermin [12] and mersacidin (Fig. S1 in Supporting information) [13]. The C-terminal AviCys motif is also found in other RiPPs classes including thioamitides [14], lipolanthines [15], linaridins [16] and lanthidins [17]. So far, compounds containing AviCys motifs show potent biological activities [10], indicating AviCys motif is of biological importance.
To identify new lanthipeptides containing AviCys motif, we carried out a genome-wide survey of AviCys containing lanthipeptides biosynthetic gene clusters (BGCs) in the AntiSMASH genome database which includes over 25, 802 bacterial genomic data (as of December 15, 2020) [18]. Among them, roughly 4939 BGCs belong to the lanthipeptide category. Previous investigation on AviCys biosynthesis indicated that the formation of AviCys macrocycle requires the collaborative action of a dehydratase and an flavin adenin dinucleotide (FAD) dependent decarboxylase [19, 20]. Briefly, the dehydratase and cysteine decarboxylase form a tight complex. The cysteine decarboxylase oxidatively decarboxylated C-terminal Cys to form a labile thioenol group; then the Ser/Thr-residues in the decarboxylated core peptide is converted into Dha/Dhb by dehydratase. Finally, the enzyme complex completes the AviCys macrocyclic ring formation via a Michael-type addition between thioenol motif and Dha/Dhb. Although the dehydratases sometimes are found outside BGCs [19], the cysteine decarboxylases are always encoded in the related BGCs. We thus performed a BLAST search for MibD, a cysteine decarboxylase in NAI-107 biosynthesis, to see if these lanthipeptide BGCs contain the MibD homologues, resulting in the discovery of 53 BGCs that may be responsible for the production of AviCys-containing lanthipeptide (Fig. S2 in Supporting information). After manual dereplication, only 19 discrete precursor peptides were obtained from these BGCs (Table S8 and Fig. S2 in Supporting information). We successfully obtained two new AviCys-containing lanthipeptides, daspyromycins A and B (1 and 2), together with two degraded products daspyromycins C and D (3 and 4), through the heterologous expression of the das gene cluster in Streptomyces albus (S. albus) J1074 followed by bioassay guided fractionation. Daspyromycins A and B showed potent antimicrobial activity against a spectrum of Gram-positive and -negative bacteria including MRSA.
To investigate whether the obtained actinobacterial strains including Actinokineospora diospyrosa NBRC 15665, Marininema halotolerans DSM 45789, Marininema mesophilum DSM 45610, Actinoplanes teichomyceticus ATCC 31121 and Alloactinosynnema iranicum IBRC-M 10403 could produce AviCys containing lanthipeptides (Table S8), we cultivated these strains on F medium. We hypothesized that the produced lanthipeptides will show potent antibacterial activity. Thus, the susceptibility of the test strain Micrococcus luteus to various potential lanthipeptide producing strains were carried out using a standard disk diffusion assay. A big and clear inhibition zone was observed around a disk from Actinokineospora diospyrosa NBRC 15665 strain (Fig. S3 in Supporting information), whereas, no or only weak inhibition zones were observed on disk inoculated with other strains, indicating that only NBRC 15665 might have the ability to produce antimicrobial compounds under the current culture condition.
Thus, a large scale fermentation (10 L) was carried out, followed by the bioactivity-guided fractionation and high performance liquid chromatography (HPLC) purification. A tiny peak, with an [M + H]+ ion at 2096.8, collected from semi-preparative HPLC showed most prominent anti Micrococcus luteus activity. However, the isolated material is not sufficient for nuclear magnetic resonance (NMR)-based structural characterization. Attempts to increase the titer using different fermentation conditions were not successful.
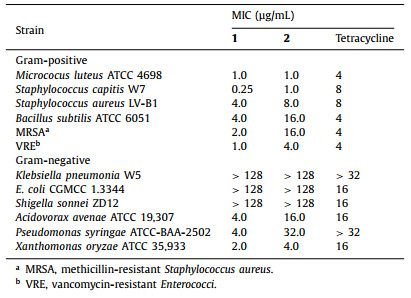
To increase the low titer of target compound, we turned to clone and express the entire gene cluster in heterologous surrogate hosts. The das gene cluster (GenBank Accession No. MW686201) contains six genes (Fig. 1A), and encodes for a precursor peptide (DasA), posttranslational modification enzymes including a lantibiotic dehydratase (DasB), a cyclase (DasC) and a FAD-dependent cysteine decarboxylase, as well as two transporters (DasT and DasU). A pJTU2554 vector-based cosmid library was then constructed to capture the das BGC [21]. One cosmid, 6I13, covering the intact das BGC was obtained and introduced into S. albus J1074 by conjugation.
|
Download:
|
| Fig. 1. Biosynthesis of daspyromycins A and B. (A) The gene cluster for daspyromycin. (B) Biosynthetic pathway for daspyromycins A and B. | |
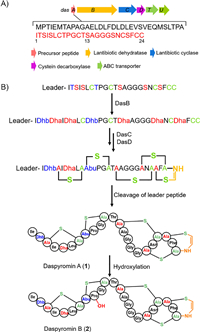
The recombinant strain S. albus J1074/cosmid 6I13 was fermented with the original S. albus J1074 as a control. One major peak that has an [M + H]+ ion at m/z 2096.8 consistent with that produced in wild-type strain, together with several minor peaks was observed in liquid chromatography–mass spectrometry (LC–MS) analysis (Fig. 2A). The titer for 1 in the heterologous host is much higher than that in the native host. We thus performed a 5-L scale fermentation of S. albus J1074/cosmid 6I13. Four compounds 1–4 were thus isolated and characterized (Fig. 2A).
|
Download:
|
| Fig. 2. LC–MS analyses of metabolic extracts. (A) LC–MS analyses of metabolic extracts from S. albus J1074 and S. albus J1074 carrying cosmid 6I13. (B) LC–MS detection of 2 upon feeding 1 into S. albus J1074. | |
Compound 1 was obtained as an amorphous powder. Based on the high resolution mass analysis (Fig. S4 in Supporting information), its molecular formula is determined to be C88H129N25O25S5, indicating 37 degrees of unsaturation. Analysis of its 1H, 13C, heteronuclear single quantum correlation (HSQC) and 1H–1H correlation spectroscopy (COSY) NMR data revealed characteristic signals for the aromatic/olefinic proton signals for Phe (δH 7.22, 7.32 and 7.20), Dhb (δH 6.25, 1.74), Dha (δH 5.42, 6.07) and cis double bond in AviCys moiety (δH 6.87, 5.47, J = 7.0 Hz). The deduced 24-aa core peptide accounts for at least 32 unsaturated degrees including one phenyl, one Pro, 23 peptide bonds, one terminal amide bond in Gln, and three double bonds. Thus, five rings are required to consume the remaining 5 unsaturated degrees, which is in agreement with the five Cys residues observed in core peptide that finally may form three lanthionine rings, one methyllanthionine ring and one AviCys ring through Michael-type additions. Based on the above analysis and the reported cyclization pattern for NAI-107 [22], the structure for daspyromycin A (1) was proposed as shown in Fig. 1B.
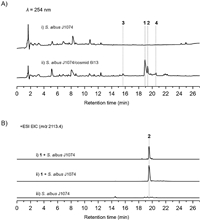
To confirm the structure of 1, an MS/MS analysis was performed (Fig. 3). The ion signals at m/z 1983.3 (y22) and 197.1 (b2) correspond to the loss of N-terminal Ile-and Ile-Dhb, respectively. The peaks at m/z 664.4 (b7), 467.6 (b7-b2) and 1431.5 (y16) clearly indicated the formation of a thioether bridge between Ala3 and Ala7. In addition, ion peaks at m/z 1004.5 (b11) and 664.4 (b7) revealed the formation of a second thioether ring between Abu8 and Ala11. The clear ion peaks of 991.1 (y11), 1105.5 (b12), 242.1 (b17-b13) and no other fragmental signals indicated the remaining three rings are twisted together to form the Ala13-S-Ala20, Ala18-S-Ala23 and AviCys ring between Ala21 and the C-terminal Cys, which is also observed in NAI-107. Careful analysis of 1D and 2D NMR data established the structure of 1 (Table S4).

|
Download:
|
| Fig. 3. MS/MS fragmentation analyses of 1–4. | |
The molecular formula of daspyromycin B (2) was determined to be C88H129N25O26S5 by the high resolution electrospray ionization mass spectroscopy (HRESIMS), indicating one more oxygen atom relative to 1. The MS/MS analysis showed the b2, b7 and y11 ion signals are the same as those observed in 1, whereas b11 and b12 signals are 16 Da greater than those in 1, indicating one hydroxyl group is substituted in the 2nd lanthionine ring. Further scrutiny of the 1D and 2D NMR data of 2 indicated an oxygenated methine proton δH 4.05 showed 1H–1H COSY correlations with a nitrogenated methylene at δH 3.33 and an aliphatic methylene at δH 1.94/2.08, which indicated the additional hydroxyl group is located at C-4 position of Pro9. As no other hydroxylation related enzymes are encoded in das gene cluster, we hypothesized if the hydroxylation was generated by the heterologous host. We thus fed 1 to the S. albus J1074 strain. After fermentation, a new peak that has the same retention time and molecular weight to 2 was detected in LC–MS analysis (Fig. 2B), whereas the S. albus J1074 strain without supplementation of 1 has no such peak, revealing the hydroxylase for hydroxylation at Pro9 is encoded in the genome of S. albus J1074.
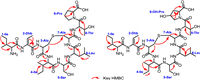
Compound 3 was obtained as a colorless powder. The molecular formula of 3 was determined to be C40H67N9O12S on the basis of HRESIMS. Analysis of the 1H, 13C and 1H–1H COSY spectra indicated the presence of several amino acid residues including 2 Ile, 1 Dhb, 1 Ser, 1 Leu, 1 Thr and 1 Pro, as well as a lanthionine moiety (Table S6 in Supporting information). Heteronuclear multiple bond correlation (HMBC) correlations revealed the sequence of these amino acid residues, which is consistent with the N-terminal core peptide (Fig. 4). In addition, the HMBC correlations of δH 2.68 with δC 35.8 clearly established the ether bridge between Ala3 and Ala7. Compound 4 was determined to have a molecular formula of C40H67N9O13S. 1H and 13C NMR spectra of 4 are highly similar to those of 3, except for the presence of an additional oxygenated methine proton (δH 4.05) (Table S7 in Supporting information) which showed clear HMBC correlations with C-2, C-5 position of Pro9, indicating the OH group was located at C-4 position of Pro9. The hydroxyl group substitution pattern in 4 is consistent with that in 2. Detailed explanation of MS/MS (Fig. 3), 1D and 2D NMR spectra established the structures of 3 and 4.
|
Download:
|
| Fig. 4. Key HMBC correlations of 3 and 4. | |
Based on the bioinformatics analysis of the das gene cluster and the characterized structures, the biosynthetic pathway for 1 was proposed. The biosynthesis starts with the production of precursor peptide DasA (Fig. 1B). DasB dehydrates seven Ser/Thr-residues to afford Dha/Dhb within the core peptide in a glutamyl-tRNAglu-dependent manner. Then, DasC catalyzes the Michael-type addition of Cys residue to Dha/Dhb to give lanthio-nine/methyllanthionine moieties. DasD catalyzes the oxidative decarboxylation of the C-terminal Cys to form a thioenol. A subsequent Michael type addition of the thioenol to the nearby Dha yields the AviCys moiety (Fig. 1B). After protease cleavage, the mature daspyromycin A is formed. Though the AviCys formation in thioamitides has been well established [19, 20], it remains elusive whether it is formed through a similar manner, i.e., DasB and DasD form a complex and collaboratively catalyze the macrocyclic AviCys ring formation.
Finally, all the isolated compounds (1–4) were evaluated for the antibacterial activity against an array of pathogenic bacteria. Though 3 and 4 were inactive to all tested strains [minimum inhibitory concentrations (MICs) > 128 µg/mL], 1 and 2 showed potent antibacterial activities against all Gram-positive bacteria (Table 1). This result is consistent with the reported mode of action for the NAI-107 that blocks peptidoglycan biosynthesis in Gram-positive bacteria and causes accumulation of the soluble peptidoglycan precursor in the cytoplasm [11]. In addition, 1 and 2 were also active to three tested Gram-negative bacteria including Acidovorax avenae, Pseudomonas syringae and Xanthomonas oryzae, but were inactive to Klebsiella pneumonia, Escherichia coli and Shigella sonnei. Compound 1 was particularly active to Staphylococcus capitis, which is associated with prosthetic valve endocarditis. More importantly, 1 showed potent activities against MRSA and VRE with MIC values of 2 and 1 µg/mL, respectively. From Table 1, the activities of 1 were generally superior to those of 2, suggesting that the hydroxylation on Pro9 might be a detoxification step during the heterologous production of 1. Most of lanthipeptides are only active towards Gram-positive bacteria [23], while few of them appear to be selective for Gram-negative strains [24]. Daspyromycins A and B showed broad spectrum antibacterial activity, providing an unusual example of an antibiotic that are active towards both Gram-positive and -negative bacteria.
|
|
Table 1 Antimicrobial activities of 1 and 2. |
In summary, our genome mining study revealed the widespread occurrence of AviCys containing lanthipeptides in the bacterial kingdom. Heterologous expression of the das gene cluster led to the production of daspyromycins A–D (1–4). Their structures were determined through extensive analysis of bioinformatics, NMR, HRMS and MS/MS data. Daspyromycin A (1) showed remarkable antibacterial activities against a broad spectrum of pathogenic bacteria including MRSA and VRE, opening the path to future development for pharmaceutical application.
Declaration of competing interestWe further confirm that the authors declare no competing interest.
AcknowledgmentsThis work was financially supported by Ministry of Science and Technology (MOST) of China (Nos. 2018YFA0902000, 2018YFC1706200 and 2019YFC0312500), National Natural Science Foundation of China (NSFC, Nos. 81925033, 21861142005, 81773591, 81991524, 81991522, 81803380 and 21661140001), and the Fundamental Research Funds for the Central Universities (Nos. 14380092, 14380113 and 14380142, China).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.010.
| [1] |
R. Laxminarayan, A. Duse, C. Wattal, et al., Lancet Infect. Dis. 13 (2013) 1057-1098. |
| [2] |
A. Zipperer, M.C. Konnerth, C. Laux, et al., Nature 535 (2016) 511-516. DOI:10.1038/nature18634 |
| [3] |
C.A. Arias, B.E. Murray, Nat. Rev. Microbiol. 10 (2012) 266-278. DOI:10.1038/nrmicro2761 |
| [4] |
S.B. Levy, B. Marshall, Nat. Med. 10 (2004) S122-S129. DOI:10.1038/nm1145 |
| [5] |
A. Parmar, R. Lakshminarayanan, A. Iyer, et al., J. Med. Chem. 61 (2018) 2009-2017. DOI:10.1021/acs.jmedchem.7b01634 |
| [6] |
S. Cascioferro, B. Parrino, D. Carbone, et al., J. Med. Chem. 63 (2020) 7923-7956. DOI:10.1021/acs.jmedchem.9b01245 |
| [7] |
L.M. Repka, J.R. Chekan, S.K. Nair, W.A. van der Donk, Chem. Rev. 117 (2017) 5457-5520. DOI:10.1021/acs.chemrev.6b00591 |
| [8] |
C. Chatterjee, M. Paul, L. Xie, W.A. van der Donk, Chem. Rev. 105 (2005) 633-684. DOI:10.1021/cr030105v |
| [9] |
P.G. Arnison, M.J. Bibb, G. Bierbaum, et al., Nat. Prod. Rep. 30 (2013) 108-160. |
| [10] |
M.M. Lopez, T.A. Scott, S. Ramesh, et al., Nat. Prod. Rep. 38 (2021) 130-239. DOI:10.3917/mouv.108.0130 |
| [11] |
D. Munch, A. Muller, T. Schneider, B. Kohl, et al., J. Biol. Chem. 289 (2014) 12063-12076. DOI:10.1074/jbc.M113.537449 |
| [12] |
R.R. Bonelli, T. Schneider, H.G. Sahl, I. Wiedemann, Antimicrob. Agents Chemother. 50 (2006) 1449-1457. DOI:10.1128/AAC.50.4.1449-1457.2006 |
| [13] |
A.N. Appleyard, S. Choi, D.M. Read, et al., Chem. Biol. 16 (2009) 490-498. DOI:10.1016/j.chembiol.2009.03.011 |
| [14] |
L. Frattaruolo, R. Lacret, A.R. Cappello, A.W. Truman, ACS Chem. Biol. 12 (2017) 2815-2822. DOI:10.1021/acschembio.7b00677 |
| [15] |
V. Wiebach, A. Mainz, M.J. Siegert, et al., Nat. Chem. Biol. 14 (2018) 652-654. DOI:10.1038/s41589-018-0068-6 |
| [16] |
S. Ma, Q. Zhang, Nat. Prod. Rep. 37 (2020) 1152-1163. DOI:10.1039/c9np00074g |
| [17] |
F. Javier Ortiz-Lopez, D. Carretero-Molina, M. Sanchez-Hidalgo, et al., Angew. Chem. Int. Ed. 59 (2020) 12654-12658. DOI:10.1002/anie.202005187 |
| [18] |
K. Blin, S. Shaw, K. Steinke, et al., Nucleic Acids Res. 47 (2019) W81-W87. DOI:10.1093/nar/gkz310 |
| [19] |
J. Lu, Y. Wu, Y. Li, H. Wang, Angew. Chem. Int. Ed. 60 (2021) 1951-1958. DOI:10.1002/anie.202012871 |
| [20] |
Y. Qiu, J. Liu, Y. Li, Y. Xue, W. Liu, Cell Chem. Biol. 28 (2021) 675-685. DOI:10.1016/j.chembiol.2020.12.016 |
| [21] |
J. Shi, Y.J. Zeng, B. Zhang, et al., Chem. Sci. 10 (2019) 3042-3048. DOI:10.1039/c8sc05670f |
| [22] |
L.C. Foulston, M.J. Bibb, Pro. Nat. Acad. Sci. U. S. A. 107 (2010) 13461-13466. DOI:10.1073/pnas.1008285107 |
| [23] |
S.I. Maffioli, J.C. Cruz, P. Monciardini, et al., J. Ind. Microbiol. Biotechnol. 43 (2016) 177-184. DOI:10.1007/s10295-015-1703-9 |
| [24] |
M. Kyuho, X. Fei, R.S. Mohammad, Angew. Chem. Int. Ed. 58 (2019) 5973-5977. DOI:10.1002/anie.201901342 |
 2022, Vol. 33
2022, Vol. 33