b Faculty of Chemistry, Northeast Normal University, Changchun 130024, China;
c Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, Guangzhou University, Guangzhou 510006, China;
d Key Laboratory of Preparation and Application of Environmental Friendly Materials (Jilin Normal University), Ministry of Education, Changchun 130103, China;
e Key Laboratory of Eco-materials Advanced Technology, College of Materials Science and Engineering, Fuzhou University, Fuzhou 350108, China;
f Key Laboratory for UV Light-Emitting Materials and Technology, Northeast Normal University, Ministry of Education, Changchun 130024, China
Recently, due to the fluctuation of fossil energy prices, global warming caused by the destruction of ecosystems, and fossil fuels are gradually drying up. Researchers have conducted a lot of work in the development of sustainable energy [1]. Solar energy, wind energy, and tidal energy stand out owing to their renewability, but there are many limitations in actual commercial applications [2]. Therefore, it is particularly important to use cheap, efficient and safe equipment to store the generated energy. Among the numerous energy storage systems, lithium-ion batteries (LIBs) are well developed with the advantages of light density, small size and high energy density. Also, LIBs have been widely applied in many commercial fields, from small digital products to automobiles [3]. However, there have obvious shortcomings of LIBs, such as the scarce resources and uneven distribution of lithium salts in the earth's crust. With the large-scale commercial application of LIBs, the price of lithium resources rises quickly, which significantly limits the applicability of LIBs in stationary energy storage [4-6]. Therefore, sodium-ion, potassium-ion batteries [7] and aqueous zinc-ion batteries [8] have gradually developed.
Sodium-ion batteries (SIBs) have become a promising candidate to LIBs owing to the similar electrochemical reaction mechanism [9]. In terms of material cost, the high abundance of sodium-based resources makes it indisputable have an advantage over lithium-based materials. However, Na+/Na couple has higher redox potential than that of Li+/Li. Due to the large mass and atomic radius of sodium, the energy density of SIBs is low and their cycling performance is poor [10-12]. Therefore, it is significant to find more suitable electrode materials [13], especially anode materials [14]. An ideal anode material should have the advantages of economy, high-capacity storage property, good rate capability and low reaction potential [15]. The electrode materials based on conversion reaction mechanism, especially the metal sulfides and phosphides [16] are the promising anode materials of SIBs. However, their drastic volume expansion and chemical instability seriously impede the rate and cycling performance, limiting their practical application. Because of the high theoretical specific capacity and relatively low redox potential, alloy anodes have attracted much attention for SIBs. At present, the research hotspots of alloy anodes mainly include the following two elements, Sn and Sb [17, 18]. But there is a serious problem in both of them. As the charge and discharge processes proceed, the electrode materials undergo huge volume change, which lead to the cracking of the electrode materials and the deterioration of battery cycling stability. Therefore, exploring novel anode materials with excellent electrochemical performance is urgently needed for practical applications.
Thiophosphate has come into the field of researchers due to its high conductivity and low Vander Waals force. It is widely used in supercapacitors, photocatalysts, and electrocatalysts [19, 20]. However, when used as electrode material, due to the presence of phosphorus and sulfur, it is accompanied by drastic volume change during cycling process. Therefore, alleviating volume change based on its excellent electrochemical performance is the top priority of current research. Among them, the modification of the material itself and the coating of carbon materials are considered effective methods. In this work, we use SbPS4 as an anode for SIBs [21]. Due to the combination of alloying and conversion mechanisms, the theoretical specific capacity of SbPS4 is 1335 mAh/g. In order to alleviate its volume effect, the tube cluster SbPS4 is fragmented to form the short tube and composited with graphene oxide (GO). When used as an anode of SIBs, a splendid reversible capacity of 772.2 mAh/g was obtained at 0.1 A/g. While further improving the current density to 5 A/g, a charge capacity of 360 mAh/g has remained. When it returns original current density, the SbPS4/GO can obtain a capacity of 637.33 mAh/g, proving the structural stability of the SbPS4/GO at a high rate. The resultant SbPS4/GO is considered to improve the electron/Na+ transport and relieve the volume change during charging/discharging process. The SbPS4/GO material displays high competitiveness in comparison with binary metal sulfides and phosphides for SIBs.
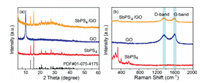
Scheme 1 shows the detailed preparation process of SbPS4/GO. Firstly, the tube cluster SbPS4 is synthesized by the high-temperature calcination method. After that, SbPS4 is transformed into a short tube by fragmentation. Lastly, SbPS4/GO is obtained by composite with GO and freeze-drying. During the preparation process, the short tubular SbPS4 is embedded in the spongy GO. Through the addition of GO, the conductivity of the material can be improved, the transmission distance of sodium ions and electrons can also be shortened, and the volume expansion during charging and discharging can be alleviated, resulting in the improvement of the electrochemical performance. The chemical composition and structure of the product are discussed in detail in Fig. 1a, and the diffraction peaks of SbPS4 prepared by high-temperature calcination are well matched. The diffraction peak intensity of SbPS4 decreased after adding GO, which was due to the reduction of the size of SbPS4 and the compound of carbon. Raman results on SbPS4/GO and GO show that the intensity ratio of D-band and G-band (ID/IG) of the two samples are 1.01 and 0.98, respectively (Fig. 1b). The higher ID/IG ratio indicates the amorphous state of the carbonaceous materials owing to the porous feature [22].

|
Download:
|
| Scheme 1. The synthesis process of SbPS4/GO composite. | |
|
Download:
|
| Fig. 1. The structural characterizations of SbPS4/GO: (a) XRD patterns, (b) Raman spectra. | |
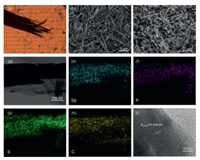
Tube cluster SbPS4 can be clearly observed in the upright metallurgical microscope image (Fig. 2a). After fragmenting, its original tubules remain almost intact, and the size of each unit is significantly reduced (Fig. 2b). As displayed in the SEM image (Fig. 2c), GO glues together the nanosized short tubes of SbPS4 to form a continuous conductive network that can significantly promote electron transfer. In addition, due to the presence of spongy graphene, there are obvious pore channels to guarantee the electrolyte complete penetration and the rapid ions migration. SbPS4/GO has a large number of pore channels, which can also be determined through the pore size distribution diagram (Fig. S1 in Supporting information). The element mappings of SbPS4/GO in Figs. 2d–h confirm the uniform distribution of Sb, P, S and C, which is consistent with the SEM image shown in Fig. S2 (Supporting information). The lattice spacing of 0.564 nm in the HRTEM image coincides with the (004) plane of the crystal SbPS4 (Fig. 2i), indicating the good purity of SbPS4.
|
Download:
|
| Fig. 2. (a) Upright metallographic microscope image of tube cluster SbPS4. SEM images of (b) SbPS4 after fragmentation and (c) SbPS4/GO. TEM image of (d) SbPS4/GO and elemental mapping of (e-h) SbPS4GO. (i) HRTEM image of SbPS4/GO. | |
Fig. 3a shows the discharge/charge profiles of SbPS4, SbPS4-X45 and SbPS4/GO composite in the initial five cycles. SbPS4-X45 was selected as the comparison material because it has the best electrochemical performance as shown in Fig. S3 (Supporting information). SbPS4 composited with 10% GO has the highest ionic conductivity and the lowest resistance value (Fig. S4 in Supporting information). The specific capacities of SbPS4, SbPS4-X45, and SbPS4/GO at 0.1 A/g are 356.6, 717.2 and 772.71 mAh/g, respectively. The improved electrochemical performance of SbPS4/GO is ascribed to the nanominiaturization of tubular materials and the appropriate amount of composite with carbon materials (GO).

|
Download:
|
| Fig. 3. (a) Galvanostatic curves of SbPS4, SbPS4-X45, SbPS4/GO at 0.1 A/g for the initial five cycles. (b) CV profiles of SbPS4/GO for the five cycles at 0.2 mV/s. (c) Long cycling performance of SbPS4, SbPS4-X45, SbPS4/GO and corresponding Coulombic efficiency at 1 A/g. (d) Nyquist plots. (e) Rate performance at various current densities. | |
The electrochemical properties of SbPS4/GO are studied by the CV tests at a sweep rate of 0.2 mV/s (Fig. 3b). At the first cathodic process, a peak located at 1.4 V can be indexed as the intercalation of Na+ into the SbPS4/GO, while the distinct peak near 1.2 V is attributed to the conversion reaction of Na2S. The peak at 0.8 V corresponds to the breaking of P-S bond and the production of Na3P and Sb, while the peak at 0.23 V ascribes to the production of Na3Sb [23]. During the initial anodic scan, the weak broad peak located at 0.25 V indicates Na3P desodiation, and the peak at 0.77 V attributed to Na3Sb dealloying. The next 1.5 V and 1.6 V peaks ascribe to Na2S and Na+ further desodiation reaction [24]. Remarkably, the CV profiles remain almost overlapped, suggesting the excellent electrochemical reversibility. Therefore, the corresponding redox reactions mechanism of SbPS4 can be listed as follows:
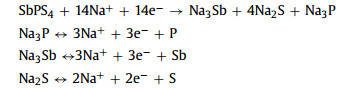
|
Fig. 3c shows the cycling performance of different samples at 1 A/g. The reversible capacity of SbPS4/GO is 420.52 mAh/g after 100 cycles with the initial coulombic efficiency of 57%, which is superior to SbPS4–X45 and SbPS4. The Nyquist plots of the three samples consist of a depressed semicircle at the high frequency corresponding to the charge transfer process and an oblique line at the low frequency corresponding to the charge transfer process and an oblique line at the low frequency representing Na+ diffusion process, confirming a faster electronic and ionic transport of SbPS4/GO than that of SbPS4-X45 and bare SbPS4 (Fig. 3d). The EIS plots of materials with different mixed graphene content are shown in Fig. S5a (Supporting information). It can be seen that SbPS4 composited with 10% GO has the smallest resistance value. As expected, it exhibits the best electrochemical performance, which is consistent with the data shown in Fig. S4. The EIS analysis in Fig. S5b (Supporting information) shows that the charge transfer resistance (Rct) of the 100th cycle is lower than that of before cycling state, which may be due to the formation of the stable SEI layer between the electrode material and the electrolyte after repeated cycles, which is conducive to the transport of Na+. As shown in Fig. 3e, the SbPS4/GO has the capacity of 359.58 mAh/g at 5 A/g. Importantly, the capacity of SbPS4/GO can return to 637.33 mAh/g when the current density returns to 0.1 A/g, indicating the structural stability of SbPS4/GO at a high rate.
To further explore the possible reaction mechanism during the Na-insertion/extraction process, ex situ XRD and XPS analysis of SbPS4/GO were performed at specific distinct potentials of the first charge/discharge curve. As shown in Fig. 4, when the cell was discharged to 1.3 V, the Na2S was found [25]. The presence of Na2S reveals that sodium ions react with sulfur after insertion into SbPS4. When the discharge voltage reaches 0.9 V, the characteristic peak of Sb has appeared. Further discharging to 0.3 V, Na3Sb was detected. After it was discharged to 0.05 V, the diffraction peak of Na3Sb becomes stronger and Na2S still exists. In the charging process, the diffraction peak of Sb appears again when the cell was charged to 1.0 V. With the extraction of sodium ions, the diffraction peaks of Na3Sb and S indicated that the desodiation reaction of Na2S and the dealloying process are not completed [26]. After charging to 2 V, the signal of Na3Sb almost disappears. Compared with the original state, the diffraction peaks of fully charged state hardly change, further confirming the excellent reversibility of the SbPS4/GO.

|
Download:
|
| Fig. 4. Ex situ XRD patterns of SbPS4/GO taken at different stages at 1 A/g. | |
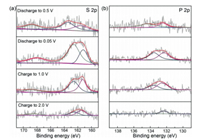
XPS studies of the electrodes were carried out at various discharge/charge states in the first cycle. Fig. 5 shows the high-resolution S 2p and P 2p spectra of SbPS4/GO electrode with the SnP2S6 and Cu3PS4 as the references. In Fig. 5a, the peaks corresponding to the S 2p3/2 and S 2p1/2 signals at 161.6 and 162.8 eV can be assigned to Na2S [27]. The binding energy of 168.8 eV corresponds to S. It indicates that Na2S and S exist in the whole charge and discharge processes. Both the peak intensities of Na2S and S increase during the discharge process [28]. There is still a tiny amount of Na2S and S at the end of the charging stage, indicating that the transformation reaction of S is not completely reversible. In Fig. 5b, the two splitting peaks located at 132.6 and 133.7 eV were ascribed as the P-S bonding and 2p1/2 of the P 2p spectrum, respectively [29]. It can be seen that P elemental substance increases with the discharge process. Upon charging, the amount of P is gradually decreased with forming P-S bonding. As shown in Fig. S6a (Supporting information), the Sb 3d spectrum overlaps with O 1s spectrum and shows two peaks at 531 and 539.8 eV, which are attributed to the presence of Sb 3d3/2 [30]. In addition, 535 and 532.5 eV correspond to the presence of O-C and O = C functional group, respectively [31]. It can be seen from Fig. S6 (Supporting information) that a large amount of Sb is generated as discharged to 0.5 V. With further discharging to 0.05 V, the amount of Sb decreases due to the occurrence of alloying reaction. When charged to 1.0 V, the dealloying of Na3Sb leads to an increase of Sb content. In the end, the charging is completed to 2.0 V, Sb still exists but the amount becomes less. In the C 1s spectrum of Fig. S6b, the peaks at 287.7 eV, 285.5 eV and 284.3 eV can be ascribed to O-C=O, C-O and C-C bonds, respectively.
|
Download:
|
| Fig. 5. XPS analysis of the S 2p and P 2p signal for the electrodes at selected potentials in the first cycle. | |
To reveal the origin of high rate performance of SbPS4/GO, the sodium storage mechanism was studied by analyzing CV profiles at various scan rates from 0.1 mV/s to 1.0 mV/s (Fig. 6a). The charge storage mechanism of SbPS4/GO can be quantified by dividing the current (i) at a fixed potential (v) into two mechanisms: k1v and k2v0.5. Here, a and b are adjustable parameters, v is scan rate, and k1 and k2 are constants.
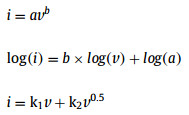
|

|
Download:
|
| Fig. 6. (a) CV curves at diverse sweep rates. (b) Linear relationship of log(i) vs. log(v). (c) Proportion of capacitive (shaded region) contribution at 0.1 mV/s. (d) Contribution percentage of capacitive and diffusion-controlled capacities vs. scan rates. | |
If the b value approaches to 1, the electrochemical process is a surface-controlled process, such as adsorption/resolution [32]. When the b value is 0.5, the process mainly relies on the diffusion-controlled process [33]. Fig. 6b shows the fitting results of log(i) to log(v). The values of b are 0.64, 1.37, 1.16 and 0.98, respectively. With the increase of scanning rate, the reaction of sodium ions is similar to the adsorption-desorption process, resulting in peak 2 being a surface-controlled process, and the same mechanism exists at peak 3 [34]. Fig. 6c shows the normalized contribution percentage of capacitive and diffusion-controlled capacities. When the scanning rate is 0.1 mV/s, the capacitive contribution ratio reaches 60.1%. The contribution rate of pseudo-capacitance is predominant with the increase of current density as shown in Fig. 6d. The results indicate that pseudo-capacitance plays a decisive role in Na-ion storage at high scan rates, thus resulting in excellent rate capability [35].
Motivated by such a superior specific capacity and an excellent rate capability of the SbPS4/GO anode. Na-ion full cell was constructed by coupling with Na3V2O2(PO4)2F (NVOPF) cathode for practical applications [36]. The NVOPF cathode exhibits a high discharge capacity of ~120 mAh/g at 0.1 C in a half-cell configuration. The typical charge/discharge curve of the Na-ion full cell shows an outstanding specific capacity of 119 mAh/g at 0.8 C between 1.2 V and 4.3 V (based on the weight of cathode) (Fig. 7a). The full cell possesses two discharge plateaus located at 3.8–3.0 V and 2.5–1.8 V, respectively. Moreover, the specific capacity of 63 mAh/g could be obtained at 4 C (Fig. 7b), indicating the superior rate capability of the Na-ion full cell. Furthermore, the full cell shows 62% capacity retention in comparison of the first cycle, exhibiting good cycling performance (Fig. S7 in Supporting information). Such a superior full cell performance indicates the potential application of SbPS4/GO anode for SIBs.

|
Download:
|
| Fig. 7. (a) Initial charge/discharge profiles of the full-cell and the homemade NVPOF cathode and SbPS4/GO anode in the half-cell. (b) Rate capability of the full-cell at different rates. | |
In summary, the SbPS4/GO composite has been intensively studied as anode material for SIBs in this work. The unique structure boosts rapid reaction kinetics, inhibits electrode structural failure and agglomeration for volume change, suppresses the polysulfides shuttle behavior, resulting in excellent rate capability and good cycling performance. As an anode material for the half-cell, the SbPS4/GO exhibits a specific capacity of 772 mAh/g at 0.1 A/g. Moreover, a high capacity of 420.5 mAh/g could be also maintained after 100 cycles (1 A/g). Significantly, it has a high Na ion storage capacity of 119 mAh/g at 0.8 C in the full-cell configuration. The favorable advantages including low-cost composition, facile synthesis process, and excellent electrochemical performance enable the SbPS4/GO to be a potential anode material for high performance SIBs.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 91963118, 51801030). This study is also supported by the Open Project Program of Key Laboratory of Preparation and Application of Environmental Friendly Materials (Jilin Normal University, No. 2020004).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.065.
| [1] |
Y. Wu, C. Zhang, H. Zhao, Y. Lei, J. Mater. Chem. A 9 (2021) 9506-9534. DOI:10.1039/D1TA00831E |
| [2] |
T.G. Allen, J. Bullock, X. Yang, A. Javey, S. De Wolf, Nat. Energy 4 (2019) 914-928. DOI:10.1038/s41560-019-0463-6 |
| [3] |
J. Ding, W. Hu, E. Paek, D. Mitlin, Chem. Rev. 118 (2018) 6457-6498. DOI:10.1021/acs.chemrev.8b00116 |
| [4] |
Y. Li, Y. Lu, C. Zhao, et al., Energy Storage Mater. 7 (2017) 130-151. DOI:10.1016/j.ensm.2017.01.002 |
| [5] |
M. Yang, Q. Ning, C. Fan, X.L. Wu, Chin. Chem. Lett. 32 (2021) 895-899. DOI:10.1016/j.cclet.2020.07.014 |
| [6] |
W.H. Li, H.J. Liang, X.K. Hou, et al., J. Energy Chem. 50 (2020) 416-423. DOI:10.1016/j.jechem.2020.03.043 |
| [7] |
H. Huang, X. Luo, Y. Yao, et al., InfoMat 3 (2021) 421-431. DOI:10.1002/inf2.12176 |
| [8] |
Y. Heng, Z. Gu, J. Guo, X. Wu, Acta Phys. Chim. Sin. 37 (2021) 2005013. |
| [9] |
S. Geng, T. Zhou, M. Jia, et al., Energy Environ. Sci. 14 (2021) 3184-3193. DOI:10.1039/D1EE00193K |
| [10] |
V. Palomares, M. Casas-Cabanas, E. Castillo-Martínez, M.H. Han, T. Rojo, Energy Environ. Sci. 6 (2013) 2312-2337. DOI:10.1039/c3ee41031e |
| [11] |
H. Pan, Y.S. Hu, L. Chen, Energy Environ. Sci. 6 (2013) 2338-2360. DOI:10.1039/c3ee40847g |
| [12] |
X. Hou, W. Li, Y. Wang, et al., Chin. Chem. Lett. 31 (2020) 2314-2318. DOI:10.1016/j.cclet.2020.04.021 |
| [13] |
Z.Y. Gu, J.Z. Guo, X.X. Zhao, et al., InfoMat 3 (2021) 694-704. DOI:10.1002/inf2.12184 |
| [14] |
Y. Jiang, Y. Wang, J. Ni, L. Li, InfoMat 3 (2021) 339-352. DOI:10.1002/inf2.12175 |
| [15] |
H.J. Liang, B.H. Hou, W.H. Li, et al., Energy Environ. Sci. 12 (2019) 3575-3584. DOI:10.1039/C9EE02759A |
| [16] |
S. Haghighat-Shishavan, M. Nazarian-Samani, M. Nazarian-Samani, K.B. Kim, Energy Storage Mater. 39 (2021) 96-107. DOI:10.1016/j.ensm.2021.03.037 |
| [17] |
K.T. Chen, S. Chong, L. Yuan, Y.C. Yang, H.Y. Tuan, Energy Storage Mater. 39 (2021) 239-249. DOI:10.1016/j.ensm.2021.04.019 |
| [18] |
B. Dou, J. Yan, Q. Chen, et al., Sens. Actuator. B. Chem. 328 (2021) 129082. DOI:10.1016/j.snb.2020.129082 |
| [19] |
A. Kato, H. Kowada, M. Deguchi, et al., Solid State Ionics 322 (2018) 1-4. DOI:10.1016/j.ssi.2018.04.011 |
| [20] |
A. Qiao, H. Tao, Y. Yue, J. Non-Cryst. Solid 521 (2019) 119476. DOI:10.1016/j.jnoncrysol.2019.119476 |
| [21] |
C.D. Malliakas, M.G. Kanatzidis, J. Am. Chem. Soc. 128 (2006) 6538-6539. DOI:10.1021/ja057943m |
| [22] |
H.J. Liang, Z.Y. Gu, X.Y. Zheng, et al., J. Energy Chem. 59 (2021) 589-598. DOI:10.1016/j.jechem.2020.11.039 |
| [23] |
Z. Yi, N. Lin, W. Zhang, et al., Nanoscale 10 (2018) 13236-13241. DOI:10.1039/C8NR03829E |
| [24] |
L. Yang, W. Hong, Y. Tian, et al., Chem. Eng. J. 385 (2020) 123838. DOI:10.1016/j.cej.2019.123838 |
| [25] |
C. Kang, S. Zhou, J. Liu, et al., J. Mater. Chem. A 9 (2021) 2152-2160. DOI:10.1039/D0TA10556B |
| [26] |
L. Zhou, Z. Cao, J. Zhang, et al., Adv. Mater. 33 (2021) 2005993. DOI:10.1002/adma.202005993 |
| [27] |
C. Lin, Y. Wang, F. Zhong, et al., Chem. Eng. J. 407 (2021) 126991. DOI:10.1016/j.cej.2020.126991 |
| [28] |
F. Xie, L. Zhang, Q. Gu, et al., Nano Energy 60 (2019) 591-599. DOI:10.1016/j.nanoen.2019.04.008 |
| [29] |
B.H. Hou, Y.Y. Wang, Q.L. Ning, et al., Nanoscale 11 (2019) 1304-1312. DOI:10.1039/C8NR08849G |
| [30] |
H. Huang, R. Xu, Y. Feng, et al., Adv. Mater. 32 (2020) 1904320. DOI:10.1002/adma.201904320 |
| [31] |
O.A. Jaramillo-Quintero, R.V. Barrera-Peralta, A.G. El Hachimi, et al., J. Colloid Interface Sci. 585 (2021) 649-659. DOI:10.1016/j.jcis.2020.10.044 |
| [32] |
H. Lu, F. Ai, Y. Jia, et al., Small 14 (2018) 1802694. DOI:10.1002/smll.201802694 |
| [33] |
A. Xu, C. Huang, G. Li, et al., J. Mater. Chem. A 9 (2021) 12169-12178. DOI:10.1039/D1TA01930A |
| [34] |
B.H. Hou, Y.Y. Wang, Q.L. Ning, et al., Adv. Mater. 31 (2019) 1903125. DOI:10.1002/adma.201903125 |
| [35] |
H.Y. Yu, H.J. Liang, Z.Y. Gu, et al., Electrochim. Acta 361 (2020) 137041. DOI:10.1016/j.electacta.2020.137041 |
| [36] |
X. Li, S. Jiang, S. Li, et al., J. Mater. Chem. A 9 (2021) 11827-11838. DOI:10.1039/D1TA02250D |
 2022, Vol. 33
2022, Vol. 33 

