b Jiangxi Engineering Technology Research Center of Nuclear Geoscience Data Science and System, East China University of Technology, Nanchang 330013, China;
c Key Laboratory of Cluster Science of Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China
Polyoxometalates (POMs) constitute a characteristic class of molecular metal-oxygen clusters and have drawn extensive attentions not only due to the fascinating structural diversity but also the application in multifarious fields including catalysis, electrochemistry, biomedicine, photoelectricity, nanomaterials and so on [1-7]. Keggin-type, Well-Dawson-type and many other classic basic building units have been extensively studied and reported [8, 9]. Based on the basic building units or beyond, chemists have been committed to exploring new functional POMs [10-16]. Considering the fascinating architectures with potential functions, development of new families of POMs is still an attractive and challenging research field. Recent years, the structural diversities of POMs had been greatly enriched [17], and many new non-classical POMs and oxo-clusters were reported [18-23]. Being different from the extensively reported POMs-based inorganic-organic hybrid frameworks, pure inorganic POMs- or oxo-clusters-based frameworks are few reported due to the uncertainties in chemical behavior of inorganic linkers and/or other factors. While inorganic POMs- or oxo-clusters-based frameworks always show more impressive physical, chemical and other properties. For example, Xu's group reported a non-classical 3D POMs named as CoP4Mo6 for water purification [24]. Li and co-workers reported two inorganic 3D frameworks based on [MnV13O38]7− clusters and lanthanide ions, which shown excellent proton conductivities [25]. Zheng's group reported a series of 3D porous lanthanide-substituted POMs-based frameworks based on {Ln6W8O28} cage-shaped clusters [26]. Kang's group synthesized a series of non-classical POMs frameworks and explored the influences of transition metals to the molybdenum clusters [27].
Here, we represent a new 3D metal-oxo-clusters-based inorganic framework, [NaCo2Mo2O7(OH)3]n (NaCoMo), named as 3D platelike ternary-oxo-cluster. Being different to the traditional POMs and other oxo-clusters, there are no classical building units in NaCoMo. The Na/Co/Mo atoms in NaCoMo are connected by sharing oxygen edges, corners of oxygen atoms and bridging hydroxyls to form a new 3D inorganic framework, in which 1D chains of Co(II) octahedrons are formed by sharing the edges of bridging oxygen atoms, and are extended to 2D planes by sharing bridging oxygen atoms corners with Mo(VI) tetrahedrons. The 3D structure of NaCoMo is constructed by sharing bridging oxygen atoms corners of 2D planes and Na(I) octahedrons. In a word, NaCoMo represents a new metal-oxo-clusters-based inorganic framework which is different from all known POMs and oxo-clusters.
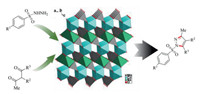
POMs had been widely reported as a class of highly efficient and green catalysts in the synthesis of nitrogen heterocyclic compounds. Pyrazoles play a key role in the fields of pharmaceutical chemistry, agricultural chemistry and synthetic organic chemistry [28-31]. The pyrazole motif could be found in many biologically active molecules and marketed drugs [32-34]. As part of our ongoing research on polyoxometalates chemistry [35-40], we herein reported the first NaCoMo as an efficient catalyst for the synthesis of pyrazoles. In addition, the DFT of NaCoMo was performed to study the crystal formation process and stability. To the best of our knowledge, this new metal-oxo-clusters-based inorganic framework is the first ternary-oxo-cluster catalyst for synthesizing the pyrazoles by the condensation cyclization of sulfonyl hydrazines with 1, 3-diketones (Scheme 1).
|
Download:
|
| Scheme 1. The structure and catalytic application of NaCoMo. | |
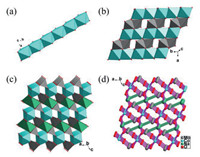
Single-crystal diffraction analysis reveals that NaCoMo crystallizes in the monoclinic space group C2/m with a unique 3D inorganic framework. The asymmetric unit of NaCoMo consists of a quarter Na(I), half Co(II), half Mo(VI), one and a half of μ3-O (O1/O3), one half of μ2-OH (O2) and a μ2-O (O4) (Fig. S1 in Supporting information). Na1 is coordinated with four μ3-O1 (O1/O1#6/O1#7/O1#8) and two μ2-OH (O2#5/O2#9) in an octahedral geometry with the Na-O bond lengths of 2.4326(18) or 2.533(3) Å. Co1 is also in an octahedral geometry and is coordinated with two μ3-O1 (O1/O1#3), two μ3-O3 (O3#4/O3#5) and two μ2-O (O4/O4#3). Mo1 shows an unusual tetrahedral geometry and is coordinated with three μ3-O (O1/O1#2/O3) and one μ2-OH (O2). One μ3-O1 connects one Co1, one Na1 and one Mo1 almost in a plane. O1 lies on the plane formed by Na/Co/Mo with a distance of 0.0718 Å. One μ3-O3, two Co(II) atoms (Co1#1/Co1#4) and one Mo1 are also nearly coplanar. The distance is 0.0660 Å from O3 to the plane constructed by two Co1 and Mo1 atoms (Fig. S2a in Supporting information). One Na(I) octahedron connects six Mo(VI) tetrahedrons and four Co(II) octahedrons by sharing corners (four O1 atoms and two O2 atoms) (Fig. S2b in Supporting information). One Co(II) octahedron connects four Mo(VI) tetrahedrons, two Na(I) octahedrons and two adjacent Co(II) octahedrons by sharing four corners (two O1 atoms and two O3 atoms) and sharing edges (O3/O4) (Fig. S2c in Supporting information). Similarly, one Mo(VI) tetrahedron is linked with four Co(II) octahedrons and three Na(I) octahedrons by sharing four corners (two O1, one O3 and one O2) (Fig. S2d in Supporting information). And a 1D chain is formed by two adjacent Co(II) octahedrons, sharing the edges O3/O4 (Fig. 1a). Adjacent 1D chains of Co(II) octahedrons are connected by Mo(VI) tetrahedrons to form a 2D plane (Fig. 1b). However, Adjacent 2D planes are linked by Na(I) octahedrons to form the 3D packing structure of NaCoMo (Fig. 1c).
|
Download:
|
| Fig. 1. Schematic depiction of the (a) connection of the Co(II) octahedrons 1D chain. (b) 2D plane constructed by Co(II) octahedrons and Mo(VI) tetrahedrons. (c) 3D structure, (d) (3, 3, 4, 6, 6)-connected 3D network of NaCoMo (Color code: Na, green; Co, turquiose; Mo, gray; O, red). | |
Topologically, Na1 and Co1 can be treated as 6-connected nodes. 4-coordinated Mo1 is a 4-connected node. μ3-O (O1/O3) can be simplified as 3-connected nodes. The 3D structure of NaCoMo can be simplified as a 5-node (3, 3, 4, 6, 6)-connected 3D network with a point symbol of (3·52)2(52·6)4(52·62·82)2(56·64·82·93)(32·56·62·83·92)2 (Fig. 1d).
As a new type of metal-oxo-clusters-based inorganic framework, the significant difference is that, there are no Mo-O-Mo bond angles in NaCoMo, which are common in traditional POMs. Traditional multidimensional POMs are always constructed by classical building units and some bridging ligands (such as organic/inorganic ligands, multi-coordinated atoms or clusters). But NaCoMo is totally different and there are no classical building units in NaCoMo, and the asymmetric units of NaCoMo are directly extended into a new 3D platelike structure. Due to the similarity of NaCoMo and inorganic mineral, this new type of metal-oxo-clusters-based inorganic framework is named as 3D platelike-ternary-oxo clusters.
The uniformity of NaCoMo achieved by the one-pot synthesis method was also verified by EDS elemental mapping, which demonstrates the uniform distribution of Na, Co, Mo and O throughout the crystal (Fig. 2 and Fig. S3 in Supporting information). The experimental and simulated PXRD patterns of NaCoMo were in good agreement, indicating the phase purity of NaCoMo samples (Fig. S4 in Supporting information). The FT-IR spectrum of NaCoMo is shown in Fig. S5 (Supporting information). The characteristic bands around 3300 and 3100 cm−1 are attributed to the ν(O-H) vibrations [41]. The other bands in the range of 1600–500 cm−1 are attributed to the ν(metal-Ot) and ν(metal-O-metal) vibrations. Bond valence sum (BVS) calculations for NaCoMo are consistent with Na, Co and Mo being in the +1, +2 and +6 oxidation states, respectively (Table S3 in Supporting information). The XPS spectra of NaCoMo was also recorded to further consolidate the chemical valences of Na, Co and Mo atoms, which matched well with BVS results (Fig. S6 in Supporting information). Besides, the thermostability of NaCoMo was evaluated using TGA (Fig. S7 in Supporting information). The frameworks of NaCoMo can remain stable until 320 ℃.

|
Download:
|
| Fig. 2. (a) SEM image of NaCoMo. (b-e) EDS mappings of NaCoMo for Na (green), Co (turquiose), Mo (yellow), O (red). | |
To obtain the ground state of the crystal, the crystal of NaCoMo is optimizing in three axes (Fig. S8 in Supporting information). The lattice constants are 5.57 Å, 7.52 Å and 10.39 Å, respectively. And the formation energy of NaCoMo has been considered as E = Etotal -2ENa -4ECo -4EMo -20EO -6EH, which ENa, ECo and EMo are the energy of the atoms (Na, Co and Mo) in metal crystal, and EO, EH are the energy of O, H in O2 and H2, respectively. The average formation energy of the crystal is −1.73 eV. The negative formation energy indicates that the crystal formation process is exothermic and the structure is very stable. It can be found that Co atoms are located in the center of O octahedron, while Mo atoms are located in the center of O tetrahedron, which means that all Co atoms adopt a 6-coordinated mode and all Mo atoms adopt a 4-coordinated mode (Fig. S9 in Supporting information). It can also be found that there are four kinds of O atoms with different surroundings in the crystal structure from Fig. S8. Among them, the first kind of O atom forms chemical bond with only one Mo atom, while the second kind of O atom forms chemical bonds with Mo, Co and H atoms, respectively. The third O atom forms chemical bond with two Co atoms and two H atoms. And the fourth middle O atom forms chemical bond with one Mo and one Co 0.968e, respectively. From the crystal structure, it can be seen that Na is located in the channel composed of O tetrahedron and O octahedron, and loses 0.841e.
In addition, the electronic structure of the crystal has been analyzed (Fig. S10 in Supporting information). The Fermi surface is located in the conduction band, which means the presence of free electrons in the low conduction band. Further analysis of the density of states shows that the density of states near the Fermi surface is mainly contributed by the s orbital of Mo atom, the s orbital of Co atom and the p orbital of O atom (Fig. S11 in Supporting information).
To further evaluate the catalytic activity of the cluster catalyst, the condensation cyclization of sulfonyl hydrazines with 1, 3-diketones for synthesizing the pyrazoles was chose as the model reaction. p-Toluenesulfonyl hydrazide 1a and acetylacetone 2a were used as the substrates. The catalysis of NaCoMo ternary- oxygen-clusters under various reaction conditions was studied, and the optimal conditions were explored. As shown in Table 1, in the absence of catalyst, only 31% yield of the desired product 3a could be obtained at room temperature for 15 min (entry 1). The raw materials CoCl2·6H2O (4 mol%) and Na2MoO4·2H2O (4 mol%) used to synthesize NaCoMo were subjected to the reaction as catalyst, affording 3a in 38% and 44% yield, respectively (entries 2 and 3). When the NaCoMo ternary-oxo-cluster (2 mol%) was employed as catalyst to carry out the reaction at room temperature for 15 min, 3a was observed in 84% yield (entry 4). Increasing the catalyst loading to 3 mol%, the yield of 3a was not improved (entry 5). However, increasing the reaction temperature to 50 ℃ and 80 ℃, it could be clearly observed that the yield of 3a were 89% and 91%, respectively (entries 6 and 7). Subsequently, extending the reaction time for 30 min and 60 min at 80 ℃, the yield of 3a increase to 93% and 98%, respectively (entries 8 and 9). Thus, 2 mol% NaCoMo, 80 ℃ and 60 min were chosen as optimal conditions for this condensation reaction.
|
|
Table 1 Condition optimization.a |
The scope of this pyrazole synthesis protocol was investigated under the optimized reaction conditions. As can be seen in Table 2, p-toluenesulfonyl hydrazide 1a reacted with acetylacetone 2a, 3-chloropentane-2, 4-dione 2b, 3-methylpentane-2, 4-dione 2c and 1-cyclopropylbutane-1, 3-dione 2d under 80 ℃ or 120 ℃, giving the desired products 3a-3d in 98%, 91%, 94% and 90% yields, respectively (entries 1–4). The results indicated that the substrate 2b containing electron-withdrawing group and substrate 2d containing cyclopropane showed lower efficiency than the substrate 2c containing electron-donating group. Consequently, an improved temperature (120 ℃) was found to be necessary. The reactions of benzenesulfonyl hydrazide 1b with acetylacetone 2a could give a yield of 99% under standard conditions. Further, benzenesulfonyl containing both electron-donating and electron-withdrawing groups 1c-1f with acetylacetone 2a catalyzed by NaCoMo cluster also proceeded smoothly, delivering the corresponding pyrazoles 3f-3i as desired products in 83%−97% yields (entries 6–9). The experimental results indicated that the reaction substrates containing electron-withdrawing group had low reactivity and required a higher reaction temperature. Moreover, as the performance of the electron-withdrawing group increases (NO2 > Cl > Br), the reactivity of the substrate gradually decreases (1f < 1d < 1e), and the yield of the disered product gradually decreases (3i < 3g < 3h). Furthermore, it is noteworthy to mention that naphthalene-2-sulfonohydrazide 1g was also proved to be an effective substrate to react with acetylacetone 2a towards the desired product 3j at 80 ℃ for 1.5 h, delivering 3, 5-dimethyl-1-(naphthalen-2-ylsulfonyl)-1H-pyrazole 3j in 94% yield (entry 10). Besides, the stability of NaCoMo was comfirmed by PXRD (Fig. S4) after 5-cycles.
|
|
Table 2 Substrate scope of NaCoMo-catalysed condensation cyclization reaction.a |
In summary, we have synthesized a new 3D metal-oxo-clusters-based inorganic framework NaCoMo, named as 3D platelike ternary-oxo-cluster, and which was fully characterized by various means. DFT results reveal that formation process of the crystal is exothermic and the structure is very stable. Furthermore, the pyrazoles can be synthesized using NaCoMo as an efficient catalyst under moderate conditions with excellent yields via the condensation cyclization reaction. The successfully synthesis of NaCoMo represents the discovery of a new kind of metal-oxo-clusters-based inorganic framework. Further investigations on the structure of similar 3D platelike ternary-oxo-cluster and activity of them are in progress.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 22001034, 21871026, 21804019), the Open Fund of the Jiangxi Province Key Laboratory of Synthetic Chemistry (No. JXSC202008), the Research Found of East China University of Technology (Nos. DHBK2019264, DHBK2019265, DHBK2019267).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.008.
| [1] |
S.R. Seidel, P.J. Stang, Acc. Chem. Res. 35 (2002) 972-983. DOI:10.1021/ar010142d |
| [2] |
A. Dolbecq, E. Dumas, C.R. Mayer, et al., Chem. Rev. 110 (2010) 6009-6048. DOI:10.1021/cr1000578 |
| [3] |
T.R. Cook, P.J. Stang, Chem. Rev. 115 (2015) 7001-7045. DOI:10.1021/cr5005666 |
| [4] |
Y. Sun, C.Y. Chen, J.B. Liu, et al., Chem. Soc. Rev. 49 (2020) 3889-3919. DOI:10.1039/d0cs00038h |
| [5] |
Q.G. Zhai, C. Mao, X. Zhao, et al., Angew. Chem. Int. Ed. 54 (2015) 7886-7890. DOI:10.1002/anie.201503095 |
| [6] |
S.S. Wang, G.Y. Yang, Chem. Rev. 115 (2015) 4893-4962. DOI:10.1021/cr500390v |
| [7] |
Y.M. Chen, S.Y. Sun, D. Lu, et al., Chin. Chem. Lett. 30 (2019) 37-43. DOI:10.1080/09593330.2017.1377769 |
| [8] |
H.N. Miras, J. Yan, D.L. Long, L. Cronin, Chem. Soc. Rev. 41 (2012) 7403-7430. DOI:10.1039/c2cs35190k |
| [9] |
O. Sadeghi, L.N. Zakharov, M. Nyman, Science 347 (2015) 1359-1362. DOI:10.1126/science.aaa4620 |
| [10] |
C.F. Li, N. Mizuno, K. Yamaguchi, K. Suzuki, J. Am. Chem. Soc. 141 (2019) 7687-7692. DOI:10.1021/jacs.9b02541 |
| [11] |
B.J. Yan, X.S. Du, R.W. Huang, et al., Inorg. Chem. 57 (2018) 4828-4832. DOI:10.1021/acs.inorgchem.8b00702 |
| [12] |
S. Chen, W.H. Fang, L. Zhang, J. Zhang, Inorg. Chem. 57 (2018) 8850-8856. DOI:10.1021/acs.inorgchem.8b00751 |
| [13] |
X.L. Lin, B.B. Liu, H. Huang, et al., Inorg. Chem. Front. 5 (2018) 723-731. DOI:10.1039/C7QI00693D |
| [14] |
S. Chen, Z.N. Chen, W.H. Fang, et al., Angew. Chem. Int. Ed. 58 (2019) 10932-10935. DOI:10.1002/anie.201904680 |
| [15] |
X. Fan, J.H. Wang, K.F. Wu, L. Zhang, et al., Angew. Chem. Int. Ed. 58 (2019) 1320-1323. DOI:10.1002/anie.201809961 |
| [16] |
P.T. Ma, F. Hu, J.P. Wang, J.Y. Niu, Coord. Chem. Rev. 378 (2019) 281-309. DOI:10.1016/j.ccr.2018.02.010 |
| [17] |
X.Q. Huang, N. Zhen, Y.L. Chi, C.W. Hu, Sci. Sin. Chim. 50 (2020) 1064-1092. DOI:10.1360/SSC-2020-0083 |
| [18] |
W.H. Fang, L. Zhang, J. Zhang, Chem. Soc. Rev. 47 (2018) 404-421. DOI:10.1039/C7CS00511C |
| [19] |
L. Vilà-Nadal, L. Cronin, Nat. Rev. Mater. 2 (2017) 17054. DOI:10.1038/natrevmats.2017.54 |
| [20] |
C. Falaise, K. Kozma, M. Nyman, Chem. Eur. J. 24 (2018) 14226-14232. DOI:10.1002/chem.201802671 |
| [21] |
T. Boyd., Y.F. Song, L. Cronin, et al., J. Am. Chem. Soc. 139 (2017) 5930-5938. DOI:10.1021/jacs.7b01807 |
| [22] |
Y.X. Wang, Z.T. Tao, S.A. Wang, Inorg. Chem. 54 (2015) 10023-10029. DOI:10.1021/acs.inorgchem.5b01801 |
| [23] |
Y.Y. Zhou, S. Yao, Z.M. Zhang, et al., Dalton Trans. 44 (2015) 20435-20440. DOI:10.1039/C5DT03397G |
| [24] |
Q. De, X.X. Xu, Chem. Eur. J. 26 (2020) 7923-7929. DOI:10.1002/chem.202001031 |
| [25] |
J.X. Wang, H.Y. Zang, Y.G. Li, et al., Inorg. Chem. Front. 5 (2018) 1213-1217. |
| [26] |
J.H. Liu, Y.Q. Sun, S.T. Zheng, et al., Inorg. Chem. 58 (2019) 14734-14740. DOI:10.1021/acs.inorgchem.9b02413 |
| [27] |
W.S. Zhang, Y. Liu, H.L. Hu, Z.H. Kang, et al., Dalton Trans. 42 (2013) 1760-1769. DOI:10.1039/C2DT31595E |
| [28] |
F. Meng, H. Zhang, Y. Zhu, et al., Adv. Synth. Cat. 362 (2020) 248-254. DOI:10.1002/adsc.201901104 |
| [29] |
G.P. Yang, S.X. Shang, C.W. Hu, et al., Inorg. Chem. Front. 5 (2018) 2472-2477. DOI:10.1039/c8qi00678d |
| [30] |
B. Yu, L.N. He, ChemSusChem 8 (2015) 52-62. DOI:10.1002/cssc.201402837 |
| [31] |
B. Zhang, A. Studer, Chem. Soc. Rev. 44 (2015) 3505-3521. DOI:10.1039/C5CS00083A |
| [32] |
Z. Xu, C. Gao, Z.S. Lv, et al., Eur. J. Med. Chem. 139 (2017) 429-440. DOI:10.1016/j.ejmech.2017.07.059 |
| [33] |
A. Ansari, A. Ali, M. Asif, New. J. Chem. 41 (2017) 16-41. DOI:10.1039/C6NJ03181A |
| [34] |
D. Sar, R. Bag, T. Punniyamurthy, Org. Lett. 17 (2015) 5308-5311. DOI:10.1021/acs.orglett.5b02669 |
| [35] |
G.P. Yang, N. Zhang, C.W. Hu, et al., Adv. Synth. Cat. 359 (2017) 926-932. DOI:10.1002/adsc.201601231 |
| [36] |
G.P. Yang, D. Dilixiati, C.W. Hu, et al., Appl. Organomet. Chem. 32 (2018) e4450. DOI:10.1002/aoc.4450 |
| [37] |
G.P. Yang, N. Jiang, C.W. Hu, et al., Mol. Cat. 468 (2019) 80-85. |
| [38] |
M.T. Lv, Y.F. Liu, K. Li, G.P. Yang, Tetrahedron. Lett. 65 (2021) 152757-152760. DOI:10.1016/j.tetlet.2020.152757 |
| [39] |
G.P. Yang, X. Wu, C.W. Hu, et al., ACS Sustain. Chem. Eng. 7 (2019) 3727-3732. DOI:10.1021/acssuschemeng.8b06445 |
| [40] |
G.P. Yang, Y. Liu, K. Li, et al., Chin. Chem. Lett. 31 (2020) 3233-3236. DOI:10.1016/j.cclet.2020.07.018 |
| [41] |
J. Wang, J.Y. Niu, J.P. Wang, et al., Inorg. Chem. 58 (2019) 57-60. DOI:10.1021/acs.inorgchem.8b02856 |
 2022, Vol. 33
2022, Vol. 33