The selective hydrogenation of C≡C to C=C bonds is an important step in chemical industry such as the synthesis of vitamins, pharmaceuticals, and polymers [1-3]. 2-Methyl-3-buten-2-ol (MBE), an important intermediate in the manufacture of vitamins A and E, is generally produced by selective hydrogenation of 2-methyl-3-butyn-2-ol (MBY) under pressurized H2 in batch reactor [3]. The main challenge in the MBE production is to prevent over-hydrogenation (to 2-methyl-2-butanol (MBA)) and/or oligomerization of C=C bonds. Palladium based catalysts show unique catalytic performance in this process. Extensive studies on the structure-activity relationship of Pd catalysts have shown that the selectivity is largely influenced by two factors: (i) palladium hydride species formed over supported Pd catalysts [4-7] and (ii) adsorption strength of alkenol and alkynol on active sites [3]. Many open literatures have shown that both factors are strongly related to the structural and electronic properties of Pd nanoparticles [8, 9]. Thus, several strategies have been brought forward to manipulate the catalytic properties of Pd nanoparticles by (i) adjusting the morphology (shape and size) of the Pd nanoparticles [10, 11] and (ii) tuning the electronic property through coordinating with organic capping reagent [12] or alloying with second metal M (Pb, Zn, Ag, Cu, Au, In, Ga, etc.) [13-20]. The second metal plays comprehensive roles in controlling the catalytic performance depending on the interaction between Pd-M [21]. Some metals such as Pb [22], Zn [23], Ga [24] and In [25] may sit on the defect sites of Pd nanoparticles, thus inhibiting the overhydrogenation. In some other cases, the Pd ensemble size may be largely decreased by forming alloy or intermetallic structure, which hinder the formation of unselective Pd hydride phase [4]. Among these metals, Cu has been recognized as a promising element which can significantly improve the selectivity in semi-hydrogenation of acetylene and other alkynes due to its low-price and relatively large abundance on earth [8, 16]. Moreover, single atom alloy Pd-Cu structure containing Pd in very low concentration can catalyze the semi-hydrogenation of several alkynes efficiently under mild reaction conditions where monometallic copper is inert [8, 16]. Both experimental and theoretical results have shown that single Pd atom isolated by Cu atoms can promote the H2 dissociation while H atoms may spillover onto Cu surface where hydrogenation occurs [15, 16, 26]. These merits of PdCu alloy structure have stimulated many efforts on the development of synthetic methodologies of heterogeneous supported PdCu catalysts [2]. Boucher et al. deposited trace amount of Pd exclusively onto the pre-formed Cu nanoparticles by the galvanic replacement reaction [16]. McCue et al. prepared CuPd catalyst with Cu: Pd up to 50 by a simple sequential impregnation methodology, giving > 99% acetylene conversion and > 70% ethylene selectivity at 373 K [27]. Cao et al. systematically compared the effect of preparation methods on the structure and catalytic performance of PdCu catalysts [28]. The results suggested that surface PdCu alloy was formed via a modified sequential impregnation recipe and showed preferable stability and better resistance against the deposition of coke. Apparently, further study on the influence of preparation procedure on the structure and performance of PdCu catalyst is highly desired. In this study, we have prepared a PdCu catalyst which showed promising semi-hydrogenation activity for the production of MBE. The preparation contains two sequential steps. In the first step, CuO/SiO2 was prepared by incipient impregnation and calcination. In the second step, trace amount of Pd (about 500 ppm) was loaded by deposition-reduction on CuO/SiO2 by using H2PdCl4 solution as precursor and NaBH4 as reductant, respectively. It was found that the pH value at which Pd was deposited plays a crucial role in determining the catalytic performance of PdCu catalyst. Characterization results suggested that partially dissolved Cu2+ from CuO were co-precipitated and co-reduced with Pd2+, thus forming highly selective hydrogenation nanoparticles with metallic alloy structure.
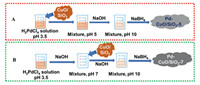
The CuO/SiO2 support with CuO loading of 0.5 wt% was used in this study, which was prepared using Cu(NO3)2·3H2O as a precursor in presence of ethylene glycol (EG). EG is believed to favor the homogeneous distribution of CuO species on silica surface. The detailed synthesis procedure of CuO/SiO2 has been described in Supporting information. Pd catalysts with loading of 0.05 wt% were prepared according to Scheme 1. In synthesis procedure A, 1.85 g of 0.5CuO/SiO2 was distributed in H2O (80 mL) with vigorous stirring for 1 h. An appropriate amount of H2PdCl4 aqueous solution (2.1512 gPd/L, pH 3.5) was added into the mixture, leading to a mixture with pH value about 5. After stirring for 3 h, the final pH value of suspension was adjusted to 10 by adding NaOH aqueous solution (1 mol/L). Then, NaBH4 aqueous solution (NaBH4/Pd = 15, molar ratio) was added into the suspension to reduce the Pd(OH)2 species. The slurry was stirred for another 1 h, filtered, washed with deionized water, and finally dried at 90 ℃ overnight. Thus obtained catalyst was denoted as 0.05Pd-0.5CuO/SiO2–5. In synthesis procedure B, the H2PdCl4 solution was firstly neutralized to pH value of 7 by adding 1 mol/L NaOH solution. Then, 0.5CuO/SiO2 composites (1.85 g) were added and the pH value of the mixture was adjusted to 10. After vigorous stirring for 3 h, the precipitated Pd(OH)2 were reduced by NaBH4 aqueous solution and the catalyst was named as 0.05Pd-0.5CuO/SiO2–7.
|
Download:
|
| Scheme 1. A schematic illustration of the preparation procedure of Pd-CuO/SiO2–5 (A) and Pd-CuO/SiO2–7 (B). | |
The catalysts were characterized by several techniques including XRD, TEM, H2-TPR and FT-IR (see Supporting information). Catalytic reactions for semi-hydrogenation of MBY to MBE were conducted in a Teflon-lined (100 mL) steel batch reactor at a stirring speed of 500 rpm for 40 min. The reactor was charged with MBY (0.2 mL), ethanol (9.8 mL), and Pd catalysts (50 mg). After being purged five times with H2, the reactor was pressurized with 0.3 MPa of H2. The mixture was stirred in an oil bath at 30 ℃. Once the reaction was completed, the products were diluted with ethanol and analyzed with a Tianmei 7900 GC equipped with a flame ionization detector (FID) and a capillary column of DM-FFAP. The detailed information on the product analysis and kinetics study have been described in Supporting information.
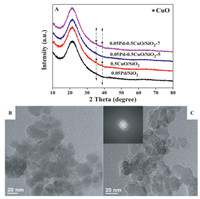
Fig. 1A shows the XRD patterns of 0.5CuO/SiO2 support and the supported Pd catalysts. Two diffraction peaks at 35.6° and 38.7o assigned to CuO phase were observed for 0.5CuO/SiO2 support. Both peaks were also distinguished for 0.05Pd-0.5CuO/SiO2–7, while they disappeared for 0.05Pd-0.5CuO/SiO2–5. We surmise that large CuO particles were likely dissolved by the acidic solution of H2PdCl4 during the preparation procedure in the case of 0.05Pd-0.5CuO/SiO2–5, thus became invisible by XRD. The dissolution of CuO in acidic H2PdCl4 solution was confirmed by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry). In an independent control experiment, we found that about 10% of CuO were dissolved by the acidic Pd solution. These Cu2+ were again precipitated by NaOH and subsequently reduced by NaBH4 together with Pd(OH)2. Cu nanoparticles could be formed by reducing Cu2+ ions with NaBH4 in alkaline solution [29], whilst the formation of Cu2O cannot be excluded. As shown in Table S1 (Supporting information), the Cu and Pd loading of both catalysts prepared at different pH values equaled to each other. The dissolution of CuO did not occur during the preparation of 0.05Pd-0.5CuO/SiO2–7, where the pH value of Pd solution was 7. Due to the very low loadings of Pd and Cu, we did not see any diffractions attributed to Pd(Cu) nanoparticles.
|
Download:
|
| Fig. 1. XRD patterns of 0.5CuO/SiO2 support and supported Pd catalysts with different deposition pH values (A) and TEM images of 0.05Pd-0.5CuO/SiO2–5 (B) and 0.05Pd-0.5CuO/SiO2–7 (C). | |
To provide the particle size and morphology of Pd and Cu species of 0.05Pd-0.5CuO/SiO2 catalysts prepared by two different methods in more details, we have performed TEM analysis on two catalysts and the results are shown in Figs. 1B and C. We did not see any visible Pd nanoparticles on both catalysts by HRTEM, meaning that the Pd species likely existed as clusters in sub-nanometer. One interesting finding was that clear SAED (Selected Area Electron Diffraction) of CuO phase was observed in the case of 0.05Pd-0.5CuO/SiO2–7 (inset in Fig. 1C), whereas it was not found on 0.05Pd-0.5CuO/SiO2–5. This result points to the different nature of CuO species on both catalysts. As we have mentioned early, some CuO particles on 0.05Pd-0.5CuO/SiO2–5 were dissolved by the residual acid in H2PdCl4 solution and then turned to Cu clusters together with Pd in presence of NaBH4. While in the 0.05Pd-0.5CuO/SiO2–7, the dissolution of CuO did not occur, thereby the CuO phase was clearly detected by HRTEM. This result is consistent with the XRD findings. We tried to characterize the supported Pd and Cu species by XPS and CO IR under ambient conditions, but no meaningful information (not shown) could be derived due to the very low loadings of both Pd and Cu.
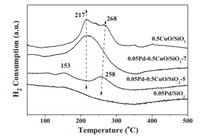
The H2 temperature programmed reduction of 0.5CuO/SiO2 and supported Pd catalysts were displayed in Fig. 2. For 0.5CuO/SiO2 support, two overlapped reduction peaks in the range of 200–300 ℃ were observed and the H/Cu ratio was estimated to be 1.96. This value is consistent with the reduction of CuO to Cu. The higher-temperature shoulder (Tm = 275 ℃) may arise from the contribution of a decaying rate stage in the reduction of the larger CuO particles [30]. As for 0.05Pd/SiO2, we cannot find any reduction peak due to ultralow Pd content. When palladium was loaded on 0.5Cu/SiO2 at pH 5, two reduction peaks centered at 153 and 258 ℃ were noticed. It is worth noting that the intensity of both peaks was very weak compared with the 0.5CuO/SiO2 support. The H/Cu ratio is estimated to be about 0.36, which is much lower than the theoretical value of 2. This observation is consistent with our assumption that some Cu2+ species were reduced by NaBH4 during the preparation. The small reduction peak at 153 ℃ might be due to the reduction of PdCu species formed in the preparation procedure, while the reduction peak at 258 ℃ could be due to the reduction of CuOx species. In the case of 0.05Pd-0.5CuO/SiO2–7, much different reduction behavior to that of 0.05Pd-0.5CuO/SiO2–5 was found. This catalyst showed a strong reduction peak centered at 217 ℃ as observed for 0.5CuO/SiO2 support, which pointing to a much weak interaction between Pd and CuO species prepared at pH 7. Taking into account of the above mentioned results, we may deduce that on 0.05Pd-0.5CuO/SiO2–5, the CuO was partly reduced to metallic Cu which leading to the formation of PdCu alloys.
|
Download:
|
| Fig. 2. H2-TPR profiles of 0.5CuO/SiO2, 0.05Pd/SiO2, 0.05Pd-0.5CuO/SiO2–5 and 0.05Pd-0.5CuO/SiO2–7. | |
The catalytic performances of MBY semi-hydrogenation over the 0.05Pd-0.5CuO/SiO2 catalysts and their monometallic counterparts at 30 ℃ are compared in Fig. S1 (Supporting information). The 0.5CuO/SiO2 support showed very low MBY conversion at 30 ℃ and the reaction was dominated by dimerization, suggesting that CuO was almost inert toward MBY hydrogenation under mild conditions. Monometallic 0.05Pd/SiO2 catalyst gave 87% conversion of MBY after reaction of 40 min, with 92% selectivity to MBE (Fig. S1B). Further prolonging the reaction period to 2 h led to full conversion of MBY and significant decrease of MBE selectivity from 92% to 44%. This behavior has been commonly observed for monometallic Pd nanoparticles in case of semi-hydrogenation of triple bonds [31, 32]. The 0.05Pd-0.5CuO/SiO2–7 catalyst showed even higher activity than 0.05Pd/SiO2, with 100% MBY conversion within 40 min but with only 54% MBE selectivity. In contrast, 0.05Pd-0.5CuO/SiO2–5 catalyst showed distinct performance, over which the MBY conversion and MBE selectivity were found to be 42% and 95% after reaction for 40 min, respectively. Further extending the reaction period to 2 h, the MBY was fully converted while the MBE selectivity could remain as high as 85%. We have investigated the influence of reaction time on the MBY conversion and MBE selectivity on both catalysts. Tables S2 and S3 (Supporting information) suggest that the triple bond hydrogenation rate decreased by two fold while the double bond saturation rate was severely hindered within 2 h over 0.05Pd-0.5CuO/SiO2–5 catalyst. Then we compared its performance with a benchmark Lindlar catalyst. The results shown in Table S4 (Supporting information) indicate the 0.05Pd-0.5CuO/SiO2–5 was indeed more selective than Lindlar catalyst toward semi-hydrogenation of MBY.
This interesting result encouraged us to compare the reaction kinetics of MBY semi-hydrogenation over 0.05Pd/SiO2 and 0.05Pd-0.5CuO/SiO2–5 catalysts by changing several variables, namely the initial concentration of MBY, H2 pressure and reaction temperature. The following equation was proposed to reveal the influence of each variable:
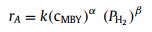
|
(1) |
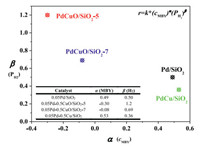
The reaction orders in MBY and H2 over four catalysts, namely 0.05Pd/SiO2, 0.05Pd-0.5Cu/SiO2, 0.05Pd-0.5CuO/SiO2–5 and 0.05Pd-0.5CuO/SiO2–7 were estimated. The detailed data are shown in Fig. 3 and Fig. S2 (Supporting information). For comparison, a Pd doped metallic 0.5Cu/SiO2 was prepared and the synthesis procedure is described in the ESI. The reaction order with respect to initial concentration of MBY and PH2 over 0.05Pd/SiO2 was calculated to be 0.49 and 0.50, respectively. Both positive numbers are well consistent with previous finding that MBY and H2 undergo competitive adsorption on Pd surface. Under the similar reaction conditions, the reaction order with respect to initial cMBY and PH2 over 0.05Pd-0.5CuO/SiO2–5 was calculated to be ‒0.30 and 1.2, respectively. The changes of reaction orders are normally associated with the variation of reaction conditions (temperature and hydrogen pressure) and adsorption behavior of molecules (reactant, intermediate and product). Therefore, the great change in reaction orders in both MBY and H2 clearly means that the PdCu-related species in Pd-CuO/SiO2 altered the adsorption behavior of MBY and/or H2. For monometallic Pd catalysts, the MBY and H2 undergo competitive adsorption on Pd surface, thus leading to both positive reaction orders. In contrast, negative reaction orders (‒0.3 and ‒0.08) in MBY along with more positive reaction orders of H2 over Pd catalysts containing Cu/CuO species were observed. This result demonstrates that the presence of metal oxides or PdCu alloy may affect the adsorption behavior and reaction kinetics of alkynols hydrogenation over Pd catalysts.
|
Download:
|
| Fig. 3. The detailed data of the reaction orders in α(cMBY) and β(PH2) over four catalysts, namely 0.05Pd/SiO2, 0.05Pd-0.5CuO/SiO2–5, 0.05Pd-0.5CuO/SiO2–7 and 0.05Pd-0.5Cu/SiO2. | |
Generally, the presence of large amount of MBY can significantly slow down the hydrogenation rate of MBE. To our surprise, we found in this case that the hydrogenation rate of MBE over 0.05Pd-0.5CuO/SiO2–5 was still very low even when trace amount of MBY was present. This finding prompted us to compare the reaction rate constants of the two sequential steps as shown below. Before calculating the k1 and k2, we determined the reaction orders in terms of MBE concentration and H2 pressure on two catalysts and the results are shown in Table S5 (Supporting information). Based on these reaction orders, the reaction rate constant of MBY hydrogenation (k1) and MBE saturation (k2) was deduced (Table S5 in Supporting information) by taking into account of the MBE concentration and H2 concentration in ethanol. The H2 concentration in ethanol was calculated according to Henry rule, where K = 452 MPa was employed according to literature [33]. One can see that over 0.05Pd/SiO2, the k1/k2 is about 3.3, which means that the reaction rate constants of C≡C and C=C bond were in the same magnitude. Surprisingly, the k1/k2 over 0.05Pd-0.5CuO/SiO2–5 is as high as 129. This finding well explains the high semi-hydrogenation selectivity of 0.05Pd-0.5CuO/SiO2–5 catalyst, though the over-hydrogenation reaction is favored from a kinetic point of view according to the thermodynamic understanding [34]. The reaction kinetics may be influenced by several parameters. The adsorption strength of MBY/MBE on Pd surface has been obviously affected due to the formation of PdCu alloy, as observed by Vernuccio and coworkers on the PdZn catalyst [35]. The reaction order of 1 in terms of H2 over 0.05Pd-0.5CuO/SiO2–5 also suggests that the hydrogenation step likely took place on PdCu alloy or Cu sites, which showed rather lower ability in hydrogenation of alkenes under ambient conditions compared with monometallic Pd sites. This assumption has been further confirmed by testing the semi-hydrogenation activity over 0.05Pd-0.5CuO/SiO2–5 catalyst with varying Cu content (Fig. S3 in Supporting information). Increasing the Cu content higher than 0.5 wt% in PdCu alloy results in dramatic decrease of hydrogenation activity.
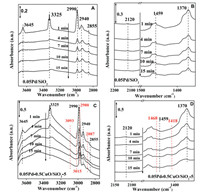
To shed light on the relationship between reaction kinetics and adsorption behaviors of MBY and MBE, we have followed the temperature programmed desorption of MBY on 0.05Pd/SiO2 and 0.05Pd-0.5CuO/SiO2–5 by in situ FTIR (Fig. S4 in Supporting information) at different evacuation temperatures (30, 50, 100, 150 and 200 ℃). Some feature vibrational bands due to C≡C stretch and –OH [36] are summarized in Table S6 (Supporting information). The results suggested that MBY desorbed at lower temperature on 0.05Pd-0.5CuO/SiO2–5, meaning that the formation of PdCu alloy in sub-nanometer significantly changed the adsorption strength of MBY on metal surface. We then followed the evolution of semi-hydrogenation process by recording the in situ FTIR spectra of the 0.05Pd/SiO2 and 0.05Pd-0.5CuO/SiO2–5 after MBY adsorption in flowing H2 at 30 ℃ (Fig. 4). For Pd/SiO2 catalyst, the intensity of bands assigned to adsorbed MBY decreased with time on stream without forming any new bands. In the case of 0.05Pd-0.5CuO/SiO2–5, the intensity of bands due to MBY decreased quickly after introducing H2. Meanwhile, new bands (1418, 1468, 2887, 2980, 3015 and 3093 cm−1) assigned to MBE were clearly seen. This observation is consistent with the finding that higher MBE selectivity was obtained on 0.05Pd-0.5CuO/SiO2–5.
|
Download:
|
| Fig. 4. The in situ FTIR spectra of the 0.05Pd/SiO2 (A, B) and 0.05Pd-0.5CuO/SiO2–5 (C, D) during MBY hydrogenation in flowing H2 at 30 ℃. | |
We have also studied the solvent effect on MBY conversion and MBE selectivity over 0.05Pd-0.5CuO/SiO2–5 catalyst (Fig. S5 in Supporting information). We can easily find that the hydrogenation activity in polar solvents (isopropanol, ethanol, tetrahydrofuran, except water) was generally higher than that in non-polar solvents (toluene, cyclohexane, heptane), which is related to the solvent properties such as dipole moment, dielectric constant, hydrogen solubility and solvent-catalyst interaction [37]. Fig. S6 (Supporting information) shows the reusability of 0.05Pd-0.5CuO/SiO2–5 catalyst. No significant loss of reactivity and selectivity was noticed for the first three runs. However, the MBY conversion dropped sharply from 85% to 45% in the 4th run, whereas the MBE selectivity remained about 95%. We speculate that the microenvironment of PdCu alloy on 0.5CuO/SiO2 might change to some extent during the hydrogenation process, which is in line with the fact that the preparation method of PdCu nanoparticles significantly affected their semi-hydrogenstion performance [17]. Therefore, further detailed studies on the structure of PdCu atom alloy and its change in the hydrogenation process are highly desired in the future.
In conclusion, we have prepared two 0.05Pd-0.5CuO/SiO2 catalysts via deposition-precipitation of H2PdCl4 onto 0.5CuO/SiO2 support by varying the pH value of PdCl42− solution where Pd was deposited. When the pH value was about 5, the CuO was partially dissolved during the deposition and then co-reduced with Pd2+ by NaBH4, forming PdCu alloy in sub-nanometer size. Thus formed PdCu alloy changed the adsorption strength of MBY on metal surface and altered the reaction kinetics of MBY semi-hydrogenation, leading to very high selectivity of desired product, namely, 2-methyl-3-buten-2-ol (MBE). The results clearly demonstrated that the pH value where Pd was deposited played a crucial role in determining the catalytic performance of PdCu catalyst in semi-hydrogenation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe acknowledge the financial support from the National Natural Science Foundation of China (No. 21773067) and the Open Research fund of Shanghai Key Laboratory of Green Chemistry and Chemical Processes.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.012.
| [1] |
F. Zaera, ACS Catal. 7 (2017) 4947-4967. DOI:10.1021/acscatal.7b01368 |
| [2] |
J.A. Delgado, O. Benkirane, C. Claver, D. Curulla-Ferré, C. Godard, Dalton Trans. 46 (2017) 12381-12403. DOI:10.1039/C7DT01607G |
| [3] |
J.A. Delgado, C. Godard, Progress in the selective semi-hydrogenation of alkynes by nanocatalysis, in: P.W.N.M. van Leeuwen, C. Claver (Eds. ), Recent Advances in Nanoparticle Catalysis, Springer Molecular Catalysis 1, Spain, 2020, pp. 303-344.
|
| [4] |
M. García-Mota, B. Bridier, J. Pérez-Ramírez, N. López, J. Catal. 273 (2010) 92-102. DOI:10.1016/j.jcat.2010.04.018 |
| [5] |
D. Teschner, J. Borsodi, A. Wootsch, et al., Science 320 (2008) 86-89. DOI:10.1126/science.1155200 |
| [6] |
M. Armbrüster, M. Behrens, F. Cinquini, et al., ChemCatChem 4 (2012) 1048-1063. DOI:10.1002/cctc.201200100 |
| [7] |
S.F. Parker, H.C. Walker, S.K. Callear, et al., Chem. Sci. 10 (2019) 480-489. DOI:10.1039/c8sc03766c |
| [8] |
G. Pei, X. Liu, X. Yang, et al., ACS Catal 7 (2017) 1491-1500. DOI:10.1021/acscatal.6b03293 |
| [9] |
M.R. Ball, K.R. Rivera-Dones, E.B. Gilcher, et al., ACS Catal 10 (2020) 8567-8581. DOI:10.1021/acscatal.0c01536 |
| [10] |
M. Crespo-Quesada, A. Yarulin, M. Jin, Y. Xia, L. Kiwi-Minsker, J. Am. Chem. Soc. 133 (2011) 12787-12794. DOI:10.1021/ja204557m |
| [11] |
R. Ma, N. Semagina, J. Phys. Chem. C 114 (2010) 15417-15423. DOI:10.1021/jp103754b |
| [12] |
X. Zhao, L. Zhou, W. Zhang, et al., Chem 4 (2018) 1080-1091. DOI:10.1016/j.chempr.2018.02.011 |
| [13] |
W. Niu, Y. Gao, W. Zhang, N. Yan, X. Lu, Angew. Chem. Int. Ed. 54 (2015) 8271-8274. DOI:10.1002/anie.201503148 |
| [14] |
M.W. Tew, H. Emerich, J.A. van Bokhoven, J. Phys. Chem. C 115 (2011) 8457-8465. DOI:10.1021/jp1103164 |
| [15] |
G. Pei, X. Liu, A. Wang, et al., ACS Catal. 5 (2015) 3717-3725. DOI:10.1021/acscatal.5b00700 |
| [16] |
G. Kyriakou, M.B. Boucher, A.D. Jewell, et al., Science 335 (2012) 1209-1212. DOI:10.1126/science.1215864 |
| [17] |
M.B. Boucher, B. Zugic, G. Cladaras, et al., Phys. Chem. Chem. Phys. 15 (2013) 12187-12196. DOI:10.1039/c3cp51538a |
| [18] |
X. Li, Z. Wang, Z. Zhang, et al., Mater. Horiz. 4 (2017) 584-590. DOI:10.1039/C6MH00478D |
| [19] |
Q. Feng, S. Zhao, Y. Wang, et al., J. Am. Chem. Soc. 139 (2017) 7294-7301. DOI:10.1021/jacs.7b01471 |
| [20] |
M. Armbrüster, K. Kovnir, M. Behrens, et al., J. Am. Chem. Soc. 132 (2010) 14745-14747. DOI:10.1021/ja106568t |
| [21] |
S. Furukawa, T. Komatsud, K. Shimizu, J. Mater. Chem. A 8 (2020) 15620-15645. DOI:10.1039/d0ta03733h |
| [22] |
J.A. Anderson, J. Mellor, R.P.K. Wells, J. Catal. 261 (2009) 208-216. DOI:10.1016/j.jcat.2008.11.023 |
| [23] |
L. Shen, S. Mao, J. Li, et al., J. Catal. 350 (2017) 13-20. DOI:10.1016/j.jcat.2017.01.021 |
| [24] |
M. Krajčí, J. Hafner, J. Phys. Chem. C 118 (2014) 12285-12301. DOI:10.1021/jp5025075 |
| [25] |
S. Mao, B. Zhao, Z. Wang, et al., Green Chem. 21 (2019) 4143-4151. DOI:10.1039/c9gc01356c |
| [26] |
A.J. McCue, A.M. Shepherd, J.A. Anderson, Catal. Sci. Technol. 5 (2015) 2880-2890. DOI:10.1039/C5CY00253B |
| [27] |
A.J. McCue, C.J. McRitchie, A.M. Shepherd, J.A. Anderson, J. Catal. 319 (2014) 127-135. |
| [28] |
X. Cao, A. Mirjalili, J. Wheeler, W. Xie, B.W.L. Jang, Front. Chem. Sci. Eng. 9 (2015) 442-449. DOI:10.1007/s11705-015-1547-x |
| [29] |
Q. Liu, D. Zhou, Y. Yuya, I. Ryoichi, O. Masazumi, Trans. Nonferrous Met. Soc. China 22 (2012) 117-123. DOI:10.1016/S1003-6326(11)61149-7 |
| [30] |
S.J. Gentry, P.T. Walsh, J. Chem. Soc., Faraday Trans. I 78 (1982) 1515-1523. DOI:10.1039/f19827801515 |
| [31] |
T. Mitsudome, T. Urayama, K. Yamazaki, et al., ACS Catal. 6 (2016) 666-670. DOI:10.1021/acscatal.5b02518 |
| [32] |
F.P. da Silva, J.L. Fiorio, R.V. Goncalves, E. Teixeira-Neto, L.M. Rossi, Ind. Eng. Chem. Res. 57 (2018) 16209-16216. DOI:10.1021/acs.iecr.8b03627 |
| [33] |
M.S. Walnwright, T. Ahn, D.L. Trimm, N.W. Cant, J. Chem. Eng. Data 32 (1987) 22-24. DOI:10.1021/je00047a006 |
| [34] |
Á. Molnár, A. Sárkány, M. Varga, J. Mol. Catal. A: Chem. 173 (2001) 185-221. DOI:10.1016/S1381-1169(01)00150-9 |
| [35] |
S. Vernuccio, R. Goy, P.R. von Rohr, J. Medlock, W. Bonrath, React. Chem. Eng. 1 (2016) 445-453. DOI:10.1039/C6RE00093B |
| [36] |
F. Ferrante, A. Prestianni, D. Duca, J. Phys. Chem. C 118 (2014) 551-558. DOI:10.1021/jp410878j |
| [37] |
L. Nikoshvili, E. Shimanskaya, A. Bykov, et al., Catal. Today 241 (2015) 179-188. DOI:10.1016/j.cattod.2014.01.045 |
 2022, Vol. 33
2022, Vol. 33 

