b Department of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China;
c School of Physics and Technology, Wuhan University, Wuhan 430072, China
pH Value is one of the important physical and chemical parameters in the fields of industry, agriculture, medicine, environmental protection and scientific research [1]. pH is also associated with many health complaints, including cancer and neurodegenerative diseases, because slightly disrupted pH balance leads to cell dysfunctions [2-4]. Thus, high-precision pH sensing and monitoring is essential for understanding its effect on related cellular functions and pathological processes. Fluorescence probes have recently shown great potential for monitoring pH fluctuations because of its high spatiotemporal resolution, high sensitivity, non-invasiveness and easy operation [5]. Various fluorescent probes have been developed for pH sensing [6-13]. Among them, ratiometric fluorescent probes that report solution pH by exhibiting changes of the measured intensity ratio at two excitation or emission wavelengths are superior in quantitative pH analysis compared to probes with single detection window [14-17]. The intrinsic self-calibration capability of ratiometric fluorescence probes avoided the interference caused by external factors such as fluctuation of probe concentration, environmental conditions and instrument sensitivity, accordingly enhancing the measurement accuracy [18-20]. Additionally, red/near-infrared fluorescent sensors are highly desirable for in vivo physiological pH measurement since longer wavelengths photons can more easily penetrate into deep tissues and were less interfered by autofluorescence [21-27].
Most of the reported fluorescent pH chemosensors were obtained by complex, environmentally-unfriendly and costly chemical synthesis, which limited their widespread use [28, 29]. Fluorescent molecules and dyes from natural sources are often easily obtained and environmentally friendly [30], and their photophysical properties have therefore attracted increasing attention from the research community. Accordingly, several fluorescence probes based on natural products have been recently reported [31-33], showing great promise as biocompatible and biodegradable alternatives in the development of new pH sensors. Curcumin (CUR), a fluorescent polyphenol extracted from Curcuma longa roots, has been widely investigated for its pharmacological activity as, for example, an antioxidant or anticancer drug [34, 35]. The excellent optical characteristics also allowed CUR to be used in sensor development [36, 37]. Till now, several CUR analogues have been developed for Aβ plaque and cyanide detection [38-40]. It has also been suggested that CUR could be used in qualitative pH sensing test strips due to the colorimetric behavior [36]. However, the short emission wavelength of CUR has prevented the use of CUR-based fluorescent probes in biological applications.
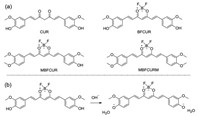
To obtain longer emission wavelengths, a CUR derivative (BFCUR, Scheme 1) was prepared by introducing boron trifluoride moiety in the middle of CUR molecule. This pH-sensitive fluorescent BFCUR exhibited absorption and emission maxima at longer wavelengths than those of CUR. The underlying mechanism for the photophysical behavior was further explored by singly or doubly methylating the phenolic hydroxyl groups of BFCUR to obtain MBFCUR and MBFCURM, respectively. The acid-base response of BFCUR, MDFCUR and MBFCURM demonstrated that the deprotonation process of phenol hydroxyl groups was responsible for the fluorescence intensity changes caused by a change in solution pH. BFCUR demonstrated the strongest response to pH changes and was evaluated as a ratiometric colorimetric and fluorescent pH sensing probe by monitoring the absorbance ratio A500/A650 and the fluorescence intensity ratio I622/I743, realizing further test strip-based detection and cell imaging.
|
Download:
|
| Scheme 1. (a) Structures of CUR, BFCUR, MBFCUR and MBFCURM. (b) Proposed response mechanisms of BFCUR to pH. | |
The phenol groups in the CUR structure are easily protonated and deprotonated, which cause clear color changes in response to the changing pH conditions. Therefore, CUR has been considered as an optical acid-base sensor [41]. However, the short UV-vis absorption and fluorescence emission wavelength of CUR has so far limited its widespread use. CUR features a D-π-A-π-D conjugated system with two intramolecular charge transfer (ICT) interactions [42]. The electron acceptors and donors are respectively in the middle and at both ends of the molecule. Enhancing the electron-withdrawing ability is an effective strategy to shift the absorption and emission peaks to longer wavelengths. Therefore, boron trifluoride with high electronegativity was introduced into CUR to obtain the derivative BFCUR via a one-pot synthesis with high yield and longer excitaion/emission wavelengths (Scheme 1, Figs. S1-S3 in Supporting information). Subsequently, singly or doubly methylated MBFCUR and MBFCURM were also synthesized to provide mechanism insight into the pH sensitivity and to compare the sensing performance (Scheme 1, Figs. S4-S9 in Supporting information).
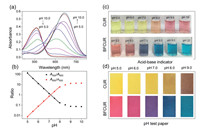
UV-vis spectra of BFCUR at diverse pH values were studied. Compared with CUR, the absorption maxima wavelength of BFCUR shifted from 430 nm to 500 nm under acidic and neutral conditions and from 530 nm to 650 nm under alkaline conditions (Fig. S10). BFCUR displayed a strong absorbance at 500 nm and no obvious absorption peak at 650 nm at pH 5.0. As the pH increased from 5.0 to 8.0, the absorbance maximum at 500 nm disappeared gradually, which coincided with a remarkable absorbance turn-on response at 650 nm (Fig. 1a). The ratio of absorbance (A500/A650) was linearly correlated with the pH between 5.0 and 8.0 (R2 = 0.99, Fig. 1b). Moreover, clear color changes from red to purple to blue were observed as the pH of BFCUR solutions in B-R buffer changed from 5.0 to 8.0 (Fig. 1c). In order to exploit this, BFCUR-based test strips for the qualitative pH determination were prepared. As the pH gradually increased from 5.0 to 8.0, the color of the BFCUR-based test papers expectedly changed from red (pH < 7) to purple (pH 7) to blue (pH > 7) (Fig. 1d). For comparison, the corresponding colorimetric response of the CUR-based test strips was much less clear, where the color changed from yellow to sandy brown.
|
Download:
|
| Fig. 1. (a) UV-vis spectra of BFCUR (10 µmol/L) at different pH values (5.0-10.0) in the B-R buffer/EtOH (1/1, v/v) solution. (b) The plot of the absorbance ratios of BFCUR at A500/A650 and A650/A500 vs. pH values including the linear relationships of both plots ranging from pH 5.0 to 8.0. (c) The color changes of CUR and BFCUR solutions at different pH values (5.0-9.0). (d) The observed color changes of CUR- and BFCUR-coated test strips after soaking in aqueous solution at different pH values (5.0-9.0) by naked eyes. | |
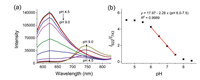
The fluorescence emission spectra of BFCUR varied with different excitation wavelengths (Fig. S11 in Supporting information). When λex = 500 nm, the fluorescence intensity at 622 nm increased markedly as pH value decreased from 8 to 6. Meanwhile, when λex = 650 nm, the fluorescence intensity at 743 nm increased significantly as pH increased from 7 to 9. Notably, when λex = 550 nm, the fluorescence intensity at 622 nm also decreased as the pH increased, while a new emission peak at 743 nm gradually emerged (Fig. 2a). The pH values in the range of 6.0-7.5 and the ratios of fluorescence intensities (I622/I743) showed a strong linear relationship (Fig. 2b). This suggested that BFCUR could be applied as a ratiometric fluorescence sensor for detecting changes of pH. The large pKa of 6.75 indicated its capability of BFCUR to test weak acid.
|
Download:
|
| Fig. 2. (a) Fluorescence titration spectra of BFCUR (10 µmol/L) at different pH values (4.5-9.0) in the B-R buffer/EtOH (1/1, v/v) solution (λex = 550 nm). (b) The plot of the fluorescence intensity ratios of BFCUR at I622 /I743 vs. pH values. | |
Since both CUR and BFCUR could respond to pH in real time, we hypothesized that the underlying response mechanism was related to the increased electron donating ability of BFCUR after deprotonation under basic condition. Deprotonation of the phenolic hydroxyl groups enhanced the intramolecular charge transfer (ICT), leading to a red shift of UV-vis and fluorescence spectra. In order to confirm this, another two derivatives MBFCUR and MBFCURM were further synthesized by singly or doubly methylating the phenolic hydroxy groups on BFCUR, respectively. By diminishing the extent of deprotonation, a change in response to pH was expected. UV-vis and fluorescence spectra obtained from MBFCUR showed less red-shift in response to an increasing pH compared to BFCUR, while MBFCURM showed only negligible response to an increased pH (Fig. S13 in Supporting information). These data proved the rationality of the hypothesized response mechanism.
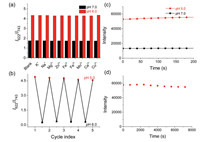
The selectivity of BFCUR was evaluated via measuring the fluorescence spectra of BFCUR in the presence of relevant metal ions, such as K+, Na+, Mg2+, Zn2+, at physiological concentrations. None of the tested metal ions had any effect on the fluorescent properties of BFCUR at pH 6.0 or 7.0 (Fig. 3a). The reversibility of BFCUR was examined by cycling the pH between 5.0 and 8.0 in B-R buffer, where only negligible changes in fluorescent properties were observed after four cycles (Fig. 3b). The time-dependent fluorescence of BFCUR at pH 5.0 and 7.0 was investigated by monitoring the fluorescence intensity at 622 nm. After BFCUR was added to B-R buffer solutions at pH 5.0 or 7.0, the fluorescence intensity at 622 nm rapidly reached a stable state, which indicated a real-time response to pH change (Fig. 3c). After continuous light irradiation (λex = 550 nm) for 2 h, the fluorescence intensity of BFCUR at 622 nm showed only a slight decrease, indicating excellent photo-stability (Fig. 3d).
|
Download:
|
| Fig. 3. (a) The fluorescence intensity ratio of BFCUR (10 µmol/L) at I622/I743 containing different metal cations in the solution (B-R buffer/EtOH: 1/1, v/v) at pH 6.0 and 7.0 (λex = 550 nm). The ions are KCl (150 mmol/L), NaCl (15 mmol/L), MgCl2 (2 mmol/L), ZnCl2 (2 mmol/L), FeCl2 (50 µmol/L), FeCl3 (50 µmol/L), MnCl2 (50 µmol/L), CaCl2 (2 mmol/L) and CuCl2 (50 µmol/L) at intracellular physiological concentrations. (b) Fluorescence reversibility of BFCUR (10 µmol/L) in the solution (B-R buffer/EtOH: 1/1, v/v) between pH 5.0 and 8.0. (c) Time courses of the fluorescence intensity at 622 nm (λex = 550 nm) of BFCUR (10 µmol/L) in the solution (B-R buffer/EtOH: 1/1, v/v) at pH 5.0 and 7.0. (d) The fluorescence intensity at 622 nm (λex = 550 nm) of BFCUR in the continuous excitation light irradiation for 120 min. | |
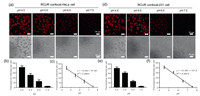
Inspired by the excellent pH-dependent fluorescence properties of BFCUR, we wondered if it can be applied in vivo as a real-time pH probe. The cytotoxicity of BFCUR was first assessed against HeLa cells and MDA-MB-231 cells, where cell viabilities exceeded 75% in groups treated with up to 5 µmol/L BFCUR for 24 h (Fig. S14 in Supporting information). Confocal micrographs showed that the red fluorescence intensity increased as the BFCUR concentration increased, suggesting excellent cell permeability (Fig. S15 in Supporting information). Evaluation of the intracellular pH reporting capabilities was performed using an intracellular pH calibration buffer kit [27]. A clear decrease in fluorescence intensity was observed as the pH increased from 4.5 to 7.5, where the average fluorescence intensity per cell was linearly related to intracellular pH values (Fig. 4). Moreover, the same response was observed in both cell types, indicating the good stability and universality of BFCUR in reporting intracellular pH.
|
Download:
|
| Fig. 4. Images of (a) HeLa cells and (d) MDA-MB-231cells with BFCUR incubated at pH 4.5, 5.5, 6.6, 7.5. (b, c, e, f) The fluorescence intensity quantitation was analyzed by the Image J. | |
In this work, three CUR derivatives were designed with different numbers of phenolic hydroxyl groups, and the role of phenolic hydroxyl in pH sensing was explored. BFCUR displayed pH reporting capabilities through ratiometric analysis of absorption spectra between pH 5.0 and 8.0 and fluorescence emission spectra between pH 6.0 and 7.5 with a large pKa of 6.75; and through colorimetric analysis between pH 5.0 and 8.0. Qualitative pH test papers prepared by BFCUR displayed significantly different colors depending on the solution pH. Moreover, BFCUR was also used to report the intracellular pH of different cell lines with a high accuracy. Owing to the high sensitivity, excellent stability and reversibility, and crystal-clear color changes observed by eye in response to subtle changes in pH, BFCUR shows great promise as a powerful and universal tool for the rapid and accurate reporting of pH.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (Nos. 51922111, 21774054, 21574061), the Science and Technology Development Fund, Macau SAR (No. 083/2017/A2), Guangdong-Hong Kong-Macao Joint Laboratory of Optoelectronic and Magnetic Functional Materials (No. 2019B121205002), and funded by Shenzhen Fundamental Research Programs (No. JCYJ20170412152922553).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.076.
| [1] |
S. Dong, M. Luo, G. Peng, W. Cheng, Sens. Actuator. B 129 (2008) 94-98. DOI:10.1016/j.snb.2007.07.078 |
| [2] |
T.A. Davies, R.E. Fine, R.J. Johnson, et al., Biochem. Biophys. Res. Commun. 194 (1993) 537-543. DOI:10.1006/bbrc.1993.1853 |
| [3] |
M. Schindler, S. Grabski, E. Hoff, S.M. Simon, Biochemistry 35 (1996) 2811-2817. DOI:10.1021/bi952234e |
| [4] |
S. Sinharay, M.D. Pagel, Annu. Rev. Anal. Chem. 9 (2016) 95-115. DOI:10.1146/annurev-anchem-071015-041514 |
| [5] |
J. Han, K. Burgess, Chem. Rev. 110 (2010) 2709-2728. DOI:10.1021/cr900249z |
| [6] |
S. Takahashi, Y. Kagami, K. Hanaoka, et al., J. Am. Chem. Soc. 140 (2018) 5925-5933. DOI:10.1021/jacs.8b00277 |
| [7] |
X. Liu, Y. Su, H. Tian, et al., Anal. Chem. 89 (2017) 7038-7045. DOI:10.1021/acs.analchem.7b00754 |
| [8] |
F. Su, S. Agarwal, T. Pan, et al., ACS Appl. Mater. Interfaces 10 (2018) 1556-1565. DOI:10.1021/acsami.7b15796 |
| [9] |
D. Iacopini, A. Moscardini, F. Lessi, et al., Bioorg. Chem. 105 (2020) 104372. DOI:10.1016/j.bioorg.2020.104372 |
| [10] |
M.J. Marin, F. Galindo, P. Thomas, D.A. Russell, Angew. Chem. Int. Ed. 51 (2012) 9657-9661. DOI:10.1002/anie.201203866 |
| [11] |
C.B. He, K.D. Lu, W.B. Lin, J. Am. Chem. Soc. 136 (2014) 12253-12256. DOI:10.1021/ja507333c |
| [12] |
J. Joniak, H. Stankovicova, J. Filo, et al., Sens. Actuator. B 307 (2020) 127646. DOI:10.1016/j.snb.2019.127646 |
| [13] |
X. Zhao, C. Wang, G. Yuan, et al., Sens. Actuator. B 290 (2019) 79-86. DOI:10.1016/j.snb.2019.03.122 |
| [14] |
A.P. de Silva, H.Q. Gunaratne, T. Gunnlaugsson, et al., Chem. Rev. 97 (1997) 1515-1566. DOI:10.1021/cr960386p |
| [15] |
F. Galindo, M.I. Burguete, L. Vigara, et al., Angew. Chem. Int. Ed. 44 (2005) 6504-6508. DOI:10.1002/anie.200501920 |
| [16] |
M. Lee, N.G. Gubernator, D. Sulzer, D. Sames, J. Am. Chem. Soc. 132 (2010) 8828-8830. DOI:10.1021/ja101740k |
| [17] |
Y. Yan, X. Zhang, X. Zhang, et al., Chin. Chem. Lett. 31 (2020) 1091-1094. DOI:10.1016/j.cclet.2019.10.025 |
| [18] |
X. Liu, Q. Yang, W. Chen, et al., Org. Biomol. Chem. 13 (2015) 8663-8668. DOI:10.1039/C5OB00765H |
| [19] |
M. Wang, G. Meng, Q. Huang, et al., Chem. Commun. (Camb.) 47 (2011) 3808-3810. DOI:10.1039/c0cc05371f |
| [20] |
X. Feng, T. Zhang, J.T. Liu, J.Y. Miao, B.X. Zhao, Chem. Commun. (Camb.) 52 (2016) 3131-3134. DOI:10.1039/C5CC09267A |
| [21] |
L. Yuan, W. Lin, K. Zheng, L. He, W. Huang, Chem. Soc. Rev. 42 (2013) 622-661. DOI:10.1039/C2CS35313J |
| [22] |
D.S. Koktysh, Mater. Res. Bull. 123 (2020) 110686. DOI:10.1016/j.materresbull.2019.110686 |
| [23] |
Z.P. She, Y. Tian, Y.S. Xia, et al., Dyes Pigm. 179 (2020) 108402. DOI:10.1016/j.dyepig.2020.108402 |
| [24] |
W. Shen, L. Wang, S. Zhu, et al., Anal. Biochem. 596 (2020) 113609. DOI:10.1016/j.ab.2020.113609 |
| [25] |
H.X. Li, H. Dong, M.M. Yu, et al., Anal. Chem. 89 (2017) 8863-8869. DOI:10.1021/acs.analchem.7b01324 |
| [26] |
J.F. Yang, M. Li, W.H. Zhu, Res. Chem. Intermed. 44 (2018) 3959-3969. DOI:10.1007/s11164-018-3334-z |
| [27] |
J. Li, X.K. Li, J.B. Jia, et al., Dyes Pigm. 166 (2019) 433-442. DOI:10.1016/j.dyepig.2019.03.060 |
| [28] |
F.Y. Su, S. Agarwal, T.T. Pan, et al., ACS Appl. Mater. Interfaces 10 (2018) 1556-1565. DOI:10.1021/acsami.7b15796 |
| [29] |
X.J. Liu, Y.A. Su, H.H. Tian, et al., Anal. Chem. 89 (2017) 7038-7045. DOI:10.1021/acs.analchem.7b00754 |
| [30] |
Y. Gu, Z. Zhao, H. Su, et al., Chem. Sci. 9 (2018) 6497-6502. DOI:10.1039/C8SC01635F |
| [31] |
A. Mars, M. Hamami, L. Bechnak, D. Patra, N. Raouafi, Anal. Chim. Acta 1036 (2018) 141-146. DOI:10.1016/j.aca.2018.06.075 |
| [32] |
W. Qin, P.F. Zhang, H. Li, et al., Chem. Sci. 9 (2018) 2705-2710. DOI:10.1039/C7SC04820C |
| [33] |
F. Zsila, Z. Bikadi, M. Simonyi, Org. Biomol. Chem. 2 (2004) 2902-2910. DOI:10.1039/B409724F |
| [34] |
M.T. Huang, Y.R. Lou, W. Ma, et al., Cancer Res. 54 (1994) 5841-5847. |
| [35] |
H. Hatcher, R. Planalp, J. Cho, F.M. Tortia, S.V. Torti, Cell. Mol. Life Sci. 65 (2008) 1631-1652. DOI:10.1007/s00018-008-7452-4 |
| [36] |
M. Pavai, J. Mihaly, A. Paszternak, Food Anal. Meth. 8 (2015) 2243-2249. DOI:10.1007/s12161-015-0102-1 |
| [37] |
B. Kuswandi, T.S.Larasati Jayus, A. Abdullah, L.Y. Heng, Food Anal. Meth. 5 (2012) 881-889. DOI:10.1007/s12161-011-9326-x |
| [38] |
C.Z. Ran, X.Y. Xu, S.B. Raymond, et al., J. Am. Chem. Soc. 131 (2009) 15257-15261. DOI:10.1021/ja9047043 |
| [39] |
X.L. Zhang, Y.L. Tian, Z. Li, et al., J. Am. Chem. Soc. 135 (2013) 16397-16409. DOI:10.1021/ja405239v |
| [40] |
A. Chaicham, S. Kulchat, G. Tumcharern, T. Tuntulani, B. Tomapatanaget, Tetrahedron 66 (2010) 6217-6223. DOI:10.1016/j.tet.2010.05.088 |
| [41] |
D. Xiang, Q. Meng, H. Liu, M. Lan, G. Wei, Talanta 146 (2016) 851-856. DOI:10.1016/j.talanta.2015.05.066 |
| [42] |
L. Zhou, L. Xie, C. Liu, Y. Xiao, Chin. Chem. Lett. 30 (2019) 1799-1808. DOI:10.1016/j.cclet.2019.07.051 |
 2022, Vol. 33
2022, Vol. 33 

