b School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China
Lung fibrosis is an important cause of lung injury aggravation. Abnormal proliferation of fibroblasts after lung injury is a significant period in pulmonary fibrosis. While fibroblasts are pivotal in wound healing after injury as well as in connective tissue production under normal circumstance [1]. Patients with pulmonary fibrosis often have a poor prognosis, and drugs that inhibit the pathogenetic pathway of pulmonary fibrosis are being sought to reduce or delay the progression of the disease [2,3].
Fortunately, plumbagin (PLB) has the potential to be a treatment for pulmonary fibrosis, which can inhibit the epithelial-mesenchymal transition of breast cancer cells [4,5]. Moreover, PLB plays a significant role in altering the expression of four EMT markers (E-cadherin, N-cadherin, β-catenin and vimentin) of human tongue squamous cells, which can hinder the occurrence of EMT and thus effectively inhibit lung injury [6-8]. However, PLB has low water solubility, short median elimination half-life (9.6 h) and quick average residence time (5.0 h) [9]. Recently, unremitting attempts have been dedicated to improve plumbagin's half-life and bioavailability through nanocarrier mediated delivery such as protein microspheres, and nano-silver material [10,11]. Thus, it is imperative to seek novel materials with impressive properties to enhance the drug release performances.
As three-dimensional porous materials, metal organic frameworks (MOFs) feature high porosity, large surface areas, adjustable functionality, and ordered pore structures [12-14]. Benefited from its unique properties, MOFs hold great potential in vast applications including storage and separation, catalysis, and sensors [13-17]. Zeolitic imidazolate framework-8 (ZIF-8) is one of the most prospective representatives of MOFs built from zinc ions and 2-methylimidazolate [18,19]. ZIF materials have also been applied in encapsulation of drug molecules, enzymes, and even noble metal nanoparitcles with new functions and biological application [20-23]. Nevertheless, there are no reports on ZIF-8 encapsulated PLB nanocomposites as a drug delivery system for the treatment of acute lung injury up to now.
Here, we have developed a simple process for the encaplsulation of anticancer drug PLB in the ZIF-8 nanoparticles. Then the obtained PLB@ZIF-8 nanoparticles were used as an efficient drug delivery system for acute lung injury therapy using a mouse model constructed by lipopolysaccharide (LPS). Intervention treatment, immunohistochemistry and ELISA experiments showed that PLB@ZIF-8 nanoparticles can detect the expression of inflammatory factors and collagen. This study offered a new path to develop functional materials for applications in effectively lung injury.
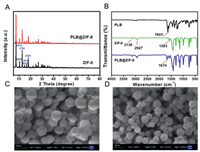
According to a typical preparation method reported in our previous study [24], ZIF-8 nanoparticles were synthesized via a simple solvothermal method. Subsequently, the as-synthesized ZIF-8 nanoparticles were simply mixed with PLB to prepare PLB@ZIF-8 via adsorption with ethanol at room temperature for 9 h. XRD revealed ordered crystal structure of ZIF-8 and PLB@ZIF-8 nanoparticles. As shown in Fig. 1A, ZIF-8 exhibited six typical peaks between 5° and 60°, including (011), (002), (112), (022), (013) and (222), which indicates successful formation of highly crystalline ZIF-8 nanoparticles [25]. With the adsorption of PLB, the XRD pattern of PLB@ZIF-8 nanoparticles was quite similar with original peak for ZIF-8, suggesting ZIF-8 maintains its crystallinity. In FTIR spectrum of PLB (Fig. 1B), a characteristic peak at about 1645 cm−1 corresponded to the amide C = O stretching [26]. In the curve of ZIF-8, two peaks at 3136 cm−1 and 2927 cm−1 were associated with stretching vibration of the C-H for imidazole. The band at 1583 cm−1 is related to the C = N stretch mode. In the regions of 500–1350 cm−1 and 1350–1500 cm−1, a convolutional spectrum was caused by the plane bending and stretching of the imidazole ring [27]. Compared with the spectrum of PLB, the amide C = O stretching of PLB in the PLB@ZIF-8 was shifted from 1645 cm−1 to 1674 cm−1, while the spectra of PLB@ZIF-8 showed main characteristic peaks of ZIF-8, indicating the success of the preparation of PLB@ZIF-8. Smaller crystal sizes at the nanoscale may offer broad prospects for the development of biological fields. The SEM images (Figs. 1C and D) showed the hexagonal shape of both ZIF-8 and PLB/ZIF-8 crystal in an average of ~150 nm.
|
Download:
|
| Fig. 1. (A) XRD crystallinity patterns of pure ZIF-8 and PLB@ZIF-8. (B) FTIR spectra of PLB, ZIF-8 and PLB@ZIF-8. SEM micrographs of (C) pure ZIF-8 and (D) PLB@ZIF-8 nanoparticles. Scale bars: 200 nm. | |
UV–vis absorption spectrum was performed to assess the loading and controlled release behavior of the PLB@ZIF-8 nanoparticles. With PLB loading on, an additional peak appeared at 480 nm was related to the characteristic of PLB, proving the existence of PLB in the ZIF-8 (Fig. S1A in Supporting information). After the measurement of the characteristic PLB optical absorption at 480 nm, the loading rate about 12.8% was obtained. There are two reasons for the encapsulation of PLB into ZIF-8 nanoparticles: (1) ZIF-8 nanoparticles with high specific surface area can effectively improve the adsorption capacity of PLB. (2) PLB can be adsorbed onto the ZIF-8 nanoparticles surface through π-π stacking interaction. The release profile of PLB from PLB@ZIF-8 was exhibited in Fig. S1B (Supporting information). Approximately 59.1% of PLB was significantly released within 1 h, followed by a more stable low-dose release observed in about a week or so, with over 84.1% PLB release.
The effect of PLB on the viability of HPMEC cells was tested by CCK8 assay (Fig. 2A), PLB@ZIF-8 can alleviate the damage of LPS to cells and maintain cell viability. At the same time, the culture supernatant ELISA test also found that PLB@ZIF-8 can better reduce the secretion of inflammatory factor TGF-β and IL-6 (Figs. 2B and C).

|
Download:
|
| Fig. 2. (A) In vitro cell viabilities of HPMEC cells measured by CCK8 assay. (B, C) The levels of inflammation cytokines (TGF-β and IL-6) tested by ELISA, respectively (*P < 0.05, **P < 0.01, ***P < 0.001, and ns is not significant). | |
A lung injury model (Fig. 3A) was constructed through LPS, and treated with PLB@ZIF-8 to prevent the development of lung injury. C57 animals at the age of 6 weeks were placed in a continuous temperature and humidity environment, with 12 h of brightness/darkness a day and free to drink and eat. The mice were randomly divided into 5 groups (10 mice/group), including control group, LPS group, LPS+ZIF-8 group, LPS+PLB and the LPS+PLB@ZIF-8 group. LPS was intraperitoneally injected at 20 mg/kg, while the control group was added with the same amount of normal saline; 0.5 h after LPS treatment, 1.5 mg/100 g of pentobarbital was used to anesthetize the mice. Then they were administered via tracheal nebulization with the following doses: 2 mg/kg PLB in the LPS+PLB group, 12.8 mg/kg PLB@ZIF-8 in the LPS+PLB@ZIF-8 group; 12.8 mg/kg in the ZIF-8 group mg/kg ZIF-8, the same volume of normal saline were added into the control group and LPS group. After 24 h, the mice were sacrificed and their lung tissues were collected. The HE and Masson staining showed that PLB combined with ZIF-8 could delay pneumonia response and decrease the generation of collagen fibers and thus control lung injury in comparison with the control group (Fig. 3B).

|
Download:
|
| Fig. 3. (A) Schematic diagram of animal model construction. (B) Animal tissue H&E and Masson staining. Scale bars: 50 µm. | |
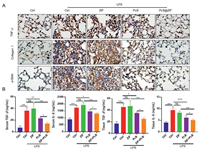
The expression of collagen I, TNF-α and α-SMA was detected by immunohistochemistry (Fig. 4A). First, 4% paraformaldehyde was used to fix the tissue, followed by embedding in paraffin and sectioning. Sections were treated with hematoxylin and eosin (H&E), TNF-α (1:400; Proteintech, China) according to standard procedures. We performed Masson staining to test the degree of pulmonary fibrosis on the basis of the manufacturer's instructions. The results indicated that the pure PLB and PLB@ZIF-8 groups can effectively reduce the expression of collagen I, α-SMA and TNF-α caused by LPS compared with the control group. PLB@ZIF-8 group can better reduce the secretion of inflammatory factor TGF-β and IL-6 both in serum and tissue (Fig. 4B). Taken together, ZIF-8 encapsulated with PLB can efficiently prevent the progression of lung injury.
|
Download:
|
| Fig. 4. (A) The TNF-α, collagen I and α-SMA immunohistochemistry detection. Scale bars: 20 µm. (B) The ELISA assay for serum and tissue IL-6 and TGF-β (*P < 0.05, **P < 0.01, ***P < 0.001, and ns is not significant). | |
In summary, ZIF-8 nanoparticles with a uniform particle size were successfully synthesized and applied in PLB drug delivery. The data obtained in the present work suggested that ZIF-8 encapsulation of PLB enhance the anti-inflammatory efficacy of PLB. Animal models experiments improved that PLB@ZIF-8 can be used as potent therapeutic agent, which can better control the development of inflammation and collagen expression than free PLB. Therefore, ZIF-8 embedded PLB may become a new remedy for severe lung injury.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Open Project of the State Key Laboratory of Trauma, Burn and Combined Injury, Third Military Medical University (No. SKLKF201704), and Postdoctoral Science Foundation of China (No. 2018M633759). The animal experiments have been approved by the Ethics Committee of the Third Military Medical University.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.080.
| [1] |
R. Malaviya, V.R. Sunil, J. Cervelli, et al., Toxicol. Appl. Pharmacol. 248 (2010) 89-99. DOI:10.1016/j.taap.2010.07.018 |
| [2] |
M.A. Matthay, R.L. Zemans, Annu. Rev. Pathol. 6 (2011) 147-163. DOI:10.1146/annurev-pathol-011110-130158 |
| [3] |
R. Marshall, G. Bellingan, G. Laurent, Thorax. 53 (1998) 815-817. DOI:10.1136/thx.53.10.815 |
| [4] |
P. Premakumari, K. Rathinam, G. Santhakumari, Indian J. Med. Res. 65 (1977) 829-838. |
| [5] |
I. Sharma, D. Gussain, V.P. Dixit, Indian J. Physiol. Pharmacol. 35 (1991) 10-14. |
| [6] |
A.A. Powolny, S.V. Singh, Pharma. Res. 25 (2008) 2171-2180. DOI:10.1007/s11095-008-9533-3 |
| [7] |
R. Gomathinayagam, S. Sowmyalakshmi, F. Mardhatillah, et al., Anticancer Res. 28 (2008) 785-792. |
| [8] |
J.P. Sun, R.J. McKallip, Leukemia Res. 35 (2011) 1402-1408. DOI:10.1016/j.leukres.2011.06.018 |
| [9] |
V.R. Sunil, K.N. Vayas, E.V. Abramova, et al., Toxicol. Appl. Pharmacol. 387 (2020) 114798. DOI:10.1016/j.taap.2019.114798 |
| [10] |
J.A. Kropski, W.E. Lawson, L.R. Young, T.S. Blackwell, Dis. Model. Mech. 6 (2013) 9-17. DOI:10.1242/dmm.010736 |
| [11] |
N. Sakunrangsit, K. Wannarasmi, Pharmacol Res. 150 (2019) 104517. DOI:10.1016/j.phrs.2019.104517 |
| [12] |
S. Zhang, X.B. Pei, H.L. Gao, S. Chen, J. Wang, Chin. Chem. Lett. 31 (2020) 1060-1070. DOI:10.1016/j.cclet.2019.11.036 |
| [13] |
Y. Du, X. Gao, S.W. Li, L. Wang, B. Wang, Chin. Chem. Lett. 31 (2020) 609-616. DOI:10.1016/j.cclet.2019.06.013 |
| [14] |
S.F. Zhao, L.Z. Zeng, G. Cheng, L. Yu, H.Q. Zeng, Chin. Chem. Lett. 30 (2019) 605-609. DOI:10.1016/j.cclet.2018.10.018 |
| [15] |
M.X. Zhang, W. Zhou, T. Pham, et al., Angew. Chem. Int. Ed. 56 (2017) 11426-11430. DOI:10.1002/anie.201704974 |
| [16] |
Z.Y. Dong, Y.Z.S. Sun, J. Chu, X.Z. Zhang, H.X. Deng, J. Am. Chem. Soc. 139 (2017) 14209-14216. DOI:10.1021/jacs.7b07392 |
| [17] |
X. Lin, E.L. Ning, X.M. Li, Q.W. Li, Chin. Chem. Lett. 31 (2020) 813-817. DOI:10.1016/j.cclet.2019.05.055 |
| [18] |
A. Schejn, L. Balan, V. Falk, et al., CrystEngComm 16 (2014) 4493-4500. DOI:10.1039/C3CE42485E |
| [19] |
C. Yang, J. Xu, D.D. Yang, et al., Chin. Chem. Lett. 29 (2018) 1421-1424. DOI:10.1016/j.cclet.2018.02.014 |
| [20] |
P.Z. Li, X.J. Wang, J. Liu, et al., J. Am. Chem. Soc. 138 (2016) 2142-2145. DOI:10.1021/jacs.5b13335 |
| [21] |
B. Li, J.G. Ma, P. Cheng, Angew. Chem. Int. Ed. 57 (2018) 6834-6837. DOI:10.1002/anie.201801588 |
| [22] |
B.L. Xu, H. Wang, W.W. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 4911-4916. DOI:10.1002/anie.201813994 |
| [23] |
Y.Y. Zheng, C.X. Li, X.T. Ding, et al., Chin. Chem. Lett. 28 (2017) 1473-1478. DOI:10.1016/j.cclet.2017.03.014 |
| [24] |
Y.L. Chen, W. Huang, K.J. Chen, et al., Sens. Actuators B: Chem. 290 (2019) 434-442. DOI:10.1016/j.snb.2019.04.006 |
| [25] |
A. Schenjn, L. Balan, V. Falk, et al., CrystEngComm 16 (2014) 4493-4500. DOI:10.1039/C3CE42485E |
| [26] |
N. Duraipandy, R. Lakra, S.K. Vinjimur, et al., Metallomics 6 (2014) 2025-2033. DOI:10.1039/C4MT00165F |
| [27] |
Y. Hu, H. Kazemian, S. Rohani, Y.N. Huang, Y. Song, Chem. Commun. 47 (2011) 12694-12696. DOI:10.1039/c1cc15525c |
 2022, Vol. 33
2022, Vol. 33 

