The sulfonium ylides have been widely recognized as important synthetic precursors in a plethora of organic transformations [1-9]. Sulfonium ylides can be considered as positive charged sulfur atom stabilized by an adjacent carbanion. The substituents, attached to the sulfonium center with non-ylidic carbon-sulfur bond, not only stabilized the ylides but also governed the reactivity of ylides. While activation of ylidic C-S bonds was well-established, efforts towards intermolecular cleave of non-ylidic C-S bond was far less documented [10]. In 1973, Fujisawa reported an intriguing acidic methyl esterification enabled by non-ylidic S-methyl bond cleavage of sulfonium ylide 1 (Scheme 1a) [11]. A year later, Oae et al. demonstrated that reaction of sulfonium ylides 2a/b with potassium hydroxide in refluxing methanol generated a dimethyl ether or benzyl methyl ether [12]. Unfortunately, there has no progress towards these intriguing discoveries since then.

|
Download:
|
| Scheme 1. Non-ylidic bond cleavage of sulfonium ylides. | |
Until recently, the concept of non-ylidic C-S bond cleavage has been further enriched by Maulide et al. [13], Liu et al. [14] and Shen [15-20] respectively. Our group also reported a mild glycosylation method through the selective cleavage of the S-glycosyl bond of in situ generated glycosyl sulfonium ylide 3 (Scheme 1a) [21]. It is noteworthy that when the sulfur atom is directly attached to alkyl group and aryl group, all the cleavages occurred at non-ylidic S-alkyl bond, that of non-ylidic S-aryl bond was barely touched. In 2013, Maulide et al. reported an unprecedented Pd(Ⅱ)-catalyzed dearylation of diphenyl sulfonium ylide 4, which is the only example for activation of non-ylidic S-aryl bond so far (Scheme 1b) [13]. Inspired by the previous approaches, we envisioned that if non-ylidic C-S bonds could be differentiated and activated, a new chemistry might be developed. More importantly, this chemistry would offer a powerful tool for functionalization of biological important molecules such as carbohydrates and pharmaceuticals. Herein, we reported the development of efficient alkylation and arylation reactions enabled by discriminating non-ylidic C-S bond cleavages (Scheme 1c).
Given the stability of benzyl ethers, benzylation of alcohols becomes one of the most common used protection methods for hydroxy groups [25-26], but mild conditions for benzylation of base-labile substrates are highly desired [27-35]. Drawing inspiration from our recent Rh(Ⅱ)/H+-catalyzed glycosylation method hinged on protonation of glycosyl sulfonium ylide (3) to facilitate transient glycosyl sulfonium species [21-24], we commenced to examine the benzylation reaction in acidic conditions (Table 1). As depicted in Table 1, sulfonium ylides (2f-i) produced the desired benzylated sugar 6a from 5a in good yields in the presence of 1.2 equiv. of DTBMP·TfOH. Among them, sulfonium ylides possessing electron deficient phenyl groups gave forth better results (entries 1–4). Specifically, the nitro group on the phenyl ring greatly enhanced the activity of 2i to accelerate the generation of 6a in 91% yield within 10 min (entry 4). Interestingly, upon using 2.0 equiv. of 2i (entry 5), 0.25 equiv. of DTBMP·TfOH was sufficient to uphold the high reaction efficiency, albeit the attendant much longer reaction time (24 h). We reckoned such mildly acidic conditions should augur well for extremely acid-labile substrates. Aside from weakly acidic DTBMP·TfOH salt, TfOH itself and Lewis acid LiBF4 promoted the reaction too, but with lower product yields (entries 6 and 8). Meanwhile, camphorsulfonic acid (CSA) was found inept for this benzylation reaction (entry 7). Worth-mentioningly, ylide 2i could be scaled up to ten grams in 92% yield after recrystallization, and it has no stability issue and decomposition was virtually unnoticeable even after 6-months storage under ambient conditions.
|
|
Table 1 Acidic benzylation reaction development. |
Alongside product 6a, it is worth noting that sulfide 7 and benzylated sulfide 8 were also isolated from this benzylation reaction (entry 4). Mechanism studies revealed that protonation of ylide occurred first to deliver sulfonium salt intermediate (I) which was stable at cryogenic condition but gradually decomposed to benzyl triflate and co-product 7 when warmed up to room temperature. These intermediates were observed by 1H NMR spectroscopy. Among these intermediates, the moderate generated BnOTf acted exactly as the active benzylation reagent. Control experiments and 1H NMR studies portrayed the equilibrium between the sulfonium salt (I) and BnOTf which engendered the mild and efficient benzylation conditions. Besides, the origin of by-product sulfide 8, which was ruled out the Stevens rearrangement pathway, surprisingly, also attributed to a nucleophilic attack of negative charged carbon atom on ylide onto in situ formed BnOTf (details see Supporting information).
Benzylation of primary, secondary and tertiary alcohols with adequate sulfonium ylide 2i/2j, provided the benzyl ethers in good to excellent yields under the optimized conditions (Scheme 2). A wide variety of functional groups including alkali-labile functional groups such as acetate (6a, 6e, 6m), chloroacetyl acetate (6zi), anomeric acetate (6g, 6i, 6k), Fmoc group (6q), ketone (6y), lactone (6u), as well as acid-labile moiety t-butyldimethylsilyl group (6m), 2-deoxy sugar (6k) and Boc group (6s) were successfully endured under this mild condition. Other than alcohols, benzylation of acids possessing ketone (9a), alkenyl (9c), phenol (9e) and silyl (9f) groups and even phosphoric ester (14a) gave forth the reciprocal esters.

|
Download:
|
| Scheme 2. Substrate scope for alkylations. Alkylation with stable sulfonium ylides: 2i/2j (2.0 equiv.), DTBMP·TfOH (0.25 equiv.) or 2k/2m (2.0 equiv.), DTBMP (1.0 equiv.), TfOH (1.0 equiv.). Alkylation with in situ formed ylides: 2o/2p/2q/2r (in situ generated from corresponding sulfide, methyl 4-chlorophenyl-diazoacetate and 0.5 mol% of Rh2(oct)4), DTBMP·TfOH (0.25 equiv.), 30 or 0 ℃. c2i (1.2 equiv.), DTBMP·TfOH (1.2 equiv.); d2i (4.0 equiv.), TfOH (0.5 equiv.); eamine (5.0 equiv.), 2i (1.0 equiv.), TfOH (0.25 equiv.); f2i (2.0 equiv.), TfOH (0.25 equiv.); gsubstrate (2.0 equiv.), 2i/2l (1.0 equiv.), TfOH (1.0 equiv.). h2i (1.2 equiv.), TfOH (1.2 equiv.). | |
Amines are key building blocks of quite a few natural products with powerful biological activities. They are also highly ubiquitous in the comprehensive medicinal chemistry database [36-39]. In view of the prevalence of N-alkylation in pharmaceutical chemistry, various amines were examined and all gave at least 80% yields of corresponding products (11a–11f). Mono-/di-benzylation of primary amine (11a, 11b) and aniline (11h, 11i) could be auspiciously controlled by the amount of ylide used. An antihistamine drug, meclizine (11g) and clozapine derivative (11j) were also smoothly obtained by simple 3-methylbenzylation and benzylation. Interestingly, the benzylation reaction with benzamide and carbamazepine took place selectively on the oxygen rather than nitrogen atom to yield benzyl imidate (12, 13) in good yields. In addition to C-O and C-N bonds, S-benzylation of 2-mercaptobenzothiazole (10) and C-benzylation (15a) with electron-rich pentamethyl benzene were also accomplished efficiently.
Other sulfonium ylide derivatives, 2k–2n were also successfully synthesized following the standard protocol. Ylides 2k–2m were employed as 2-cyano-benzylation (6c), 3-methylbenzylation (11g) and isopropylation (6o, 6zh) reagents under acidic conditions. However, it was worth mentioning that methyl-substituted sulfonium ylide 2n could not be used as methylation reagent, whatever acid we employed. During the synthesis of novel ylidic reagents, it was found that Stevens rearrangement reaction occurred extremely fast for electron-richer motifs, such as p-methoxybenzyl, 4-((trimethylsilyl)methyl)benzyl [40], 2-tetrahydrofuranyl [41] and 2-tetrahydropyranyl [41] substituted ylides. This instability obliged us to introduce the protocols by in situ generation of these ylides (2o–2r) from corresponding sulfides and methyl 4-chlorophenyldiazoacetates. Sugar derivatives with silyl, alkynyl, and acetate including anomeric acetate groups demonstrated exquisite compatibility to deliver p-methoxybenzylated (6b, 6f, 6h, 6j, 6l, 6n), 2-tetrahydropyranylated (6x) and 4-((trimethylsilyl)methyl)benzylated (6d) products. Of note, the synthetically significant alkynyl benzoate glycoside 6p [42,43] manifested suitable for this transformation. A variety of alcohols even bearing Fmoc and Boc groups (6r, 6t, 6zb) proceeded well to furnish desired products, with no detection of loss of enantiomeric purity. Aryl, alkynyl (9g) and phosphoric acids (14b) furnished the corresponding p-methoxybenzyl esters in good yields (see Supporting information). Treatment of pentamethyl benzene with in situ generated ylide 2o afforded 15b efficiently. Biologically active substances such as steroid drugs (6z, 6za), podophyllotoxins (6zd, 6ze), and l-tyrosine derivatives (6zj, 6zk) were good substrates for p-methoxybenzylation, 2-tetrahydrofuranylation and 2-tetrahydropyranylation, respectively.
Non-ylidic S-alkyl bonds of ylide 2i–2m could be selective cleaved under acidic condition, and the corresponding non-ylidic S-aryl bonds were completely unperturbed. Then, we envisioned that the activation of non-ylidic S-aryl bonds of sulfonium ylides 2 would provide a complemental approach to the highly demanded arylations [44-53]. Inspired by Olofsson's arylation with electrophilic diaryiodonium salt [54], the arylation with sulfonium ylides under alkaline conditions were investigated (Table 2).
|
|
Table 2 Optimization of ylide structures for arylation. |
To probe the feasibility of arylation with sulfonium ylide, an extensive survey of structural modification on both alkyl and aryl side of the sulfonium ylides were then implemented. Encouragingly, reaction between alcohol 5b and benzyl substituted sulfonium ylide 2j in the presence of t-BuONa (1.5 equiv.) afforded p-nitrophenylated product 16a, albeit in 36% yield, meanwhile the benzylated product was not observed (Table 2, entry 1). 3-Pentyl (2s) and isopropyl (2m) substituted ylides gave improved yields of arylated product (entries 2 and 4). Eventually, it was found that both t-BuONa and NaH promoted the p-nitrophenylation reaction with methyl substituted ylide 2n to give forth nearly quantitative yields (entry 3). Occasionally, Shen's flouromethylation reagents (2d, 2e) were subjected to the newly developed arylation conditions. Surprisingly, the monofluoromethyl substituted sulfonium ylide 2e produced arylated product (16a) in 98% yield while the expected monofluoromethylation was not occurred. It seems that the substituents of the ylide 2 have great impacts on the reactivity, it is extremely hard to predict the reaction conditions and results. Considering the cost of starting material and the selectivity of reaction, sulfonium ylides with simple methyl group was then selected as arylation reagent for further investigation. Other than nitro group, it was found that arylsulfonium ylides possessing other electron-deficient substituents, such as cyano (2t) [55] or trifluoromethyl (2u) group, also showed superior reactivity, in contrast to the sulfonium ylides possessing electron-rich aryl moieties (entries 7–12). Especially, p-bromophenyl, phenyl and p-methoxyphenyl substituted sulfonium ylide 2w–2y gave forth no arylated products, however, with inadequate methylation products isolated.
Having established that ylides 2n, 2t and 2u were good arylating reagents, we next studied the arylation with a variety of nucleophiles. As depicted in Scheme 3, all of substrates including alcohols (16e, 16f), naphthalenes (16r) and aromatic heterocycles (16g, 16i) provided corresponding mono-/di-arylated products with excellent yields. Noticeably, selectively masking phenolic hydroxyl group in the presence of tertiary hydroxyl group (16s) proceeded well, which was greatly desirable in total synthesis of complex compounds. Introduction of aryl groups to sugar backbones is valuable for improving pharmaceutical properties of the parent molecules [48-52]. Exhilaratingly, carbohydrates with primary and steric hindered secondary hydroxyl groups were p-nitrophenylated perfectly under this condition (16l–16q). Functional groups such as acetonide (16p), 4-methoxybenzylidene (16m) and azide group (16n) were proved to be compatible. Particularly, anomeric O-arylation of carbohydrate derivatives (16p) also made prominent progress. Some glycosyl donors such as glycosyl fluoride (16l), glycal (16m) and thioglycoside (16t) were directly arylated, which could be synthetic useful for conjugation to other complex structures. Pharmaceutical ingredients (16h, 16u, 16v) [56-58] were also favorably prepared by direct p-nitrophenylation, p-cyanophenylation and p-trifluoromethylphenylation as well.
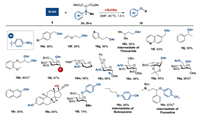
|
Download:
|
| Scheme 3. Substrate scope for arylation. Reaction conditions: 5 (1.0 equiv.), sulfonium ylide (1.2 equiv.), t-BuONa (1.5 equiv.) in DMF (0.1 mol/L) at 40 ℃ for 1.5 h. a2n (2.5 equiv.), t-BuONa (2.5 equiv.). b2u (1.5 equiv.) and t-BuONa (2.0 equiv.). | |
In summary, we described a novel non-ylidic C-S bond cleavage on sulfonium ylide to proceed selective arylation and alkylation reactions. Under weak acidic conditions, stable or in situ generated sulfonium ylides were acted as diverse alkylating agents. The smooth conversions of various nucleophiles were realized through the moderate generation of active alkyl triflates from sulfonium ylides. Under alkaline conditions, assorted O-arylations with sulfonium ylides via unprecedented non-ylidic S-aryl bond cleavages have been implemented. The chemoselective cleavages of the non-ylidic S-alkyl and aryl bonds of sulfonium ylides provided a flexible alkylation and arylation protocols, which optimistically expected to be the general reagents with broad applications.
Declaration of competing interestThe authors declare no competing financial interests.
AcknowledgmentsFinancial support from National Natural Science Foundation of China (Nos. 21877043, 21702068, 21772050, 22025102), the Fundamental Research Funds for the Central Universities, HUST (Nos. 2019kfyXKJC080, 2019JYCXJJ046) and Huazhong University of Science and Technology are greatly appreciated. We thank Mr. Xiaodi Yang from Shanghai University of Traditional Chinese Medicine for X-ray crystallographic analysis and the Analytical and Testing Center of HUST for NMR tests.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.069.
| [1] |
D. Kaiser, I. Klose, R. Oost, J. Neuhaus, N. Maulide, Chem. Rev. 119 (2019) 8701-8780. DOI:10.1021/acs.chemrev.9b00111 |
| [2] |
B.M. Trost, L.S. Melvin, Sulfur Ylides Emerging Synthetic Intermidiates, Academic Press, New York, 1975.
|
| [3] |
J.S. Clark, Nitrogen, Oxygen, and Sulfur Ylide Chemistry (Practical Approach in Chemistry Series), Oxford University Press, New York, 2002.
|
| [4] |
L.Q. Lu, T.R. Li, Q. Wang, W.J. Xiao, Chem. Soc. Rev. 46 (2017) 4135-4149. DOI:10.1039/C6CS00276E |
| [5] |
V.K. Aggarwal, C.L. Winn, Acc. Chem. Res. 37 (2004) 611-620. DOI:10.1021/ar030045f |
| [6] |
K.J. Hock, R.M. Koenigs, Angew. Chem. Int. Ed. 56 (2017) 13566-13568. DOI:10.1002/anie.201707092 |
| [7] |
Y. Zhang, J. Wang, Coord. Chem. Rev. 254 (2010) 941-953. DOI:10.1016/j.ccr.2009.12.005 |
| [8] |
J.D. Neuhaus, R. Oost, J. Merad, N. Maulide, Top. Curr. Chem. 376 (2018) 15. DOI:10.1007/s41061-018-0193-4 |
| [9] |
R. Fan, C. Tan, Y. Liu, et al., Chin. Chem. Lett. 32 (2021) 299-312. DOI:10.1016/j.cclet.2020.06.003 |
| [10] |
R. Oost, J.D. Neuhaus, J. Merad, N. Maulide, Sulfur ylides in organic synthesis and transition metal catalysis, In: V. Gessner (Ed.), Modern Ylide Chemistry: Structure and Bonding, Vol. 177, Springer, Cham, 2017, pp. 73-166. .
|
| [11] |
T. Fujisawa, T. Kojima, K. Hata, JPS4891039A, 1973. .
|
| [12] |
N. Furukawa, T. Masuda, M. Yakushiji, S. Oae, Bull. Chem. Soc. Jpn. 47 (1974) 2247. DOI:10.1246/bcsj.47.2247 |
| [13] |
X. Huang, R. Goddard, N. Maulide, Chem. Commun. 49 (2013) 4292-4294. DOI:10.1039/C2CC35762C |
| [14] |
Y.Y. Liu, X.H. Yang, X.C. Huang, et al., J. Org. Chem. 78 (2013) 10421-10426. DOI:10.1021/jo401851m |
| [15] |
Y. Liu, X. Shao, P. Zhang, L. Lu, Q. Shen, Org. Lett. 17 (2015) 2752-2755. DOI:10.1021/acs.orglett.5b01170 |
| [16] |
J. Zhu, Y. Liu, Q. Shen, Angew. Chem. Int. Ed. 55 (2016) 9050-9054. DOI:10.1002/anie.201603166 |
| [17] |
Y. Liu, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 56 (2017) 9930-9934. DOI:10.1002/anie.201704175 |
| [18] |
J. Zhu, H. Zheng, X. Xue, et al., Chin. J. Chem. 36 (2018) 1069-1074. DOI:10.1002/cjoc.201800383 |
| [19] |
Y. Liu, H. Ge, L. Lu, Q. Shen, Chin. J. Org. Chem. 39 (2019) 257-264. DOI:10.6023/cjoc201807018 |
| [20] |
X. Hong, Y. Liu, L. Lu, Q. Shen, Chin. J. Chem. 38 (2020) 1317-1331. DOI:10.1002/cjoc.202000206 |
| [21] |
L. Meng, P. Wu, J. Fang, et al., J. Am. Chem. Soc. 141 (2019) 11775-11780. DOI:10.1021/jacs.9b04619 |
| [22] |
M. Yar, E.M. McGarrigle, V.K. Aggarwal, Angew. Chem. Int. Ed. 47 (2008) 3784-3786. DOI:10.1002/anie.200800373 |
| [23] |
S.L. Riches, C. Saha, N.F. Filgueira, et al., J. Am. Chem. Soc. 132 (2010) 7626-7630. DOI:10.1021/ja910631u |
| [24] |
J. An, N.J. Chang, L.D. Song, Y.Q. Jin, et al., Chem. Commun. 47 (2011) 1869-1871. DOI:10.1039/C0CC03823G |
| [25] |
P.G.M. Wuts, Greene's Protective Groups in Organic Synthesis, 5th. Ed., John Wiley & Sons, Hoboken, 2014.
|
| [26] |
T.W. Greene, P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd. Ed., John Wiley & Sons, New York, 1999.
|
| [27] |
T. Iversen, D.R.J. Bundle, Chem. Soc. Chem. Commun. (1981) 1240-1241. |
| [28] |
K.W.C. Poon, S.E. House, G.B. Dudley, Synlett (2005) 3142-3144. |
| [29] |
T. Wang, T. Intaranukulkit, M.R. Rosana, et al., Org. Biomol. Chem. 10 (2012) 248-250. DOI:10.1039/C1OB06504A |
| [30] |
N. Asao, H. Aikawa, S. Tago, K. Umetsu, Org. Lett. 9 (2007) 4299-4302. DOI:10.1021/ol701861d |
| [31] |
K. Yamada, H. Fujita, M. Kunishima, Org. Lett. 14 (2012) 5026-5029. DOI:10.1021/ol302222p |
| [32] |
K. Yamada, Y. Tsukada, Y. Karuo, M. Kitamura, M. Kunishima, Chem. Eur. J. 20 (2014) 12274-12278. DOI:10.1002/chem.201403158 |
| [33] |
H. Fujita, S. Kakuyama, M. Kunishima, Eur. J. Org. Chem. 2017 (2017) 833-839. DOI:10.1002/ejoc.201601387 |
| [34] |
J.R. Zhao, X. Yuan, Z. Wang, et al., Org. Chem. Front. 2 (2015) 34-37. DOI:10.1039/C4QO00255E |
| [35] |
S. Chakraborty, B. Mishra, M. Neralkar, S.J. Hotha, J. Org. Chem. 84 (2019) 6604-6611. DOI:10.1021/acs.joc.9b00016 |
| [36] |
T. Henkel, R.M. Brunne, H. Müller, F. Reichel, Angew. Chem. Int. Ed. 38 (1999) 643-647. DOI:10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G |
| [37] |
T. Naito, Chem. Pharm. Bull. 56 (2008) 1367-1383. DOI:10.1248/cpb.56.1367 |
| [38] |
J.F. Hartwig, Acc. Chem. Res. 41 (2008) 1534-1544. DOI:10.1021/ar800098p |
| [39] |
R.N. Salvatore, C.H. Yoon, K.W. Jung, Tetrahedron 57 (2001) 7785-7811. DOI:10.1016/S0040-4020(01)00722-0 |
| [40] |
N. D'Alessandro, A. Albini, P.S. Mariano, J. Org. Chem. 58 (1993) 937-942. DOI:10.1021/jo00056a029 |
| [41] |
X. Si, L. Zhang, Z. Wu, et al., Org. Lett. 22 (2020) 5844-5849. DOI:10.1021/acs.orglett.0c01924 |
| [42] |
B. Yu, Acc. Chem. Res. 51 (2018) 507-516. DOI:10.1021/acs.accounts.7b00573 |
| [43] |
P. Li, H. He, L. Xu, et al., Green Synth. Catal. 1 (2020) 160-166. DOI:10.1016/j.gresc.2020.10.003 |
| [44] |
A. Zapf, M. Beller, T.H. Riermeier. Transition Metal-Catalyzed Arylation of Amines and Alcohols, in: M. Beller, C. Bolm (Eds.), Transition metals for organic synthesis-building blocks and fine chemicals (2nd ed.), Wiley, 2004, Vol. 1, pp. 231–256.
|
| [45] |
Q. Cai, W. Zhou, Chin. J. Chem. 38 (2020) 879-893. DOI:10.1002/cjoc.202000075 |
| [46] |
S.M. Roopan, J. Palaniraja, Res. Chem. Intermed. 41 (2015) 8111-8146. DOI:10.1007/s11164-014-1880-6 |
| [47] |
W. Liu, J. Li, C.Y. Huang, C.J. Li, Angew. Chem. Int. Ed. 59 (2020) 1786-1796. DOI:10.1002/anie.201909138 |
| [48] |
V. Dimakos, M.S. Taylor, Org. Biomol. Chem. 19 (2021) 514-524. DOI:10.1039/d0ob02009e |
| [49] |
A.S. Henderson, S. Medina, J.F. Bower, M.C. Galan, Org. Lett. 17 (2015) 4846-4849. DOI:10.1021/acs.orglett.5b02413 |
| [50] |
J.A. Terrett, J.D. Cuthbertson, V.W. Shurtleff, D.W.C. MacMillan, Nature 524 (2015) 330-334. DOI:10.1038/nature14875 |
| [51] |
X. Shen, C.N. Neumann, C. Kleinlein, N.W. Goldberg, T. Ritter, Angew. Chem. Int. Ed. 54 (2015) 5662-5665. DOI:10.1002/anie.201500902 |
| [52] |
W. Shang, Z.D. Mou, H. Tang, et al., Angew. Chem. Int. Ed. 57 (2018) 314-318. DOI:10.1002/anie.201710310 |
| [53] |
W. Liu, J. Li, P. Querard, C.J. Li, J. Am. Chem. Soc. 141 (2019) 6755-6764. DOI:10.1021/jacs.9b02684 |
| [54] |
G.L. Tolnai, U.J. Nilsson, B. Olofsson, Angew. Chem. Int. Ed. 55 (2016) 11226-11230. DOI:10.1002/anie.201605999 |
| [55] |
K.LeM Hoang, X. Liu, Nat. Commun. 5 (2014) 5051. DOI:10.1038/ncomms6051 |
| [56] |
R.W. Balsiger, Th. Leuenberger, W. Michaelis, O. Schindler, HeIv. Chim. Acta 52 (1969) 1323-1336. DOI:10.1002/hlca.19690520515 |
| [57] |
R. Ghosh, E. Lindstedt, N. Jalalian, B. Olofsson, ChemistryOpen 3 (2014) 54-57. DOI:10.1002/open.201402006 |
| [58] |
A. Kumar, D.H. Ner, S.Y. Dike, Tetrahedron Lett. 32 (1991) 1901-1904. DOI:10.1016/S0040-4039(00)85993-6 |
 2022, Vol. 33
2022, Vol. 33 



