Coordination-driven self-assembly is a highly modular and flexible approach for the rational construction of various metallo-supramolecular structures such as molecular cages, knots, and helicates [1-13]. In addition to these discrete molecular entities, some well-organized metallo-supramolecular polymers were also constructed, providing access to novel functional and stimuli-responsive materials [14-23]. No matter which final structures are targeted, well-preorganized metallo-ligands hold the key for the successful endeavor in these directions.
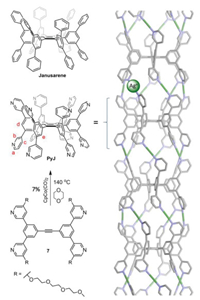
In 2017, we reported a homoditopic molecular host, named janusarene (Fig. 1, top left) [24], which can bind and align various guest compounds concurrently in the solid state. During the course of this study, we realized that a simple phenyl-to-pyridyl structural modification will result in an unusual dodecatopic pyridyl ligand PyJ, whose two-face structural feature might be particularly suitable for the construction metallo-supramolecular polymer (Fig. 1, right), taking advantage of the well-established linear Py-Ag+-Py coordination chemistry [25-28]. Therefore, we initiated this study, which is detailed below.
|
Download:
|
| Fig. 1. Synthesis and structure of the pyridine functionalized janusarene PyJ. The triethylene glycol monomethyl ether (tg) chain, attached to improve the solubility of PyJ, is omitted for clarity. Structure of the assembled PyJ-Ag+ (right) is optimized using the Spartan software, MM force field. | |
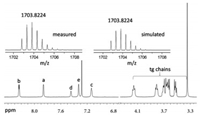
PyJ was synthesized via a cobalt catalyzed cyclotrimerization reaction (Fig. 1). Detailed synthesis and characterization are described in Supporting information. The identity of PyJ is unambiguously confirmed by using high resolution mass spectroscopy (HRMS) and a combination of different nuclear magnetic resonance (NMR) techniques (Fig. 2). HRMS shows an ionic species (m/z 1703.8224) fully consistent with the theoretical value ([M + 2H]2+, calcd. for C186H236N12O48: 1703.8224). The isotopic pattern is identical with that of the simulated one. In the 1H NMR spectrum (CDCl3), resonance signals corresponding to the hexaphenylbenzene (HPB) protons of PyJ (7.44, Hd; 7.33, He) are observed. Other resonance signals on the pyridyl unit can be well assigned with expected integrations.
|
Download:
|
| Fig. 2. High resolution mass (top) and 1H NMR spectra of PyJ (CDCl3). | |
Coordination-driven molecular assembly of PyJ was first investigated by using UV-vis absorption spectroscopy (Fig. 3A). When Ag+ is titrated to a methanol solution of PyJ, the absorption band shifts from 293 nm to 300 nm, giving a sharp isosbestic point at 303 nm, suggesting a clean transformation from PyJ to the PyJ-Ag+ assembly. The titration curve levels off after the addition 6 equiv. of Ag+ (Fig. 3A, inset), a theoretical stoichiometry for the proposed one-dimensional supramolecular assembly (Fig. 1, right).

|
Download:
|
| Fig. 3. UV-vis (A) spectral changes of PyJ (1 × 10−4 mol/L) toward AgBF4 titration in methanol. Inset, absorption at 293 nm as a function of the concentration of Ag+, and the Tyndall effect observed using a laser pointer. (B) DLS data in the absence and presence of Ag+. | |
1H NMR result (Fig. S3 in Supporting information) is consistent with the UV-vis measurement. The resonance signals of PyJ gradually vanish upon AgBF4 titration, in deuterated methanol, indicating the formation of larger assembly, which prohibits fast molecular tumbling. In addition, no evident change in the chemical shift is observed, implying a slow exchange in the NMR time scale. The absence of notable resonance signals, corresponding to the short oligomers, e.g. dimer and trimer, probably suggests a nucleation-elongation assembling process, as occurs in many supramolecular polymerization systems [29], in which the formation of short oligomers is more energetic demanding, because restricted conformational switching in the initial assembling state might promote further elongation. This is consistent with the S-shaped titration curve (Fig. 3A, inset) [30], observed in the UV-vis studies. Dynamic light scattering reveals the formation of an assembled species which has an average hydrodynamic radius of ca. 120 nm (Fig. 3B). An evident Tyndall effect is observed, accordingly (Fig. 3A, inset).
Coordination-driven one-dimensional assembly of PyJ is proved directly by using atomic force microscopy (AFM, Fig. 4A) and transmission electron microscopy (TEM, Fig. 4B). AFM image of PyJ-Ag+, obtained by casting a dilute solution of PyJ (1.0 µmol/L) and Ag+ (6.0 µmol/L) in methanol on a silica surface, shows fibrous structures of several micrometers in length, with the majority bundled in parallel. Although detailed molecular arrangement is not evident from the AFM image, the height profile of individual polymer chain (3.0 nm, Fig. 4A, inset) matches the expected value if the attached tg chains and counterion species are taken into account. In contrast, control AFM experiment using PyJ, in the absence of Ag+, reveals dot-like structures without the formation of fiber-shaped objects (Fig. S2 in Supporting information).

|
Download:
|
| Fig. 4. (A) AFM height image of PyJ-Ag+, prepared by casting a dilute solution of PyJ (1.0 µmol/L) and Ag+ (6.0 µmol/L) in methanol on a silica surface (scale 1.5 µm × 1.5 µm). Inset, cross-section profile along the white line in the image. (B) TEM image of PyJ-Ag+ negatively stained with uranyl acetate. | |
TEM examination of a negatively stained PyJ-Ag+ reveals bundles of parallelly aligned fibres (Fig. 4B; Fig. S4 in Supporting information), in agreement with that found in the AFM image. The polymer chains display a uniform diameter of ca. 4.0 nm, which is reasonable considering that PyJ-Ag+ is surrounded by a layer of tg chains and the counterions.
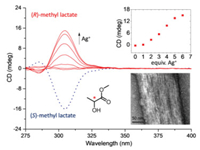
When molecular assembly is carried out in a chiral solvent like (R)-methyl lactate, optically active PyJ-Ag+ is successfully obtained [31]. As shown in Fig. 5, free PyJ is essentially optically inactive, in keeping with its dynamic conformation in solution. In a sharp contrast, evident circular dichroism (CD) signals at 285 and 305 nm emerge and develop upon Ag+ titration. The maximum spectral change is reached after 6 equiv. of Ag+ is present (Fig. 5, inset), a theoretical stoichiometry for the expected PyJ-Ag+. The corresponding UV-vis spectral changes (Fig. S5 in Supporting information) are analogous to those observed in methanol (Fig. 3A), suggesting a comparable assembling process. When the other enantiomer, (S)-methyl lactate, is used as the chiral solvent instead, a similar trend in the CD spectral change is observed, but with perfect mirror image Cotton effect (Fig. 5, dashed line). TEM image of a negatively stained sample shows bundles of parallelly aligned fibers with a uniform diameter of ca. 4.0 nm (Fig. 5, inset), reminiscent of that found for PyJ-Ag+ assembled in methanol (Fig. 4B).
|
Download:
|
| Fig. 5. CD spectral changes of PyJ (1 × 10−4 mol/L) toward AgBF4 titration in a chiral solvent of methyl lactate. Inset, plot of the CD intensity at 305 nm as a function of equiv. of Ag+ added and the TEM image of PyJ-Ag+ assembled in (S)-methyl lactate. | |
Mori et al. thoroughly investigated the propeller-chirality of a HPB derivative [32], ascertaining that (P)-HPB has a major negative Cotton effect at around 300 nm, and a minor positive one at shorter wavelength. This spectral feature is exactly reproduced in the CD spectrum of PyJ-Ag+ measured in (S)-methyl lactate (Fig. 5). Therefore, we speculate that chiral induction of PyJ-Ag+ was realized in methyl lactate, and the HPB units in the assembled PyJ-Ag+ take uniform propeller-shaped conformation. This assumption is supported by the simulated assembling structure of PyJ-Ag+ (Fig. 1, right).
In summary, we present a janusarene-based dodecatopic pyridyl ligand PyJ, which forms one-dimensional metallo-supramolecular polymer in the presence of Ag+. The two-face structural characteristic of PyJ is essential for this coordination driven self-assembly, and the propeller-shaped conformation of the HPB units in assembled PyJ-Ag+ can be biased in a chiral solvent, e.g. methyl lactate. This work may stimulate the fabrication of other metallo-supramolecular polymer with attractive chiroptical and dynamic properties.
Declaration of competing interestThere are no conflicts to declare.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21871298, 91956118) and the Sun Yat-sen University.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.057.
| [1] |
D. Fujita, Y. Ueda, S. Sato, et al., Nature 540 (2016) 563-566. DOI:10.1038/nature20771 |
| [2] |
J.J. Danon, A. Krüger, D.A. Leigh, et al., Science 355 (2017) 159-162. DOI:10.1126/science.aal1619 |
| [3] |
J.M. Lehn, A. Rigault, J. Siegel, et al., Proc. Natl. Acad. Sci. U. S. A. 84 (1987) 2565-2569. DOI:10.1073/pnas.84.9.2565 |
| [4] |
M. Ikeda, Y. Tanaka, T. Hasegawa, Y. Furusho, E. Yashima, J. Am. Chem. Soc. 128 (2006) 6806-6807. DOI:10.1021/ja0619096 |
| [5] |
J. Xu, K.N. Raymond, Angew. Chem. Int. Ed. 45 (2006) 6480-6485. DOI:10.1002/anie.200602060 |
| [6] |
M. Hutin, C.J. Cramer, L. Gagliardi, et al., J. Am. Chem. Soc. 129 (2007) 8774-8780. DOI:10.1021/ja070320j |
| [7] |
S.G. Chen, Y. Yu, X. Zhao, et al., J. Am. Chem. Soc. 133 (2011) 11124-11127. DOI:10.1021/ja205059z |
| [8] |
H. Ito, M. Ikeda, T. Hasegawa, Y. Furusho, E. Yashima, J. Am. Chem. Soc. 133 (2011) 3419-3432. DOI:10.1021/ja108514t |
| [9] |
S.E. Howson, A. Bolhuis, V. Brabec, et al., Nat. Chem. 4 (2012) 31-36. DOI:10.1038/nchem.1206 |
| [10] |
O. Gidron, M. Jirásek, N. Trapp, et al., J. Am. Chem. Soc. 137 (2015) 12502-12505. DOI:10.1021/jacs.5b08649 |
| [11] |
Y.X. Hu, X. Hao, L. Xu, et al., J. Am. Chem. Soc. 142 (2020) 6285-6294. DOI:10.1021/jacs.0c00698 |
| [12] |
Q. Ling, T. Cheng, S. Tan, J. Huang, L. Xu, Chin. Chem. Lett. 31 (2020) 2884-2890. |
| [13] |
J. Zhu. X. Liu, J. Huang, L. Xu, Chin. Chem. Lett. 30 (2019) 1767-1774. |
| [14] |
T. Wu, Z. Jiang, X. Xue, et al., Chin. Chem. Lett. 32 (2021) 1911-1914. DOI:10.1016/j.cclet.2021.01.052 |
| [15] |
T.H. Tu, T. Sakurai, S. Seki, Y. Ishida, Y.T. Chan, Angew. Chem. Int. Ed. 60 (2021) 1923-1928. DOI:10.1002/anie.202012284 |
| [16] |
C. Chakraborty, U. Rana, Y. Narayana, S. Moriyama, M. Higuchi, ACS Appl. Polym. Mater. 2 (2020) 4521-4530. DOI:10.1021/acsapm.0c00611 |
| [17] |
D. Hossain, C. Chakraborty, U. Rana, et al., ACS Appl. Polym. Mater. 2 (2020) 4449-4454. DOI:10.1021/acsapm.0c00559 |
| [18] |
J. Liu, Y. Gao, T. Wang, et al., ACS Nano 14 (2020) 11283-11293. DOI:10.1021/acsnano.0c03163 |
| [19] |
Z. Li, Y. Li, Y. Zhao, et al., J. Am. Chem. Soc. 142 (2020) 6196-6205. DOI:10.1021/jacs.0c00110 |
| [20] |
W. Zhao, B. Qiao, J. Tropp, et al., J. Am. Chem. Soc. 141 (2019) 4980-4989. DOI:10.1021/jacs.9b00248 |
| [21] |
Z. Li, J. Gu, S. Qi, et al., J. Am. Chem. Soc. 139 (2017) 14364-14367. DOI:10.1021/jacs.7b07965 |
| [22] |
D. Rais, J. Pfleger, M. Menšík, et al., J. Mater. Chem. C 5 (2017) 8041-8051. DOI:10.1039/C7TC00484B |
| [23] |
Y.J. He, T.H. Tu, M.K. Su, et al., J. Am. Chem. Soc. 139 (2017) 4218-4224. DOI:10.1021/jacs.7b01010 |
| [24] |
T. Li, L. Fan, H. Gong, et al., Angew. Chem. Int. Ed. 56 (2017) 9473-9477. DOI:10.1002/anie.201705451 |
| [25] |
H. Yamagishi, T. Fukino, D. Hashizume, et al., J. Am. Chem. Soc. 137 (2015) 7628-7631. DOI:10.1021/jacs.5b04386 |
| [26] |
M. Obana, T. Fukino, T. Hikima, T. Aida, J. Am. Chem. Soc. 138 (2016) 9246-9250. DOI:10.1021/jacs.6b04693 |
| [27] |
T. Sawada, M. Yamagami, K. Ohara, K. Yamaguchi, M. Fujita, Angew. Chem. Int. Ed. 55 (2016) 4519-4522. DOI:10.1002/anie.201600480 |
| [28] |
F. Huang, L. Ma, Y. Che, et al., J. Org. Chem. 83 (2018) 733-739. DOI:10.1021/acs.joc.7b02709 |
| [29] |
T. Aida, E.W. Meijer, S.I. Stupp, Science 335 (2012) 813-817. DOI:10.1126/science.1205962 |
| [30] |
M.I. Stefan, N.Le Novère, PLoS Comput. Biol. 9 (2013) e1003106. DOI:10.1371/journal.pcbi.1003106 |
| [31] |
M. Fujiki, Symmetry 6 (2014) 677-703. DOI:10.3390/sym6030677 |
| [32] |
T. Kosaka, Y. Inoue, T. Mori, J. Phys. Chem. Lett. 7 (2016) 783-788. DOI:10.1021/acs.jpclett.6b00179 |
 2022, Vol. 33
2022, Vol. 33 

