The continuous depletion of fossil fuels and demands for the cleaner earth has led to an imperative requirement in efficient utilization of traditional fossil resources like coal and raw oil. Coal tar is a common by-product of the coal processing industry, without suitable processing, it may cause wastes of energy resources and serious environmental pollution [1]. The high oxygen content in the composition of coal tar usually lead to high viscosity and low heating value [2], which prevent the wide utilization of it. Therefore, catalytic hydrodeoxygenation (HDO) was recognized as a promising method for upgrading of the O-containing compounds and tuning the waste to high heating value hydrocarbons [3-6]. For example, dibenzofuran (DBF) which is a representative O-containing compound of coal tar and an important intermediate in biomass gasification [7], could be upgrading to value-added hydrocarbon fuel like bicyclohexane (BCH) by HDO reaction.
The HDO reaction usually include hydrogenation, hydrogenolysis and deoxygenation, accordingly, the catalyst must be able to activate H2 while activating C-O bonds also. Cobalt molybdenum sulfide (Co-MoS2) and nickel molybdenum sulfide (Ni-MoS2) supported catalysts have been frequently used for deoxygenation of DBF due to the direct scission of the C-O bond over the MoS2 active phase and the sulfuric vacancy sites [8, 9]. Nevertheless, using these metal sulfide catalysts needs cofeeding of extra sulfide such as CS2 or H2S to maintain the activity of the catalyst [10, 11], and this may introduce sulfur into the products and results in contamination. Besides sulfide catalysts, Mo2C was recently reported as catalyst for the HDO of DBF at 350 ℃, and 4.1 MPa H2 pressure [12], HDO products including both cyclohexylbenzene (CHB) and bicyclohexane (BCH) were generated with about 50% yield. Owing to the good capability to activate H2, noble metals like Ru, Pd, Rh and Pt were reported to be efficient for the HDO of oxygen containing aromatics under milder conditions [13-15] Liang et al. [16, 17] reported the HDO of DBF at 280–300 ℃ and 3 MPa H2 over supported noble metal catalysts and acquired high efficiency. Although these seminal works reported, a high selectivity to the complete HDO product BCH seldom is focused on. Therefore, it is still in demand that an efficient catalyst which shows high selectivity to BCH in the HDO reaction of DBF.
Bimetallic nanoparticle (BMNP) catalysts, which combine two different metals, usually show performance superior to the monometallic counterparts due to the "synergistic effects" [18, 19]. Incorporation of Mo into Pt or Pd has been reported by Infantes-Molina and co-workers [20], the bimetallic catalyst was applied in the HDO of DBF and the performance was improved, however, the selectivity to BCH was still afforded lowly. Due to the high dissociation energy, the cleavage of C-O bonds is one of the most challenging steps in HDO reactions [21], and a series of reports have mentioned that Ni-based catalysts show good performance in the cleavage of C-O bonds [22-24]. The previous works of our group have demonstrated that the combination of Ni and noble metals could significantly enhance the activity in catalytic cleavage of C-O bonds [25, 26]. On the basic of the above reports, BMNPs combining Ni and noble metal, which may inherit the advantages of both metals while upgrading the catalytic performance, were expected to be promising catalysts in the HDO reactions. Herein, a series of BMNPs based on Ni and noble metals were prepared and tested for the HDO of DBF, and MgO was chosen as the catalyst support for the BMNPs to make the catalyst heterogeneous and recyclable. Pleasantly, Pt1Ni4/MgO shows good activity and the yield of desired product BCH which was up to 95% at 240 ℃ in 3 h. Alloying of those two metals inherits both great hydrogenation performance from Pt sites and good activity to C-O bonds cleavage from Ni sites. In addition, scale-up experiment proved that the catalyst has prospect for industrial application, and no significant loss in catalytic performance was observed when Pt1Ni4/MgO was recycled six times.
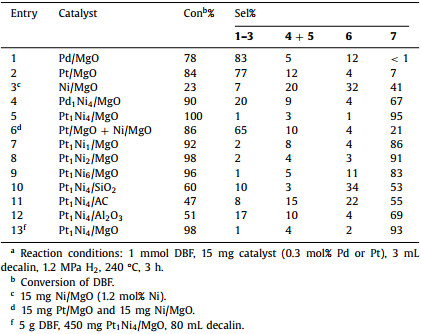
As a representative example, the HDO of DBF was carried out to evaluate the performance of the catalysts. Decalin was chosen to be the solvent in the reactions considering it has an inert chemical structure, a high boiling point and a low vapor pressure which limit the total pressure of reactor in safe range after heating. The products were identified by GC–MS analysis, and there were seven main products detected in this work. As shown in Scheme 1, compounds 1–3 retained the O-containing ring, and these products were generated from the hydrogenation of DBF without any catalytic cleavage of the C-O bond. Hydrogenolysis of the C-O bond occurred and give the compounds 4 and 5, which kept one of the C-O bonds in molecule. Compounds 6 and 7 were observed from the HDO reaction, and BCH 7 was the target product in our study.
|
Download:
|
| Scheme 1. Main products in the HDO reaction of DBF. | |
At the initial stage, monometallic nanoparticles (NPs) were supported on MgO and tested in the HDO of DBF. Monometallic noble metal catalysts showed good activity in conversion of DBF (Table 1, entries 1 and 2), which could be attributed to their excellent hydrogen activity, however the main products in these reactions were hydrogenation products (compounds 1–3), almost no target product BCH was detected in the solution catalyzed by Pd/MgO, and only 7% selectivity to BCH was achieved by Pt/MgO. The Ni monometallic catalyst afforded higher selectivity towards deoxidation products 6 and 7 (Table 1, entry 3), although the reaction had a low conversion of 23%. Since no ideal result achieved by monometallic catalysts, bimetallic catalysts alloying Ni and noble metals were prepared and used in the HDO reaction. As expected, noteworthy enhancement in catalytic performance was observed when the bimetallic catalysts were employed (Table 1, entries 4 and 5), both conversion and selectivity increased significantly. Between these two, Pt1Ni4/MgO showed a better performance, which could convert DBF completely and the selectivity towards BCH was up to 95%.
|
|
Table 1 HDO of DBF over different catalysts.a |
Physical mixtures of Pt/MgO and Ni/MgO do not lead to an apparent promotion in catalytic selectivity (Table 1, entry 6), demonstrating that it is the cooperativity inside Pt1Ni4 BMNPs, not the simple mixture of Pt and Ni, accounts for the elevation of the catalytic performance. Compositions of the BMNPs were reported to be vital for the catalytic efficiency of bimetallic catalysts, and catalysts with different Ni/Pt molar ratios were prepared and tested in the reaction (Table 1, entries 7–9). Keeping the Pt loading in the reaction constant (0.3 mol% Pt), catalysts with higher Ni contents show better selectivity to BCH. However, when the Ni/Pt molar ratio was higher than 4, selectivity to 6 increased, and a Ni/Pt ratio of 4 could be emerged as the optimized ratio. Next, Pt1Ni4 BMNPs were immobilized on different supports including SiO2, activated carbon (AC) and Al2O3, and the obtained catalysts were applied in the reaction (Table 1, entries 10–12). Lower conversions were afforded by these reference catalysts, and the superior activity of Pt1Ni4/MgO could be owing to the better dispersity of MgO in decalin. Scale-up experiment was also performed (Table 1, entry 13), Pt1Ni4/MgO showed good performance with 5 g of DBF loaded, which signified the promising application of the catalyst in industry.
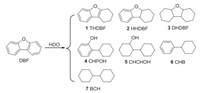
Effects of reaction temperature and initial H2 pressure were investigated and the results are presented in Fig. 1. Variations in reaction temperature had a significant influence on the conversion and selectivity for the HDO of DBF (Fig. 1a and Table S1 in Supporting information). With the reaction temperature increased, both the conversion of DBF and selectivity to BCH enhanced. When the temperature went up to 240 ℃, all DBF was converted and the selectivity to BCH reached 95%, and no improvement in the reaction selectivity was observed with further increasement in temperature. Initial H2 pressure had a lower effect on the selectivity to BCH, the raising pressure increase the conversion of DBF and afforded 100% conversion under 1.2 MPa (Fig. 1b).
|
Download:
|
| Fig. 1. Effect of the reaction conditions: 1 mmol DBF, 15 mg Pt1Ni4/MgO (0.3 mol% Pt), 3 mL decalin, 3 h, (a) effect of the reaction temperature under 1.2 MPa H2, (b) effect of the initial H2 pressure at 240 ℃. | |
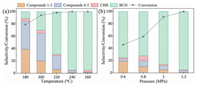
With the optimized reaction conditions established, a model reaction was conducted and tracked by GC and GC–MS to gain preliminary insights into the reaction pathway. As displayed in Fig. 2, the yields of hydrogenation products 2 and 3 increased at first and then decreased with the increase in the reaction time. Subsequently, yield of 5 increased and peaked while yield of 7 continuing growth and reaching up to 95% after 3 h. According to the above results, a plausible reaction pathway for the HDO of DBF under the current conditions is proposed in Scheme 2. DBF was hydrogenated to 1 and 2 at the initial step, then both further hydrogenation and ring-opening of 2 occurred. Considering the deoxygenation barrier is higher than that of hydrogenation for 4 [21] and the content of 6 in the products kept at a low level during the whole process (Fig. 2), it can be derived that most 4 was hydrogenated to 5 and 7 was mostly formed by the cleavage of C-O bond in 5. Moreover, because of the higher bond dissociation energy, C(sp2)-O bonds are more difficult to be cleaved than C(sp3)-O bonds [27, 28], therefore, hydrogenation of the intermediates could effectively promote the deoxygenation step. Based on the tandem pathway, it can be inferred that the Pt1Ni4/MgO should be a multifunctional catalyst which could continuously catalyze the hydrogenation and the deoxygenation. Because the Pt monometallic catalyst showed a preference for the hydrogenation reaction and the Ni monometallic catalyst showed a high selectivity to deoxygenated products (Table 1, entries 2 and 3), there might be a "relay catalysis" between Pt sites and Ni sites over the Pt1Ni4/MgO: at first, DBF was hydrogenated over the Pt sites, then, the hydrogenated intermediates were "relayed to" the Ni sites and deoxygenated.
|
Download:
|
| Fig. 2. Time-course monitoring of the reaction. Reaction conditions: 1 mmol DBF, 15 mg Pt1Ni4/MgO (0.3 mol% Pt), 3 mL decalin, 1.2 MPa H2, 240 ℃. | |
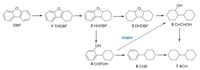
|
Download:
|
| Scheme 2. Proposed reaction pathway for the HDO of DBF over Pt1Ni4/MgO catalyst. | |
To explore the applicability of the catalytic system, substrates including diphenyl ether, phenol and benzofuran were tested. Gratifyingly, high yield of HDO products were achieved under the optimized reaction conditions (Table S4 in Supporting information), which verified the applicability of Pt1Ni4/MgO in various HDO reactions.
The morphologies of Pt1Ni4/MgO were directly observed by transmission electron microscopy (TEM). As presented in Fig. 3a, the as-prepared Pt1Ni4 BMNPs have a homogeneous distribution throughout the surface of MgO. The Pt1Ni4 BMNPs are well proportioned spherical with an average diameter of 4 nm, compared with the Pt1Ni1 BMNPs (Fig. S1 in Supporting information), the smaller size could mean more contact opportunities between the active sites and substrates. More information about the structure of the BMNPs is displayed by the high resolution TEM (HRTEM) image (Fig. 3b), and a well-resolved lattice spacing of 0.216 nm was revealed, which corresponds to the (111) plane of the Pt-Ni alloy [29]. The crystallite phases of the BMNPs were also revealed by the selected area electron diffraction (SAED) image (Fig. 3c), the diffraction pattern verified the formation of Pt-Ni alloy (JCPDS No. 65–2797). The EDS elemental maps (Figs. 3d-f) profile obviously manifested the distribution of Pt and Ni in Pt1Ni4/MgO, the distributions of Pt and Ni match well with the distribution of NPs, which further confirmed the alloy form of Pt1Ni4 BMNPs. In the XRD patterns (Fig. S3 in Supporting information) no obvious peaks for the Pt1Ni4 BMNPs, which might be attributed to the low content and small size of the Pt1Ni4 BMNPs in catalyst.

|
Download:
|
| Fig. 3. (a) TEM image and size distribution of Pt1Ni4/MgO, (b) HRTEM image of Pt1Ni4/MgO, (c) SAED image of Pt1Ni4 BMNPs, (d) STEM image of Pt1Ni4/MgO, EDS elemental maps for (e) Pt and (f) Ni. | |
X-ray photoelectron spectrum (XPS) was employed to ascertain the detailed electronic structure and chemical states of Pt1Ni4/MgO. Well-defined peaks corresponding to both Pt and Ni species can be detected in the survey spectrum (Fig. S4a in Supporting information). In the spectra of Pt 4f region (Fig S4b in Supporting information), the peaks sit at 70.8 eV and 74.2 eV which can be assigned to metallic Pt0, in contrast to that of Pt/MgO (Fig. S4d in Supporting information), a negative shift by about 0.25 eV can be observed. The shift came from the different electronegativities of Pt (2.28) and Ni (1.91) [30], and suggesting the electron-transfer effect from Ni to Pt inside the Pt1Ni4 BMNPs. The spectra of Ni 2p region (Fig. S4c in Supporting information) shows the presence of metallic Ni as well as Ni oxide, which could be the result of the fact that nickel can be easily oxidized to oxide and hydroxide when exposed to oxygen and moisture in air [25]. Besides, the Ni/Pt atomic ratio on the surface of Pt1Ni4/MgO determined by XPS was 4.8, which is close to the result of ICP-MS (Table S2 in Supporting information). It can be concluded from the characterizations results that the Pt1Ni4 alloy BMNPs are formed and have a homogeneous distribution on the surface of MgO. The formation of the Pt1Ni4 alloy BMNPs minimized the distance between Pt sites and Ni sites and increased opportunities for "relay catalysis" between the two metals sites.
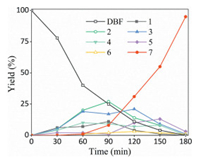
Recyclability is a key issue for a heterogeneous catalyst, and the recyclability of Pt1Ni4/MgO was tested under the scale-up experiment conditions (Table 1, entry 13). Upon the completion of the reaction, the catalyst was separated by filtration, washed by ethyl acetate and then dried under vacuum for reuse in the next cycle. As shown in Fig. 4a, the catalyst could be reused up to six times with only a minor loss in its activity, which may due to the mechanical loss of the catalyst during the recycle process. The heterogeneous nature of Pt1Ni4/MgO was demonstrated by a hot filtration experiment under the optimized conditions (Table 1, entry 5). When the reaction proceeded for 1 h, the reactor was cooled down to room temperature and the catalyst was filtered off, the isolated solution was added into the reactor which was then purged with H2 and heated for further reaction. As shown in Fig. 4b, no further increase in conversion was observed after the removal of the catalyst, and addition of the removed catalyst led to the resumption of the reaction. The TEM image of the recovered Pt1Ni4/MgO after 6 cycles (Fig. S2 in Supporting information) shows no obvious change in the morphology and the Pt1Ni4 BMNPs did not agglomerate. The XRD (Fig. S3 in Supporting information) and XPS spectra (Fig. S5 in Supporting information) of the recovered catalyst are not obviously different from the fresh samples. Moreover, ICP-MS analysis of the catalyst after 6 cycles (Table S2) and the reaction solution (Table S3 in Supporting information) revealed that the metal leaching of the catalyst is negligible during the reaction process. These results confirmed that Pt1Ni4/MgO is highly stable for the HDO of DBF.

|
Download:
|
| Fig. 4. (a) Recyclability of Pt1Ni4/MgO. (b) Hot filtration experiments of Pt1Ni4/MgO for the HDO of DBF. | |
In summary, a novel Pt-Ni bimetallic catalyst Pt1Ni4/MgO has been successfully prepared, which showed excellent selectivity to BCH in the HDO reaction of DBF. The reaction could play an important role in upgrading and utilization of the by-products from coal processing industry. With Pt1Ni4/MgO catalyzed, all DBF could be converted and the selectivity to BCH was up to 95% at 240 ℃ and 1.2 MPa of H2. The efficient catalytic performance may originate from the "relay catalysis" effect of Pt sites and Ni sites: Pt sites activate H2 and hydrogenate DBF to hydrogenated intermediates, Ni sites were believed to accelerate the cleave process of C-O bonds and finally result in desired product BCH. Characterizations of Pt1Ni4/MgO indicated the alloy form and ultrasmall size of Pt1Ni4 BMNPs on the surface of MgO. Besides, outstanding performance in scale-up experiment and recyclability test highlights the promising potential of this bimetallic catalyst in sustainable industry.
Declaration of competing interestThere are no conflicts to declare.
AcknowledgmentsWe gratefully acknowledge Key Laboratory of Biomass Energy and Material, Jiangsu Province (No. JSBEM201912) and Chinese Postdoctoral Science Foundation (Nos. 2015M571761 and 2016T90465) for financial support. This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institution and Instrument and Equipment Foundation of Nanjing University of Science & Technology.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.059.
| [1] |
P. Blum, A. Sagner, A. Tiehm, et al., J. Contam. Hydrol. 126 (2011) 181-194. DOI:10.1016/j.jconhyd.2011.08.004 |
| [2] |
J. Lu, A. Heyden, J. Catal. 321 (2015) 39-50. DOI:10.1016/j.jcat.2014.11.003 |
| [3] |
H. Wang, J. Male, Y. Wang, ACS Catal. 3 (2013) 1047-1070. DOI:10.1021/cs400069z |
| [4] |
S. Kim, E.E. Kwon, Y.T. Kim, et al., Green Chem. 21 (2019) 3715-3743. DOI:10.1039/C9GC01210A |
| [5] |
D. Leckel, Energ. Fuel. 20 (2006) 1761-1766. DOI:10.1021/ef060034d |
| [6] |
S. Yang, X. Lu, H. Yao, et al., Green Chem. 21 (2019) 597-605. DOI:10.1039/C8GC03775B |
| [7] |
C.M. Huelsman, P.E. Savage, Phys. Chem. Chem. Phys. 14 (2012) 2900-2910. DOI:10.1039/c2cp23910h |
| [8] |
S. Krishnamurthy, S. Panvelker, Y.T. Shah, AlChE J 27 (1981) 994-1001. DOI:10.1002/aic.690270616 |
| [9] |
V. LaVopa, C.N. Satterfield, Energ. Fuel. 1 (1987) 323-331. DOI:10.1021/ef00004a003 |
| [10] |
T. Mochizuki, S.Y. Chen, M. Toba, Y. Yoshimura, Appl. Catal. B: Environ. 146 (2014) 237-243. DOI:10.1016/j.apcatb.2013.05.040 |
| [11] |
S. Boullosa-Eiras, R. Lødeng, H. Bergem, et al., Catal. Today 223 (2014) 44-53. DOI:10.1016/j.cattod.2013.09.044 |
| [12] |
S. Liu, H. Wang, C.S. Kim, K.J. Smith, Appl. Catal. A: Gen. 600 (2020) 117628. DOI:10.1016/j.apcata.2020.117628 |
| [13] |
P.M. de Souza, R.C. Rabelo-Neto, L.E.P. Borges, et al., ACS Catal. 5 (2015) 1318-1329. DOI:10.1021/cs501853t |
| [14] |
J. Wildschut, F.H. Mahfud, R.H. Venderbosch, H.J. Heeres, Ind. Eng. Chem. Res. 48 (2009) 10324-10334. DOI:10.1021/ie9006003 |
| [15] |
D.A. Ruddy, J.A. Schaidle, J.R. Ferrell Iii, et al., Green Chem. 16 (2014) 454-490. DOI:10.1039/C3GC41354C |
| [16] |
L. Wang, M. Zhang, M. Zhang, G. Sha, C. Liang, Energ. Fuel. 27 (2013) 2209-2217. DOI:10.1021/ef302166q |
| [17] |
L. Wang, C. Li, S. Jin, W. Li, C. Liang, Catal. Lett. 144 (2014) 809-816. DOI:10.1007/s10562-014-1236-2 |
| [18] |
Y. Wu, S. Cai, D. Wang, W. He, Y. Li, J. Am. Chem. Soc. 134 (2012) 8975-8981. DOI:10.1021/ja302606d |
| [19] |
M. Sankar, N. Dimitratos, P.J. Miedziak, et al., Chem. Soc. Rev. 41 (2012) 8099-8139. DOI:10.1039/c2cs35296f |
| [20] |
D. Ballesteros-Plata, A. Infantes-Molina, M. Rodríguez-Cuadrado, et al., Appl. Catal. A: Gen. 547 (2017) 86-95. DOI:10.1016/j.apcata.2017.08.034 |
| [21] |
X.B. Wang, Z.Z. Xie, L. Guo, Z.Y. Du, W.Y. Li, Catal. Today 364 (2021) 220-228. DOI:10.1016/j.cattod.2020.04.044 |
| [22] |
S. Jin, Z. Xiao, X. Chen, et al., Ind. Eng. Chem. Res. 54 (2015) 2302-2310. DOI:10.1021/ie504600f |
| [23] |
V. Molinari, G. Clavel, M. Graglia, M. Antonietti, D. Esposito, ACS Catal. 6 (2016) 1663-1670. DOI:10.1021/acscatal.5b01926 |
| [24] |
A.G. Sergeev, J.D. Webb, J.F. Hartwig, J. Am. Chem. Soc. 134 (2012) 20226-20229. DOI:10.1021/ja3085912 |
| [25] |
J.W. Zhang, G.P. Lu, C. Cai, Green Chem. 19 (2017) 4538-4543. DOI:10.1039/C7GC02087B |
| [26] |
J.W. Zhang, K.K. Sun, D.D. Li, et al., Appl. Catal. A: Gen. 569 (2019) 190-195. DOI:10.1016/j.apcata.2018.10.038 |
| [27] |
J.A. Cecilia, A. Infantes-Molina, E. Rodríguez-Castellón, A. Jiménez-López, S.T. Oyama, Appl. Catal. B: Environ. 136- 137 (2013) 140-149. |
| [28] |
M. Guo, J. Peng, Q. Yang, C. Li, ACS Catal. 8 (2018) 11174-11183. DOI:10.1021/acscatal.8b03253 |
| [29] |
M. Li, Z. Zhao, T. Cheng, et al., Science 354 (2016) 1414-1419. DOI:10.1126/science.aaf9050 |
| [30] |
B. Wang, L. Xiong, H. Hao, et al., J. Alloys Compd. 844 (2020) 156253. DOI:10.1016/j.jallcom.2020.156253 |
 2022, Vol. 33
2022, Vol. 33