b College of Materials Science and Engineering, Fuzhou University, Fuzhou 350116, China
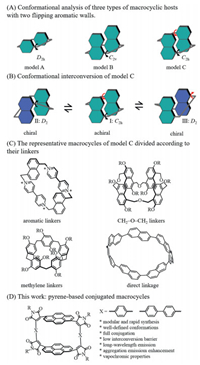
Conformationally adaptive macrocycles are adaptive to guests or environmental changes, such as temperature and solvent, which have the conformational ensemble features of bioreceptors [1, 2]. It has been demonstrated that the conformationally adaptive macrocycles have wide potential applications in chirality sensing [1], selected recognition [3], stimuli-responsive self-assembly [4-6], and molecular switches [7]. The development of conformationally adaptive macrocyclic has received extensive attention in recent years. There are three types of conformationally adaptive macrocycles with flipping aromatic sidewalls (Fig. 1A) [8]. For model C, the linking positions of aromatic sidewalls are the two centrosymmetric positions, and flipping one aromatic sidewall of I gives rise to a pair of enantiomers (Fig. 1B). The structural flexibility is dramatically affected by the linkers that connect the aromatic sidewalls. Hence, the representative macrocycles of model C can be divided based on linkers include aromatic linkers [9, 10], CH2-O-CH2 linkers [11-13], CH2 linkers [14-16] and direct linkage (Fig. 1C) [17, 18]. These successful examples have encouraged us to develop a new type of conformationally adaptive macrocycles to introduce new properties to the resulting macrocycles (Fig. 1D).
|
Download:
|
| Fig. 1. Conformational analysis of three types of conformationally adaptive macrocycles. | |
Pyrene is a widely used fluorescent chromophore that possesses unique photophysical properties, for example, high fluorescence quantum yield, the tendency for excimer formation, and long fluorescence lifetime [19-23]. Hence, pyrene was selected as the aromatic sidewall in our study. Inspired by Durola's synthesis of macrocyclic conjugated oligomers [24-26], we employ pyrene as an aromatic sidewall and aryl maleimide as a linker for the synthesis of a new type of conformationally adaptive macrocycles by Perkin reaction and imidization reaction. This new macrocycle shows vapochromic behavior and aggregation emission enhancement (AEE) effect. We have also investigated the effect of the linker on the shape and photophysical properties of the resulting macrocyclic products. Finally, the conformational interconversion barrier was determined by DFT calculations as well.
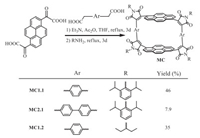
The conjugated macrocycle (MC, Scheme 1) was synthesized by the reaction of pyrenylene-1, 6-diglyoxylic acid with 1.4-phenylenediacetic acid, followed by treatment with 2, 6-diisopropylaniline in 46% isolated yield over two steps as a red solid (MC1.1). Furthermore, to prove the generality of our synthetic approach and the diversity of accessible structures, biphenyl as a subunit of the linker was tested, which provided the corresponding macrocycles MC2.1 in 7.9% yield, the lower yield is due to the more difficult macrocyclization caused by the rotation of the biphenyl. In addition to varying the linker, we have also tested different amines, such as 1-ethylpropylamine, which provided the target product MC1.2 in 35% yield. For comparison, acyclic fragment (AF) was synthesized in similar method by pyrenylene-1-glyoxylic acid and phenylacetic acid in 91% yield (Supporting information).
|
Download:
|
| Scheme 1. Synthesis of macrocycles (MC). | |
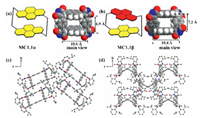
The conformationally adaptive macrocycle MC1.1 with two pyrene sidewalls has three conformations: one achiral conformation (MC1.1α) and a pair of planar-chiral ones (MC1.1β) (Fig. 2), which are unambiguously determined by single-crystal X-ray diffraction analysis. These two different single crystals of MC1.1 were obtained from different crystal growth methods. The yellow single crystal (MC1.1α) was obtained through a solvothermal method, which dissolved with the mixture solvent in CH3CN and CH3OH (v: v = 1:3) and then heated at 80 ℃ for one day. The red single crystal (MC1.1β) was obtained by its slow evaporation in the mixed solution of CHCl3 and EtOH, and the same crystal form could be obtained from other mixed solutions, such as a mixed solution of ethyl acetate and n-pentane or a mixed solution of tetrahydrofuran and n-pentane.
|
Download:
|
| Fig. 2. (a, b) The model and main view in the space-filling model of MC1.1α and MC1.1β, respectively. R group, and solvent molecules are omitted for clarity. (c, d) The packing diagram of MC1.1α, MC1.1β viewed along the a-axis, respectively; the green dash bonds were shortest hydrogen bonding. Unrelated hydrogen atoms and solvent molecules are omitted for clarity. | |
In MC1.1α, the macrocycle has two parallel and overlapping pyrene sidewalls, the distance of two pyrene's planes is 6.9 Å. Furthermore, two 1, 4-phenylene sidewalls are parallel and overlapping as well, and the distance between the centers of the benzene is 10.6 Å, the angle between two different molecular layers is 45.8° The strongest noncovalent interactions are hydrogen bonding (2.724(1) Å) between the carbonyl group on the maleimide and the isopropyl group on the adjacent molecule. Compared to MC1.1α, two pyrene units of MC1.1β are parallel but not overlapping, the torsion angle between the two pyrene units is 32.0°, the distance of the two planes increases to 7.2 Å. The two benzene units are intersectant, the distance between the centers of the benzene is 10.4 Å, and the dihedral angle between the two planes is 60.0° The dihedral angle between two different molecular layers is 0.9°, which is near parallel. The strongest noncovalent interactions are hydrogen bonding (2.466(7) Å) between the carbonyl group on the maleimide and the hydrogen on the intramolecular pyrene, which means the MC1.1β is more stable conformation. The parallel stacking of layers in MC1.1β results in a more red-shifted color than the interlaced stacking of layers in MC1.1α, explaining the difference in color between two conformations. Unfortunately, the single crystal of MC2.1 has not been obtained after many attempts due to poor solubility.
The interconversion between achiral conformation (MC1.1α) and chiral ones (MC1.1β) was further studied by DFT calculation at the B3LYP/6–31G(d) level (Fig. 3). The chiral MC1.1β is thermodynamically stable than achiral MC1.1α by 3.8 kcal/mol in terms of thermal free energies. For the interconversion process among these three conformations, the DFT calculation result provided a stepwise process where MC1.1βⅡ converts into the less stable MC1.1α as the intermediate through a transition state (TS), in which the two pyrene sidewalls have the dihedral angle of 66.8°. This process subsequently was repeated on the other pyrene sidewall to give the other enantiomer MC1.1βⅢ. The racemization barrier was estimated to be 18.4 kcal/mol at 298 K and 1 atm. Therefore, they can undergo very fast conformational interconversion at room temperature. This relatively low barrier is consistent with the result of variable temperature (VT) 1H NMR, in which the diastereomeric ratio cannot be determined (Fig. S14 in Supporting information) [27].

|
Download:
|
| Fig. 3. The interconversion process between MC1.1α and MC1.1β and related thermal free energies was performed at the B3LYP/6–31G(d) level. The different side of the pyrene is marked with red and yellow (R is replaced by -CH3 to simplify the calculation). | |
The photophysical properties of representative macrocycles were measured (Fig. 4). Compared to acyclic fragment compound AF as a reference compound (λem = 627 nm), the emission spectrum of MC1.1 (λem = 640 nm) and MC2.1 (λem = 622 nm) were observed to have a similar emission wavelength. But the fluorescence quantum yield (ΦFL) of MC is higher than AF. Due to the larger conjugate system, the absorption spectrum (λabs = 455 nm) of MC1.1 is similar to AF (λabs = 442 nm) in 455 nm with triple intensity. Compared with macrocycle with non-aromatic linkers, such as crown ethers, cyclodextrins, calixarenes, and pillararenes, the conjugated macrocycles MC with visible light absorption and fluorescence, in which the conformational transformation with color changes makes it more practical for future application.

|
Download:
|
| Fig. 4. UV–vis absorption (solid) and fluorescence spectra (short dash) of AF (black), MC1.1 (red), MC2.1 (blue) in dichloromethane. Concentration: 1 × 10−5 mol/L. Excitation wavelength: 450 nm for AF and MC1.1; 400 nm for MC 2.1. | |
In order to better understand the geometric and electronic structures of AF, MC1.1 and MC2.1 on their optical properties, the quantum chemical calculations were performed by DFT calculations at the B3LYP/6–31G(d) level. The energy levels of the frontier molecular orbitals of AF, MC1.1α, MC1.1β, and MC2.1β are shown in Fig. 5. The HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) of these four compounds are separated. The HOMO of these four compounds mainly are delocalized over the pyrene subunits, and the LUMO of these four compounds are delocalized over maleimide and benzene units, which show the clear electron donor-acceptor structure. Compared with AF, the cyclic compounds show a lower energy of HOMO and LUMO, which means the cyclic structure could reduce the energy of LUMO to increase the reactivity [28]. Meantime the Egap (Egap = ELUMO - EHOMO) of MC1.1 also decreases to 2.55 eV compared to 2.86 eV of AF.

|
Download:
|
| Fig. 5. The frontier orbitals of AF, MC1.1α, MC1.1β and MC2.1β, and corresponding energy levels and energy gap calculated in Gaussian 09 at the B3LYP/6–31G(d) level by DFT. The structures of AF, MC1.1α, MC1.1β are optimized from the initial X-ray geometries and MC2.1 is optimized from comformational searching. unit: eV. | |
Exposed the yellow crystallite (MC1.1α) to the saturated vapor of volatile solvents, such as acetonitrile (ACN), dichloromethane (DCM), acetone, ethyl acetate (EA), tetrahydrofuran (THF), toluene (Tol) for overnight, the color of crystallite changed from yellow to red except the crystallite in the saturated vapor of ACN (Fig. 5). The reason for the color of crystallite did not change in ACN vapor might be that MC1.1α is insoluble in ACN. Checked with powder X-ray diffraction (PXRD), the crystal form has changed from α to β in red crystallite (Fig. 6). The emission wavelength has also red-shifted from 585 nm to ~640 nm in red crystallite (Fig. 6 and Fig. S19 in Supporting information). It means that the color changes only when the crystal form transforms. However, the more stable β crystal could not transform to α after many attempts, which is consistent with the DFT calculation result. The vapor of solvent could induce flipping of pyrene sidewall to give another conformation along with color change, which suggests that MC would be a prominent material for environmental management and industrial production monitoring.

|
Download:
|
| Fig. 6. The powder X-ray diffraction (PXRD) and the photographs of MC1.1α before and after adsorption different solvents. The emission wavelength of solid is marked inside. Excitation wavelength: 450 nm. | |
Considering that MC1.1 has the behavior of flipping aromatic sidewalls in the solid state, we wonder if different solvent can affect the spectra behavior of MC1.1 and AF. As shown in Fig. S16 (Supporting information), both of MC1.1 and AF exhibit quite similar absorption maxima and spectral shapes in different solvent. However, the tremendous differences in different solvent appeared in their emission spectra, the emission maximum red-shifted and the fluorescent intensity decreased with the fluorescence quantum yields decreased (Table S2 in Supporting information) when increase the solvent polarity, which indicated that MC1.1 and AF underwent a typical twisted intramolecular charge transfer (TICT) process in polar solvent. To better understand the existence of TICT process in different solvents, the dependence of the Stokes shifts (Δυ) vs. the solvent polarity paramater (Δf) was investigated, the linear relationship between Δυ and Δf in higher polar solvent suggesting that the TICT process of MC1.1 and AF could be achieved in polar solvent (Fig. S17 in Supporting information).
Besides TICT effects, it seems that MCs and AF also exhibit aggregation-induced emission (AIE) or AEE features in poor solvent. Indeed, the fluorescence spectra of the three compounds in the THF (good solvent) and water (poor solvent) mixed solvents with different water fractions were investigated. As shown in Fig. 7, AF exhibits AIE feature, and MCs exhibit AEE features. As for AF, the fluorescence intensity was gradually quenched with the increase of water fraction in the mixed solvent to 70%, and then the fluorescence intensity was gradually enhanced with the further increase of water fraction. And in the case of MC1.1 and MC1.2, with the increase of the ratio of water in the mixed solvent, the fluorescence intensity first gradually decreased and then gradually increased. This is because there is a competition between TICT and AEE effects. When water fraction is low, there is no aggregation in the mixture solvent and TICT effect is dominant which induced the fluorescence intensity quenched and emission red-shifted [29, 30]. However, when water fraction was further increased, the formation of aggregates suppressed the TICT process and enhanced the AEE effect, as the result, the fluorescence intensity enhanced and emission blue-shifted.

|
Download:
|
| Fig. 7. (a-c) The fluorescence spectra of AF, MC1.1, MC2.1 in different water fraction of mixed solvent of water-THF, respectively. (d-f) The max emission intensity of AF, MC1.1, MC2.1 in different water fraction of mixed solvent of water-THF, respectively (c = 1 × 10−5 mol/L, excitation wavelength: 450 nm for AF and MC1.1; 400 nm for MC2.1). | |
In summary, we have developed an efficient synthetic strategy to synthesize a novel conformationally adaptive macrocycle linked with four maleimide units at the corners, which has two well-defined conformations (chiral and achiral one). The interconversion barriers of these conformations were estimated by DFT calculations. The particular advantages of this new conformationally adaptive macrocycles are their long-wavelength emission, AEE emission, and vapochromic behavior. In a broader perspective, different conjugated macrocycles skeleton will be synthesized and modified with various functional group in maleimides, which will have potential application in host-guest chemistry, circularly polarized luminescence (CPL) dyes and bioimage. Further exploration in this direction is ongoing in our group.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (Nos. 21971041 and 22001039), Natural Science Foundation of Fujian Province (Nos. 2018J01431, 2018J01690 and 2020J01447), and Research Foundation of Education Bureau of Fujian Province (No. JT180813).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.003.
| [1] |
L.L. Wang, M. Quan, T.L. Yang, Z. Chen, W. Jiang, Angew. Chem. Int. Ed. 59 (2020) 23817-23824. DOI:10.1002/anie.202011566 |
| [2] |
Q. Li, H. Zhu, F. Huang, J. Am. Chem. Soc. 141 (2019) 13290-13294. DOI:10.1021/jacs.9b05054 |
| [3] |
L.L. Wang, Y.K. Tu, A. Valkonen, K. Rissanen, W. Jiang, Chin. J. Chem. 37 (2019) 892-896. DOI:10.1002/cjoc.201900233 |
| [4] |
X. Yao, J. Mu, L. Zeng, et al., Mater. Horiz. 6 (2019) 846-870. DOI:10.1039/C9MH00166B |
| [5] |
A. Blanco-Gomez, P. Corton, L. Barravecchia, et al., Chem. Soc. Rev. 49 (2020) 3834-3862. DOI:10.1039/D0CS00109K |
| [6] |
L.P. Yang, L. Zhang, M. Quan, et al., Nat. Commun. 11 (2020) 2740. DOI:10.1038/s41467-020-16534-9 |
| [7] |
T. Ogoshi, R. Shiga, T.A. Yamagishi, Y. Nakamoto, J. Org. Chem. 76 (2011) 618-622. DOI:10.1021/jo1021508 |
| [8] |
X. Wang, F. Jia, L.P. Yang, H. Zhou, W. Jiang, Chem. Soc. Rev. 49 (2020) 4176-4188. DOI:10.1039/D0CS00341G |
| [9] |
B. Odell, M.V. Reddington, A.M.Z. Slawin, et al., Angew. Chem. Int. Ed. 27 (1988) 1547-1550. DOI:10.1002/anie.198815471 |
| [10] |
Q.H. Guo, J. Zhou, H. Mao, et al., J. Am. Chem. Soc. 142 (2020) 5419-5428. DOI:10.1021/jacs.0c01114 |
| [11] |
F. Jia, Z. He, L.P. Yang, et al., Chem. Sci. 6 (2015) 6731-6738. DOI:10.1039/C5SC03251B |
| [12] |
F. Jia, H.Y. Wang, D.H. Li, L.P. Yang, W. Jiang, Chem. Commun. 52 (2016) 5666-5669. DOI:10.1039/C6CC01052K |
| [13] |
F. Jia, H. Hupatz, L.P. Yang, et al., J. Am. Chem. Soc. 141 (2019) 4468-4473. DOI:10.1021/jacs.9b00445 |
| [14] |
K. Jie, M. Liu, Y. Zhou, et al., J. Am. Chem. Soc. 140 (2018) 6921-6930. DOI:10.1021/jacs.8b02621 |
| [15] |
J. Ji, Y. Li, C. Xiao, et al., Chem. Commun. 56 (2020) 161-164. DOI:10.1039/C9CC08541F |
| [16] |
X. Zhang, X. Wang, B. Wang, Z.J. Ding, C. Li, Chin. Chem. Lett. 31 (2020) 3230-3232. DOI:10.1016/j.cclet.2020.02.037 |
| [17] |
Z. Sun, P. Sarkar, T. Suenaga, S. Sato, H. Isobe, Angew. Chem. Int. Ed. 54 (2015) 12800-12804. DOI:10.1002/anie.201506424 |
| [18] |
A. Yagi, Y. Segawa, K. Itami, J. Am. Chem. Soc. 134 (2012) 2962-2965. DOI:10.1021/ja300001g |
| [19] |
S. Reiter, M.K. Roos, R. Vivie Riedle, ChemPhotoChem 3 (2019) 881-888. DOI:10.1002/cptc.201900096 |
| [20] |
A. Yagi, G. Venkataramana, Y. Segawa, K. Itami, Chem. Commun. 50 (2014) 957-959. DOI:10.1039/C3CC48198K |
| [21] |
X. Feng, Z. Xu, Z. Hu, et al., J. Mater. Chem. C 7 (2019) 2283-2290. |
| [22] |
Q. Lu, G.K. Kole, A. Friedrich, et al., J. Org. Chem. 85 (2020) 4256-4266. DOI:10.1021/acs.joc.9b03421 |
| [23] |
X. Chang, Z. Zhou, C. Shang, et al., J. Am. Chem. Soc. 141 (2019) 1757-1765. DOI:10.1021/jacs.8b12749 |
| [24] |
P. Sarkar, F. Durola, H. Bock, Chem. Commun. 49 (2013) 7552-7554. DOI:10.1039/c3cc44044c |
| [25] |
A. Robert, P. Dechambenoit, H. Bock, F. Durola, Can. J. Chem. 95 (2017) 450-453. DOI:10.1139/cjc-2016-0585 |
| [26] |
G. Naulet, A. Robert, P. Dechambenoit, H. Bock, F. Durola, Eur. J. Org. Chem. (2018) 619-626. |
| [27] |
Y. Li, A. Yagi, K. Itami, J. Am. Chem. Soc. 142 (2020) 3246-3253. DOI:10.1021/jacs.9b13549 |
| [28] |
C. Liu, G. Yang, Y. Si, Y. Liu, X. Pan, J. Mater. Chem. C 5 (2017) 3495-3502. DOI:10.1039/C7TC00337D |
| [29] |
S. Sasaki, S. Suzuki, K. Igawa, K. Morokuma, G.I. Konishi, J. Org. Chem. 82 (2017) 6865-6873. DOI:10.1021/acs.joc.7b00996 |
| [30] |
J. Li, J. Wang, H. Li, et al., Chem. Soc. Rev. 49 (2020) 1144-1172. DOI:10.1039/C9CS00495E |
 2022, Vol. 33
2022, Vol. 33 

