b Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
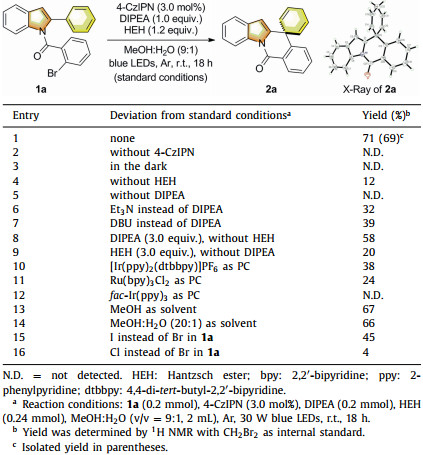
In the past decades, dearomatization reactions have been extensively studied, leading to the efficient generation of three-dimensional molecules such as spirocycles [1], which are potentially useful for drug discovery, natural product synthesis, and privileged ligand production [2]. Therefore, several strategies have been developed for dearomatizing electron-rich aromatics including phenols, naphthols, and indoles [1, 3]. By comparison, non-activated arenes such as benzene- and naphthalene-containing derivatives generally suffer from limited applicability in dearomatization methodologies due to high resonance stabilization energy of these arenes [1c, 4-6]. Recently, a number of advances in visible-light-induced dearomatization reactions were disclosed [7, 8], including several significant dearomatization protocols of non-activated arenes [9-19]. Notably, König [9] and Miyake [10] groups reported challenging Birch-type reduction of arenes promoted by visible light independently. Several dearomative hydroalkylation reactions were also achieved by the group of Zhang, Mei and You [11], as well as the groups of Stephenson [12] and Murakami [13]. Importantly, the only reductive hydroarylation of predominantly benzene derivatives via visible-light-induced dearomative spirocyclization was achieved by the Jui's group through a radical-polar crossover process (Scheme 1a) [14]. Despite these significant progresses, dearomatization of non-activated arenes to produce novel sophisticated polycyclic architectures or to avoid the use of toxic reagents (SmI2/hexamethylphosphoramide) in similar traditional transformations [20] via visible-light-induced reductive process deserves further investigation.
|
Download:
|
| Scheme 1. Visible-light-induced spirocyclizative hydroarylation of arenes. | |
Most recently, our group developed a visible-light-induced spirocyclizative remote arylcarboxylation via reductive dearomatization of non-activated arenes with CO2 through a radical-polar crossover cascade process [21]. Based on this work and inspired by Jui's results [14], we wondered whether the radical-polar crossover process could be applied for dearomative hydroarylation of corresponding non-activated arenes of our previous study. Herein, we report two novel protocols of visible-light-induced spirocyclizative hydroarylation of a series of non-activated arenes such as 2-phenyl indoles and naphthalene derivatives via reductive dearomatization under mild conditions, providing a rapid access to spirocycles with carbon−carbon double bonds that are beneficial for further transformations (Scheme 1b).
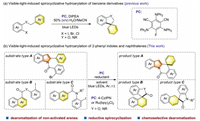
To start our investigation, bromide 1a bearing a phenyl group at the C2-position of the indole ring was selected as the model substrate. The dearomatization reaction was conducted under blue LEDs in the presence of 4-CzIPN as the photocatalyst (PC) (Table 1). After extensive evaluation of the reaction conditions, the spirocyclizative hydroarylation product 2a was obtained in 69% isolated yield by employing HEH and DIPEA as co-reductants, and MeOH: H2O (9:1) as the mixed solvent under argon for 18 h at room temperature (entry 1). Notably, it was intriguing to find 6-exo-trig cyclizative dearomatization of the non-activated 2-phenyl ring occurred selectively rather than dearomatization of the indole's ring via a 5-exo-trig cyclization at C2–C3 double bond [8]. Moreover, the main side product (about 5%) was formed via direct debromination. The structure of 2a was confirmed by X-ray crystallographic analysis. Control reactions revealed that no desired product was produced without 4-CzIPN or light, suggesting the reaction was promoted by light (entries 2 and 3). Moreover, only very low yield was obtained without HEH while using 1 equiv. of DIPEA (entry 4). And the reaction was shut down in the absence of DIPEA (entry 5). A significant decrease in the yield was observed when DIPEA was replaced by Et3N or DBU (entries 6 and 7). Notably, the yield was reduced when DIPEA was used as the sole reductant (entry 8), and only 20% yield could be received with HEH as the sole reductant (entry 9). It appeared that 4-CzIPN was the most suitable PC after comparing with other photocatalysts such as [Ir(ppy)2(dtbbpy)]PF6, Ru(bpy)3Cl2 and fac-Ir(ppy)3 (entries 10-12). A slightly lower yield of 2a was obtained when MeOH or MeOH: H2O (20:1) was used as the solvent (entries 13 and 14). Finally, the yield of 2a decreased dramatically when the iodide analog of 1a was employed (entry 15), and only trace product was detected using the chloride analog (entry 16).
|
|
Table 1 Optimization of the reaction conditions. |
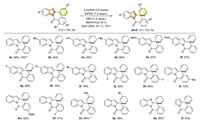
With the optimized reaction conditions in hand, the scope of 2-aryl indoles was studied (Scheme 2). First, good isolated yield (70%) was delivered with substrate 1a on a 2 mmol scale. Modest to good yields were obtained with substrates bearing electron-donating alkyl groups (2b-2d), methoxy (2e) or phenoxy (2f) group on the indole ring. Slightly better yields were received with substrates bearing halogen substituents (2g-2i). However, substitution with a fluoro group at C8 led to a lower yield of product (2j). Subsequently, the effect of substitution on the benzene ring of the 2-bromobenzoyl moiety was investigated. Pleasingly, desired products (2k-2n) could be obtained in modest to good yields with several types of substituents, though the scope was still narrow at current stage. Finally, desired products could also be delivered when a methyl or bromo group was introduced to the benzene ring at the C2-position (2o, 2p). However, other groups were not allowed on this benzene ring. Finally, desired product could be generated with a 2-phenyl benzoimidazole derivative using Ru(bpy)3Cl2 as the catalyst, albeit the yield was modest (2q), but 2-phenyl pyrrole derivative was not viable under our reaction conditions (2r). It should also be mentioned that the main side product from direct debromination of the substrates could generally be observed.
|
Download:
|
| Scheme 2. Scope of spirocyclizative hydroarylation of 2-phenyl indoles. Reaction conditions: 1 (0.2 mmol), 4-CzIPN (3.0 mol%), DIPEA (0.2 mmol), HEH (0.24 mmol), MeOH: H2O (v/v = 9:1, 2 mL), Ar, 30 W blue LEDs, r.t., 18 h. Isolated yields. 2.0 mmol scale. MeOH as the solvent. Deviation: Ru(bpy)3Cl2 (1.0 mol%), DIPEA (0.6 mmol), MeCN: H2O (v/v = 4:1, 2 mL), 24 h. N.D.: not detected. | |
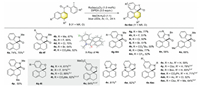
Inspired by the above results, we moved on to expand the applicability of this process by investigating other non-activated arenes such as 1-naphthalenes (Scheme 3). After modifying the reaction conditions by using Ru(bpy)3Cl2 as the PC and DIPEA as the sole reductant (see Supporting information for details), we were delighted to obtain 74% yield of desired hydroarylation product with naphthamide 3a. Moreover, the yield (70%) only decreased slightly on a larger scale with substrate 3a. Subsequently, naphthamides with a variety of substituents that were either at the meta- or para-position of the iodine atom were compatible with this transformation, leading to the desired hydroarylated products (4b-4m) in moderate to good yields (50%–77%). In addition, pyridinyl-containing substrates were also viable with this method (4n, 4v, and 4w). Methyl substituent on the naphthalenyl was also tolerated (4o and 4p). Furthermore, the versatility of this spirocyclizative hydroarylation transformation was studied with naphthalenyl ethers and desired products were obtained in good to excellent yields (4q-4w). It should be mentioned that the original products were hydrogenated before purification due to the difficulty in isolation of desired original products for examples of 4q to 4u. Finally, several aniline derivatives were also compatible with the reaction to give desired products in satisfied yields (4x-4ac). It should be noted some side products such as those from 1, 2-hydroarylation and direct de-iodination could also be detected (see section 2.1 of Supporting information).
|
Download:
|
| Scheme 3. Scope of spirocyclizative hydroarylation of 1-naphthalenes. Reaction conditions: 3 (0.2 mmol), Ru(bpy)3Cl2 (1.0 mol%), DIPEA (0.6 mmol), MeCN: H2O (v/v = 1:1, 2 mL), Ar, 30 W blue LEDs, r.t., 24 h. Isolated yields. a2.0 mmol scale. bMeCN: H2O (v/v = 4:1, 2 mL) as the solvent. cH2 (1 atm), 10% Pd/C (10 mol%), MeOH (8 mL), 40 ℃ 18 h. d48 h. | |
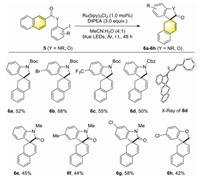
Subsequently, the generality of this hydroarylation process was further explored by employing 2-tethered naphthalenes as the substrates (Scheme 4). Pleasingly, several 2-tethered naphthalenes were found to be compatible with the reaction conditions, leading to dearomatized 1, 2-hydroarylation products (6a-6h), albeit in generally moderate yields.
|
Download:
|
| Scheme 4. Scope of spirocyclizative hydroarylation of 2-naphthalenes. Reaction conditions: 5 (0.2 mmol), Ru(bpy)3Cl2 (1.0 mol%), DIPEA (0.6 mmol), MeCN: H2O (v/v = 4:1, 2 mL), Ar, 30 W blue LEDs, r.t., 48 h. Isolated yields. Direct de-iodination side products could be detected. | |
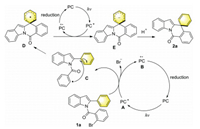
Preliminary mechanistic studies were then carried out to elucidate possible reaction mechanism (see Supporting information for details). First, the Stern-Volmer luminescence quenching experiments showed the light-activated 4-CzIPN catalyst (PC*) was readily quenched by substrate 1a. Moreover, isotope-labeling studies were also conducted, which indicated the formation of an anion intermediate. A possible catalytic cycle with substrate 1a was thus proposed based on the above mechanistic studies (Scheme 5). Upon blue light irradiation, the excited PC* (A) is generated and then quenched by 1a to give oxidized PC•+ (B) (E1/2 [PC•+/PC*] = –1.18 V vs. SCE in MeCN) [22] and corresponding aryl radical (C). Subsequently, aryl radical (C) undergoes a 6-exo-trig cyclization to afford the spirocyclic radical intermediate D, which is reduced by excited PC* (A) to generate an anionic intermediate E. Finally, protonation of E would deliver the desired dearomatized product 2a. The oxidized PC•+ (B) can be reduced by a reductant (DIPEA or HEH) to close the oxidative quenching cycle of PC.
|
Download:
|
| Scheme 5. Proposed catalytic cycle. | |
In summary, we have developed two novel protocols of visible-light-induced spirocyclizative hydroarylation of a series of non-activated aromatic precursors including 2-phenyl indoles and naphthalenes under mild conditions via reductive dearomatization. An intriguing chemoselective dearomative hydroarylation of 2-phenyl indoles was discovered. These transformations rapidly delivered valuable complex spirocycles from readily accessible non-activated arenes. Further exploration of this strategy is underway in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe gratefully thank the financial supports from the National Natural Science Foundation of China (Nos. 22022111, 21871257, 21801240), the Natural Science Foundation of Fujian Province (No. 2020J02008), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.001.
| [1] |
(a) C.X. Zhuo, W. Zhang, S.L. You, Angew. Chem. Int. Ed. 51 (2012) 12662-12686; (b) C. Zheng, S.L. You, Chem 1 (2016) 830-857; (c) W.C. Wertjes, E.H. Southgate, D. Sarlah, Chem. Soc. Rev 47 (2018) 7996-8017; (d) A.R. Pape, K.P. Kaliappan, E.P. Kündig, Chem. Rev. 100 (2000) 2917-2940. |
| [2] |
(a) Y.S. Cai, Y.W. Guo, K. Krohn, Nat. Prod. Rep. 27 (2010) 1840-1870; (b) F. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 52 (2009) 6752-6756; (c) J.H. Xie, Q.L. Zhou, Acc. Chem. Res. 41 (2008) 581-593. |
| [3] |
(a) Q.F. Wu, H. He, W.B. Liu, S.L. You, J. Am. Chem. Soc. 132 (2010) 11418-11419; (b) J. Nan, Z. Zuo, L. Luo, et al., J. Am. Chem. Soc. 135 (2013) 17306-17309; (c) C. Shen, R.R. Liu, R.J. Fan, et al., J. Am. Chem. Soc. 137 (2015) 4936-4939; (d) X. Li, B. Zhou, R.Z. Yang, et al., J. Am. Chem. Soc. 140 (2018) 13945-13951; (e) Z. Zuo, J. Wang, J. Liu, Y. Wang, X. Luan, Angew. Chem. Int. Ed. 59 (2020) 653-657; (f) R. Jiang, L. Ding, C. Zheng, S.L. You, Science 371 (2021) 380-386. |
| [4] |
P. Yang, C. Zheng, Y.H. Nie, S.L. You, Chem. Sci. 11 (2020) 6830-6835. DOI:10.1039/D0SC02816A |
| [5] |
B. Zhou, H. Wang, Z.Y. Cao, et al., Nat. Commun. 11 (2020) 4380-4839. DOI:10.1038/s41467-020-18137-w |
| [6] |
(a) B. Peng, S. Zhang, X. Yu, X. Feng, M. Bao, Org. Lett. 13 (2011) 5402-5405; (b) Z.P. Yang, R. Jiang, Q.F. Wu, et al., Angew. Chem. Int. Ed. 57 (2018) 16190-16193; (c) T. Ito, S. Harada, H. Homma, et al., J. Am. Chem. Soc. 143 (2021) 604-611; (d) M. Chen, X. Wang, Z.H. Ren, Z.H. Guan, CCS Chem. 3 (2021) 69-77. |
| [7] |
(a) M. Okumura, D. Sarlah, Eur. J. Org. Chem. (2020) 1259-1273; (b) W.C. Yang, M.M. Zhang, J.G. Feng, Adv. Synth. Catal. 362 (2020) 4446-4461; (c) Y. Chen, L.Q. Lu, D.G. Yu, C.J. Zhu, W.J. Xiao, Sci. China Chem. 62 (2018) 24-57; (d) Q.Q. Zhou, Y.Q. Zou, L.Q. Lu, W.J. Xiao, Angew. Chem. Int. Ed. 58 (2019) 1586-1604; (e) B. Hu, Y. Li, W. Dong, et al., Chem. Commun. 52 (2016) 3709-3712; (f) M.J. James, J.L. Schwarz, F. Strieth-Kalthoff, B. Wibbeling, F. Glorius, J. Am. Chem. Soc. 140 (2018) 8624-8628; (g) Q. Guo, M. Wang, H. Liu, R. Wang, Z. Xu, Angew. Chem. Int. Ed. 57 (2018) 4747-4751; (h) N. Hu, H. Jung, Y. Zheng, et al., Angew. Chem. Int. Ed. 57 (2018) 6242-6246; (i) M. Zhu, C. Zheng, X. Zhang, S.L. You, J. Am. Chem. Soc. 141 (2019) 2636-2644; (j) M. Zhu, X.L. Huang, H. Xu, et al., CCS Chem 2 (2020) 652-664; (k) L. Wu, Y. Hao, Y. Liu, H. Song, Q. Wang, Chem. Commun. 56 (2020) 8436-8439; (l) J. Ma, F. Schäfers, C. Daniliuc, et al., Angew. Chem. Int. Ed. 59 (2020) 9639-9645. |
| [8] |
W.J. Zhou, Z.H. Wang, L.L. Liao, et al., Nat. Commun. 11 (2020) 3263-3271. DOI:10.1038/s41467-020-17085-9 |
| [9] |
A. Chatterjee, B. König, Angew. Chem. Int. Ed. 58 (2019) 14289-14294. DOI:10.1002/anie.201905485 |
| [10] |
J. Cole, D.F. Chen, M. Kudisch, et al., J. Am. Chem. Soc. 142 (2020) 13573-13581. DOI:10.1021/jacs.0c05899 |
| [11] |
Y.Z. Cheng, X.L. Huang, W.H. Zhuang, et al., Angew. Chem. Int. Ed. 59 (2020) 18062-18067. DOI:10.1002/anie.202008358 |
| [12] |
R.C. McAtee, E.A. Noten, C.R.J. Stephenson, Nat. Commun. 11 (2020) 2528-2535. DOI:10.1038/s41467-020-16369-4 |
| [13] |
Y. Masuda, H. Tsuda, M. Murakami, Angew. Chem. Int. Ed. 60 (2021) 3551-3555. DOI:10.1002/anie.202013215 |
| [14] |
A.R. Flynn, K. McDaniel, M. Hughes, D. Vogt, N.T. Jui, J. Am. Chem. Soc. 142 (2020) 9163-9168. DOI:10.1021/jacs.0c03926 |
| [15] |
W. Dai, S.J. Geib, D.P. Curran, J. Am. Chem. Soc. 142 (2020) 6261-6267. DOI:10.1021/jacs.0c00490 |
| [16] |
E.H. Southgate, J. Pospech, J. Fu, D.R. Holycross, D. Sarlah, Nat. Chem. 8 (2016) 922-928. DOI:10.1038/nchem.2594 |
| [17] |
S. Stegbauer, C. Jandl, T. Bach, Angew. Chem. Int. Ed. 57 (2018) 14593-14596. DOI:10.1002/anie.201808919 |
| [18] |
V.K. Soni, H.S. Hwang, Y.K. Moon, et al., J. Am. Chem. Soc. 141 (2019) 10538-10545. DOI:10.1021/jacs.9b05572 |
| [19] |
W. Dong, Y. Yuan, X. Xie, Z. Zhang, Org. Lett. 22 (2020) 528-532. DOI:10.1021/acs.orglett.9b04283 |
| [20] |
H. Iwasaki, T. Eguchi, N. Tsutsui, H. Ohno, T. Tanaka, J. Org. Chem. 73 (2008) 7145-7152. DOI:10.1021/jo800656a |
| [21] |
Y. Gao, H. Wang, Z. Chi, et al., CCS Chem. 3 (2021) 1848-1859. |
| [22] |
E. Speckmeier, T.G. Fischer, K. Zeitler, J. Am. Chem. Soc. 140 (2018) 15353-15365. DOI:10.1021/jacs.8b08933 |
 2022, Vol. 33
2022, Vol. 33