b School of Pharmacy, Nantong University, Nantong 226001, China
Chalcogen elements (i.e., S, Se, Te) possess distinctive chemical properties owing to their large atom radiuses leading to the weak binding of the outlier electrons. Their compounds have been extensively applied in organic synthesis [1], materials science [2] and catalysis [3] due to the resulting unique chemical activities. In the field, selenium chemistry is unfolding during the past decade [4]. Selenium-containing compounds and materials are generally bioactive and may be used as the antitumor [5], antibacterial [6] and antivirus [7] reagents in medicine and biocide development. Because selenium is a necessary trace element for human beings and can be metabolized in the body [8], it is even safer than most of the transition metals [9]. Some organoselenium compounds are relatively low toxic and even non-toxic, such as diphenyl diselenide and ebselen [10]. For example, the acutely lethal dose (LD50) for diphenyl diselenide in rats treated intraperitoneally is 400 µmol/kg, while the value for ebselen is 1200 µmol/kg [11]. These results demonstrate that the organoselenium compounds and materials may represent a new class of safe reagents for antitumor and antibacterial purposes in medicine development projects.
In the past few decades, microbial pathogens that cause substantial public health threats have rapidly increased in the world, such as some bacteria-borne diseases including endocarditis, tuberculosis and rhino-sinusitis [12]. The antibacterial materials have contributed to the development of modern medicine and been utilized in the field of medicine, such as making them into surgical sutures, cardiovascular stents, artificial joints and artificial blood vessels, which play a crucial role in reducing the mortality of cardiovascular diseases, cancer, trauma and other major diseases, improving the quality of life and decreasing medical costs [13]. However, some common antibacterial materials, such as silver nanoparticles (AgNPs), show high cytotoxicity or low biocompatibility in physiological conditions [14]. In this way, the off shelf of such products brings out additional market opportunities for novel antibacterial materials. Therefore, developing the related new products safe to the environments to reduce these unsuitable effects and fill in the resulted market gap is highly demanded in recent years.
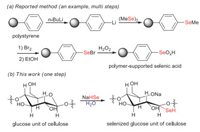
The selenium-containing materials may be good alternatives of nano-silver for their good bio-compatible features as well as the acceptable price of the element of Se. However, complex procedures for synthesizing these materials obviously limit their application scopes. For example, the preparation of polystyrene-supported selenium suffers a series of complex steps such as the lithiation, selenization, bromination and oxidation reactions, and the procedures led to a lot of wastes further impeding the large-scale applications of the related techniques (Scheme 1a) [15]. Reduction of selenium powder with NaBH4 in EtOH leads to NaHSe, which is a very strong nucleophile and could attack the positive centre of the materials to upload selenium [16]. The technique has been successfully applied in the industrial production of selenized glucose at kilogram scale [16e]. Recently, we successfully developed the reaction in aqueous solution and applied the technique in the production of selenium-containing antibacterial cotton products for medical use (Scheme 1b). Herein, we wish to report our findings.
|
Download:
|
| Scheme 1. Comparison of the preparation methods for selenium-containing materials. | |
Full cotton socks were chosen as the examples of cotton products and they were purchased from the supermarket. The selenization reagent NaHSe could be prepared via the reduction of selenium powder by NaBH4 in EtOH (Eq. 1) [16].
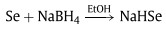
|
(1) |
The NaHSe solution in EtOH was diluted by water and the cotton socks were immersed in it to upload selenium via the mechanisms illustrated in Scheme 1b (Fig. 1a). After then, they were washed by water and dried by airing. Inductively coupled plasma-mass spectrometry (ICP-MS) analysis indicated that the selenized material contained ca. 0.15% of Se (weight content). Scanning electron microscope (SEM) was used to investigate the micro-morphologies of the materials. It was found that the selenization process did not destroy the surface structures of the materials, as the morphologies observed in the SEM images of the selenized and unselenized sock were similar (Figs. 1d and e vs. 1b and c). Fourier transform infrared (FT-IR) analysis was employed to characterize the functional groups of the materials (Fig. 1f). In the IR spectrum of unselenized cotton sock (Fig. 1f, blue line), the characterization peaks of cellulose can be observed nearing the 708 cm−1 wave number position. The peaks at 1058 cm−1, 1431 cm−1 and 2902 cm−1 attribute to the C-O-C, C-O-H and the CH2 and CH stretching vibrations, respectively. The above peaks did not change after selenization (Fig. 1f, red line vs. blue line). The stretching vibration of O-H is reflected by the absorption peak at 3297 cm−1. The signal moved to 3339 cm−1 after selenization due to the interaction of the introduced selenium groups, which affected the hydroxyl by weakening the hydrogen bonds of the materials (Fig. 1f, red line vs. blue line). Generally, the FT-IR spectrum of selenized sock did not change much, verifying that the selenization process did not cause significant damage to the functional groups of the cellulose of cotton socks. In the X-ray diffraction (XRD) pattern of unselenized sock (Fig. 1g, black line), the signal peaks at 2θ = 14.5o, 16.6o, 22.5o and 34.4o are the typical crystallization peaks of natural cellulose attributing the facets of (110), (110), (200) and (040) respectively and these results are in accordance to the structures of the materials [17]. The selenization process damaged the crystal structures of the materials, as being reflected by the weakened diffraction peaks in the XRD pattern of selenized sock (Fig. 1g, red line vs. black line). However, tension tests showed that the mechanical properties of the materials did not change much after selenization (for details see Supporting information).

|
Download:
|
| Fig. 1. Photographs and spectra: (a) Selenization of cotton socks; (b, c) SEM images of the unselenized sock; (d, e) SEM images of the selenized sock; (f) FT-IR spectra of the unselenized (blue line) and selenized (red line) socks; (g) XRD spectra of the unselenized (black line) and selenized (red line) socks. | |
Transmission electron microscope (TEM) image of the selenized sock demonstrates that selenium is not uniformly dispersed on the surface of the material (Fig. 2a). The crystal plane spacing of the facet (200) of crystal cellulose can be determined to be 0.40 nm in its high resolution transmission electron microscope (HR-TEM) image (Fig. 2b). The energy dispersive X-ray spectroscopy (EDX) attests the successful loading of selenium, while the sodium and boron elements have also been incorporated due to the selenization technique using NaBH4 as reductant (Fig. 2c). In the high-angle annular dark field scanning transmission electron microscope (HAADF-STEM) image as Z-contrast image (Fig. 2d), the bright area is attributed to the uploaded selenium because the image is highly sensitive to variations in the atomic number of atoms in the sample, and the Z contrast intensity is proportional to the square of the atomic number [18].

|
Download:
|
| Fig. 2. Composition analysis of the selenized cotton sock: (a) TEM image; (b) HR-TEM image; (c) EDX spectrum; (d) HAADF-STEM image; (e-j) elemental mapping images of Se (e), C (f), N (g), O (h), B (i) and Na (j); (k) XPS spectrum. | |
Elemental mapping analysis was performed to confirm the constituent of the material and the results were given in Figs. 2e–j. The selenium distribution is in accordance with the result of the HAADF-STEM image (Fig. 2e), while carbon formed the major skeleton of the material (Fig. 2f). Nitrogen as a widely involved element in natural products is also observed in the material (Fig. 2g). Interestingly, oxygen dispereses in the areas lack of selenium (Fig. 2h), attesting that selenium has been introduced into the material by replacing oxygen. Moreover, boron and sodium as the impurities introduced during the selenization process are also observed (Figs. 2i and j). The X-ray photoelectron spectroscopy (XPS) studies attested that selenium in the material was in the Se2− and Se− form (Fig. 2k), and the later demonstrated a slight oxidation of the species leading to Se-Se, which attributed to the red color of the selenization liquid (Fig. 1a).
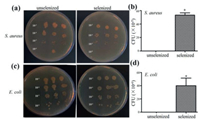
Gram-positive bacterium (S. aureus) and Gram-negative bacterium (E. coli) were used as models for studying the antibacterial activities of the unselenized and selenized socks. After overnight culturing in a 37 ℃ incubator, some viable colonies were observed on the agar plate. As photos shown in Fig. 3, the selenized socks had fewer colonies than the unselenized socks, indicating that the selenized socks had a certain degree of antibacterial effects. The percentage of bacterial inhibition of the selenized socks were 52.15% and 40% against S. aureus and E. coli in comparison with the unselenized socks, respectively. The results suggested that the selenized socks showed high bacteriostatic activity against Gram-positive bacteria. Low activity of the selenized socks against Gram-negative bacteria can be explained by different components of the cell wall of Gram-positive and Gram-negative bacteria. As Gram-positive species, the cell wall of S. aureus is composed of many layers of peptidoglycan that may be susceptible to the selenized socks. On the other hand, as Gram-negative species, E. coli consists of a dense lipopolysaccharide-lipoprotein layer which covers a thin peptidoglycan layer. Therefore, the protective effect of lipopolysaccharide-lipoprotein complexes for E. coli may reduce the antibacterial effect of the selenized socks [19].
|
Download:
|
| Fig. 3. In vitro antibacterial experiments. (a, b) Photographs of S. aureus colonies of the unselenized and selenized socks, and the bacterial inhibition (%) of unselenized and selenized socks at a concentration of 10-5–fold. (c, d) Photographs of E. coli colonies of the unselenized and selenized socks, and the bacterial inhibition (%) of unselenized and selenized socks at a concentration of 10-6–fold. Statistical significance at P < 0.05. | |
In conclusion, immersing the cotton products in the aqueous solution of the in situ generated NaHSe could easily upload the selenium species. The selenized materials were bioactive and could well restrain bacteria. Materials characterizations showed that the structures of the materials were not damaged during the processes. In comparison with the reported works of synthesizing selenium-containing polymers, this is an obvously concise, low-cost and efficient method and is fit for practical applications. The aqueous treating processes are safe and environment-friendly for large scale production [20]. The related products may be very useful for the travelers in long term field tour, which did not allow frequent washing of the wearings. Development for commercialization purpose is ongoing.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis work was financially supported by the Natural Science Foundation of Jiangsu Province (Nos. BK20190909, BK20181449), Jiangsu Provincial Six Talent Peaks Project (No. XCL-090) and Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank Jian Liu and Sichuan Selewood Technology Company Limited for support and assistances in the related product development.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.061.
| [1] |
(a) Y. Wu, J. Chen, J. Ning, et al., Green Chem. 23 (2021) 3950-3954; (b) Y. Wu, J. Chen, H. Liao, et al., Green Synth. Catal. 2 (2021) 233-236; (c) J. Chen, H. Wu, Q. Gui, et al., Chin. J. Catal. 42 (2021) 1445-1450; (d) J. Chen, C. Zhong, Q. Gui, et al., Chin. Chem. Lett. 32 (2021) 475-479; (e) J. Jiang, F. Xiao, W. He, et al., Chin. Chem. Lett. 32 (2021) 1637-1644; (f) L. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240; (g) C. Wu, L. Hong, H. Shu, et al., ACS Sustain. Chem. Eng. 7 (2019) 8798-8803; (h) Z. Cao, Q. Zhu, Y. Lin, et al., Chin. Chem. Lett. 30 (2019) 2132-2138; (i) M.X. Liu, Y.M. Li, L. Yu, et al., Sci. China Chem. 61 (2018) 294-299. |
| [2] |
(a) L. Yu, H. Cao, X. Zhang, et al., Sustain. Energy Fuels 4 (2020) 730-736; (b) X. Zhao, L. Yin, Z. Yang, et al., J. Mater. Chem. A 7 (2019) 21774-21782; (c) K. Cao, X. Deng, T. Chen, et al., J. Mater. Chem. A 7 (2019) 10918-10923. |
| [3] |
(a) C. Chen, Z. Cao, X. Zhang, et al., Chin. J. Chem. 38 (2020) 1045-1051; (b) C. Chen, Y. Cao, X. Wu, et al., Chin. Chem. Lett. 31 (2020) 1078-1082; (c) X. Deng, H. Cao, C. Chen, et al., Sci. Bull. 64 (2019) 1280-1284; (d) Y. Zheng, A. Wu, Y. Ke, et al., Chin. Chem. Lett. 30 (2019) 937-941. |
| [4] |
(a) F.V. Singh, T. Wirth, Catal. Sci. Technol. 9 (2019) 1073-1091; (b) T. Prochnow, A. Maroneze, D.F. Back, et al., J. Org. Chem. 84 (2019) 2891-2900; (c) R. Guo, L. Liao, X. Zhao, Molecules 22 (2017) 835; (d) S. Kodama, T. Saeki, K. Mihara, et al., J. Org. Chem. 82 (2017) 12477-12484; (e) C. Santi, C. Tomassini, L. Sancineto, Chimia 71 (2017) 592-595; (f) S. Santoro, J.B. Azeredo, V. Nascimento, et al., RSC Adv. 4 (2014) 31521-31535; (g) L. Yu, Y. Wu, T. Chen, et al., Org. Lett. 15 (2013) 144-147; (h) D.M. Freudendahl, S. Santoro, S.A. Shahzad, et al., Angew. Chem. Int. Ed. 48 (2009) 8409-8411. |
| [5] |
X. Song, Y. Chen, G. Zhao, et al., Carbohydr. Polym. 231 (2020) 115689. DOI:10.1016/j.carbpol.2019.115689 |
| [6] |
(a) X. Mao, P. Li, T. Li, et al., Chin. Chem. Lett. 31 (2020) 3276-3278; (b) H. Cao, Y. Yang, X. Chen, et al., Chin. Chem. Lett. 31 (2020) 1887-1889; (c) P.S. Sadalage, M.S. Nimbalkar, K.K.K. Sharma, et al., J. Colloid Interface Sci. 569 (2020) 346-357; (d) J. Yip, L. Liu, K.H. Wong, et al., J. Appl. Polym. Sci. 131 (2014) 40728. |
| [7] |
(a) M.A. Beck, Proc. Nutr. Soc. 58 (1999) 707-711; (b) L. Yu, L. Sun, Y. Nan, et al., Biol. Trace Elem. Res. 141 (2011) 254-261. |
| [8] |
M. Rayman, Lancet 379 (2012) 1256-1268. DOI:10.1016/S0140-6736(11)61452-9 |
| [9] |
US Pharmacopeial Convention (USP). USP < 232>Elemental Impurities-Limits. 40-NF 35, First Supplement, 2017.
|
| [10] |
J. Młochowski, H. Wójtowicz-Młochowska, Molecules 20 (2015) 10205-10243. DOI:10.3390/molecules200610205 |
| [11] |
F.C. Meotti, V.C. Borges, G. Zeni, et al., Toxicol. Lett. 143 (2003) 9-16. DOI:10.1016/S0378-4274(03)00090-0 |
| [12] |
X. Zhang, L. Liu, L. Huang, et al., Nanoscale 11 (2019) 9468-9477. DOI:10.1039/C9NR01284B |
| [13] |
(a) S. Ruengdechawiwat, J. Siripitayananon, R. Molloy, et al., Int. J. Polym. Mater. Po. 65 (2016) 277-284; (b) Y. Wei, Y. Ji, L. Xiao, et al., Biomaterials 34 (2013) 2588-2599; (c) L. Zhang, W. Zhang, Y. Han, et al., Appl. Surf. Sci. 361 (2016) 141-149; (d) A.F. Leitão, M.A. Faria, A.M. Faustino, et al., Macromol. Biosci. 16 (2016) 139-150. |
| [14] |
M.B. Faiz, R. Amal, C.P. Marquis, et al., Nanotoxicology 12 (2018) 263-273. DOI:10.1080/17435390.2018.1434910 |
| [15] |
Y. Wang, L. Yu, B. Zhu, et al., J. Mater. Chem. A 4 (2016) 10828-10833. DOI:10.1039/C6TA02566H |
| [16] |
(a) X. Jing, C. Chen, X. Deng, et al., Appl. Organomet. Chem. 32 (2018) e4332; (b) Y. Yang, X. Fan, H. Cao, et al., Catal. Sci. Technol. 8 (2018) 5017-5023; (c) S. Chu, H. Cao, T. Chen, et al., Catal. Commun. 129 (2019) 105730; (d) C. Liu, J. Mao, X. Zhang, et al., Catal. Commun. 133 (2020) 105828; (e) W. Zhou, P. Li, J. Liu, et al., Ind. Eng. Chem. Res. 59 (2020) 1025-1029; (f) H. Cao, R. Ma, S. Chu, et al., Chin. Chem. Lett. 32 (2021) 2761-2764; (g) X. Xiao, Z. Shao, L. Yu, Chin. Chem. Lett. 32 (2021) 2933-2938. |
| [17] |
S. Nam, M.B. Hillyer, B.D. Condon, Carbohydr. Polym. 228 (2020) 115374. DOI:10.1016/j.carbpol.2019.115374 |
| [18] |
(a) K. Sohlberg, T.J. Pennycook, W. Zhou, et al., Phys. Chem. Chem. Phys. 17 (2015) 3982-4006; (b) A. Genc, L. Kovarik, M. Gu, et al., Ultramicroscopy 131 (2013) 24-32; (c) S.J. Pennycook, Ultramicroscopy 123 (2012) 28-37. |
| [19] |
B. Żołnowska, J. Sławiński, K. Garbacz, et al., Int. J. Mol. Sci. 21 (2019) 210. DOI:10.3390/ijms21010210 |
| [20] |
(a) H. Zhang, M. Han, C. Yang, et al., Chin. Chem. Lett. 30 (2019) 263-265; (b) S. Peng, Y. Song, J. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (c) L. Xie, S. Peng, J. Tan, et al., ACS Sustain. Chem. Eng. 6 (2018) 16976-16981; (d) L. Xie, Y. Li, J. Ou, et al., Green Chem. 19 (2017) 5642-5646. |
 2022, Vol. 33
2022, Vol. 33 

