o-Carborane is an electron-deficient icosahedral boron cluster compound of formula C2B10H12, in which two carbon atoms are adjacent to each other. o-Carborane has 26 delocalized valence electrons, exhibits special three-dimensional aromaticity, and extraordinary chemical and thermal stability [1, 2]. The different electronegativity between carbon and boron makes the C-H bond of o-carboranes partially acidic, which can work as a useful reaction site to obtain functionalized o-carboranes. During the past few decades, o-carboranes and their derivatives have broad applications in many fields [3-19]. Among them, some C-alkynyl-o-carboranes have special luminochromism and can be used in optoelectronic functional materials as electron-accepting motifs to tune the LUMO and HOMO energy levels [20-25]. Some of them have remarkable aggregation-induced emission (AIE) property and/or stimuli-responsivity and/or environment-sensitivity [26-28] with potential for application in novel functional materials. In addition, alkynyl groups are fundamental structural units in organic synthesis, and can be easily further derivatized [29-33]. Thus, the synthesis and performance of C-alkynyl-o-carboranes have received considerable attention.
Currently, there are mainly three methods for the synthesis of C-alkynyl-o-carboranes. In 1964, Dupont and Hawthorne synthesized C-alkynyl-o-carboranes from decaborane and corresponding diynes for the first time (Scheme 1a) [34, 35]. In 1973, Hawthorne modified the method [36, 37]. In 1976, Zakharkin and coworkers prepared C-alkynyl-o-carboranes by the reaction of bromoalkynes with 1-Cu-o-C2B10H11 to give C-alkynyl-o-carboranes in moderate yields. 1-Cu-o-C2B10H11 was obtained from the corresponding 1-carboranyllithium and 1.25 equiv. of CuCl in a THF-ether solution (Scheme 1b) [38, 39]. In 2013, Nie and co-workers reported the cross-coupling of 1-Cu-o-C2B10H11 and Cu-CC-R to give C-alkynyl-o-carboranes with more excess amount of n-BuLi and CuCl (4 equiv. respectively, Scheme 1b) [40]. For a long period of time, C-alkynyl-o-carboranes were synthesized by these methods, the use of hypertoxic decaborane and diynes or stochiometric amounts of transition-metal salts encumbers their broader applications. Very recently, Xie and coworkers reported a very efficient approach to synthesize C-alkynyl-o-carboranes by the reaction of iodocarboranes and terminal alkynes in the presence of base under UV-light (Scheme 1c) [41]. However, this method is more suitable for o-carborane with substituents such as methyl on the ortho position. Until now, there are only a few reported mono-C-alkynyl substituted o-carboranes and the methods for synthesizing those mono-C-functional carboranes are still very limited. Therefore, it is of great significance to develop simple and efficient methods to synthesize mono-C-alkynyl-o-carboranes.

|
Download:
|
| Scheme 1. Synthesis of C-alkynyl-o-carboranes. | |
Sulfones are important intermediates in synthetic applications due to their strong electron-withdrawing property. Alkynyl sulfones have broad applications in the alkynylation and other fields including building complex organic molecules or naturally occurring products [42-47]. They could be facilely obtained from varied synthetic methods [42, 48-52]. In 2012, García Ruano's group reported a strategy that acetylenic sulfones were used as alkynylating reagents for the construction of the CAr-Csp bonds [53-55]. Inspired by these results, we wonder if arylacetylenic sulfones could be used to synthesize mono-C-alkynyl-o-carboranes. It should be noted that the carbon of o-carborane is sp hybridized, and the alkynylation of Csp with acetylenic sulfones is unknown. Herein, we wish to report our findings toward the construction of various mono-C-alkynyl-o-carboranes from o-carboranyllithiums and arylacetylenic sulfones (Scheme 1d).
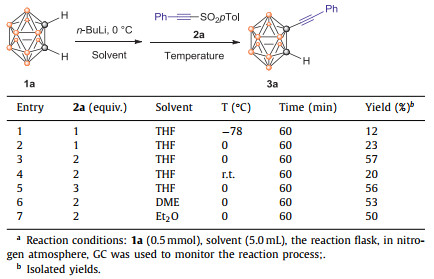
The starting arylacetylenic sulfones were synthesized according to a modified literature's procedure [48-52]. At the outset of our studies, o-carborane 1a and phenylethynyl sulfone 2a were chosen as model substrates to optimize the reaction conditions (Table 1). Initially, n-BuLi (1.2 equiv.) was added dropwise to o-carborane 1a in THF at 0 ℃, and the reaction mixture was stirred for an hour. Then phenylethynyl sulfone 2a (1 equiv.) was added and the reaction mixture was stirred for another hour at −78 ℃, the desirable product 3a was obtained with an isolated yield of 12% and some 1a was recovered (Table 1, entry 1). When the reaction temperature at the second step was kept at 0 ℃, the reaction can be finished within 2 h also, but the isolated yield of 3a is only 23% (Table 1, entry 2). If 2 equiv. of 2a was used, the reaction can be finished in 2 h also, and the yield of 3a is up to 57% (Table 1, entry 3), Adding 3 equiv. of 2a did not improve the yield and the reaction was worse when it was run at room temperature (Table 1, entries 4 and 5). At last, solvent effects on the reaction were studied, dimethyl ether (DME) and Et2O can also give comparable yields (Table 1, entries 6 and 7).
|
|
Table 1 Optimization of the reaction conditions for the synthesis of 3aa. |
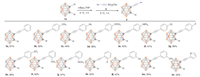
After establishing the optimized conditions (Table 1, entry 3), we examined the substrate scope and limitation of this alkynylation reaction and the results are summarized in Scheme 2. Gratifyingly, a variety of arylacetylenic sulfones 2a-2n were smoothly coupled to o-carborane 1a, delivering the corresponding mono-C-alkynyl-o-carboranes 3a-3n in moderate yields. This reaction tolerated a wide variety of functional groups, such as Me or Ph, electron-donating groups OMe, NPh2 or electron-withdrawing groups F, Cl and CF3. Moreover, the electronic properties of the substituents have no significant effect on the products yield. The positions of substituents on the Phenyl ring have no obvious impact on the yield also. In addition, alkyne with heteroaryl was compatible with this reaction, affording the product in a relatively lower yield (3n, 32%). The obtained products 3a–3n were characterized by 1H NMR, 13C NMR, 11B NMR and HRMS (Supporting information).
|
Download:
|
| Scheme 2. Synthesis of mono-C-alkynyl-o-carboranes. | |
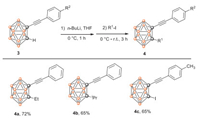
1, 2-Difunctionalized o-carboranes have some unique photoelectric properties [56-60]. After successful preparation of a variety of mono-C-alkynyl-o-carboranes, we turned our attention to synthesize 1, 2-difunctionalized o-carboranes. Unfortunately, the attempt to synthesize the bisalkynylation compound under the similar method was failed. But the alkylation and iodination reactions proceeded smoothly and provided the corresponding difuncationalized o-carboranes 4a and 4b and C-alkynyl-C'-iodocarborane 4c in 65%−72% yields (Scheme 3). Even iodoalkane bearing the hindered isopropyl group proved to be effective for furnishing the product 4b in 65% yield. The further derivatization reaction of C-I in compound 4c and applications of the new mono-C-alkynyl-o-carboranes are currently in progress in our laboratory.
|
Download:
|
| Scheme 3. Synthesis of 1, 2-difunctionalized o-carboranes. | |
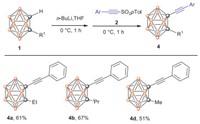
To investigate the utility of this synthetic method, methyl, ethyl and isopropyl substituted o-carboranes were synthesized according the literature [61]. When R1 is methyl or ethyl or isopropyl, 4d, 4a and 4b were obtained in yields of 51%, 61% and 67% respectively (Scheme 4). It demonstrates the new synthetic method is applicable with o-substituted o-carboranes also.
|
Download:
|
| Scheme 4. Ortho-substituted o-carborane alkynyl functionali-zation. | |
In summary, a facile synthetic route to mono-C-alkynyl-o-carboranes from o-carboranes and arylsulfonylacetylenes was developed. This new method tolerates a wide variety of functional groups, and the process occurs at mild conditions in one-pot procedure with short reaction time. The obtained mono-C-alkynyl-o-carboranes can be further derivatized to synthesize 1, 2-difunctionalized o-carboranes. This work provides a very useful tool for the functionalization and practical applications of o-carboranes.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsWe are grateful for financial support from the National Natural Science Foundation of China (Nos. 21672193, 21272218), the Key Scientific and Technological Project of Henan Province (No. 202102310327), the Ministry of Industry and Information Technology (No. Z135060009002), the Postdoctoral Research Grant in Henan Province (No. 001803004), the Programme of Introducing Talents of Discipline to Universities (111 Project, No. D20003) and Zhengzhou University of China.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.062.
| [1] |
R.N. Grimes, Carboranes. 3rd. Amsterdam: Academic Press, 20166.
|
| [2] |
M.F. Hawthorne, Z. Zheng, Acc. Chem. Res. 30 (1997) 267-276. DOI:10.1021/ar9501479 |
| [3] |
Q.Y. Wang, J. Wang, S. Wang, et al., J. Am. Chem. Soc. 142 (2020) 12010-12014. DOI:10.1021/jacs.0c04638 |
| [4] |
M. Scholz, E. Hey-Hawkins, Chem. Rev. 111 (2011) 7035-7062. DOI:10.1021/cr200038x |
| [5] |
D. Tu, P. Leong, S. Guo, et al., Angew. Chem. Int. Ed. 56 (2017) 11370-11374. DOI:10.1002/anie.201703862 |
| [6] |
M. Gon, K. Tanaka, Y. Chujo, Polym. J. 50 (2018) 109-126. DOI:10.1038/pj.2017.56 |
| [7] |
J. Guo, D. Liu, J. Zhang, et al., Chem. Commun. 51 (2015) 12004-12007. DOI:10.1039/C5CC03608A |
| [8] |
J. Zhang, K. Liu, Z. Liu, et al., ACS Appl. Mater. Interfaces. 13 (2021) 5625-5633. DOI:10.1021/acsami.0c21424 |
| [9] |
X. Wu, J. Guo, Y. Quan, et al., J. Mater. Chem. C 6 (2018) 4140-4149. DOI:10.1039/C8TC00380G |
| [10] |
J. Zhang, C. Tang, Z. Xie, Chem. Sci. 11 (2020) 9925-9929. DOI:10.1039/D0SC04465B |
| [11] |
J.A. Dupont, M.F. Hawthorne, J. Am. Chem. Soc. 86 (1964) 1643. DOI:10.1021/ja01062a042 |
| [12] |
L.I. Zakharkin, A.I. Kovredov, Russ. Chem. Bull. 25 (1976) 1593. DOI:10.1007/BF00920867 |
| [13] |
D. Bian, Y. Nie, J. Miao, Y. Wang, Z. Zhang, Chin. J. Org. Chem. 33 (2013) 1774-1781. DOI:10.6023/cjoc201302007 |
| [14] |
H. Ni, Z. Lu, Z. Xie, J. Am. Chem. Soc. 142 (2020) 18661-18667. DOI:10.1021/jacs.0c08652 |
| [15] |
M.A. Guerrero-Robles, M.A. Vilchis-Reyes, E.M. Ramos-Rivera, C. Alvarado, ChemistrySelect. 4 (2019) 13698-13708. DOI:10.1002/slct.201903728 |
| [16] |
J. Meesin, P. Katrun, C. Pareseecharoen, et al., J. Org. Chem. 81 (2016) 2744-2752. DOI:10.1021/acs.joc.5b02810 |
| [17] |
J.L. García Ruano, J. Alemán, L. Marzo, et al., Angew. Chem. Int. Ed. 51 (2012) 2712-2716. DOI:10.1002/anie.201107821 |
| [18] |
H. Naito, K. Nishino, Y. Morisaki, K. Tanaka, Y. Chujo, Chem. Asian J. 12 (2017) 2134-2138. DOI:10.1002/asia.201700815 |
| [19] |
F.A. Gomez, S.E. Johnson, M.F. Hawthorne, J. Am. Chem. Soc. 113 (1991) 5915-5917. DOI:10.1021/ja00015a086 |
| [20] |
N.S. Hosmane, Boron Science: New Technologies and Application. Boca Raton, FL: CRC Press, 2011.
|
| [21] |
A. Kataki-Anastasakou, J.C. Axtell, S. Hernandez, et al., J. Am. Chem. Soc. 142 (2020) 20513-20518. DOI:10.1021/jacs.0c09361 |
| [22] |
X. Yang, Y. Zhang, B. Zhang, et al., J. Mater. Chem. C 8 (2020) 16326-16332. DOI:10.1039/D0TC04603E |
| [23] |
A.R. Popescu, F. Teixidor, C. Viñas, Coord. Chem. Rev. 269 (2014) 54-84. DOI:10.1016/j.ccr.2014.02.016 |
| [24] |
J.F. Valliant, K.J. Guenther, A.S. King, et al., Coord. Chem. Rev. 232 (2002) 173-230. DOI:10.1016/S0010-8545(02)00087-5 |
| [25] |
C. Viñas, R. Núñez, I. Bennour, F. Teixidor, Curr. Med. Chem. 26 (2019) 5036-5076. DOI:10.2174/0929867326666190603123838 |
| [26] |
X. Yang, B. Zhang, S. Zhang, et al., Org. Lett. 21 (2019) 8285-8289. DOI:10.1021/acs.orglett.9b03047 |
| [27] |
G. Tao, F. Yang, L. Zhang, et al., Chin. Chem. Lett. 32 (2021) 194-197. DOI:10.1016/j.cclet.2020.11.018 |
| [28] |
R. Núñez, M. Tarrés, A. Ferrer-Ugalde, F.F. de Biani, F. Teixidor, Chem. Rev. 116 (2016) 14307-14378. DOI:10.1021/acs.chemrev.6b00198 |
| [29] |
R. Huang, H. Liu, K. Liu, et al., Anal. Chem. 91 (2019) 14451-14457. DOI:10.1021/acs.analchem.9b03096 |
| [30] |
H. Naito, K. Nishino, Y. Morisaki, K. Tanaka, Y. Chujo, Angew. Chem. Int. Ed. 56 (2017) 254-259. DOI:10.1002/anie.201609656 |
| [31] |
D. Tu, S. Cai, C. Fernandez, et al., Angew. Chem. Int. Ed. 58 (2019) 9129-9133. DOI:10.1002/anie.201903920 |
| [32] |
X. Wei, M. Zhu, Z. Cheng, et al., Angew. Chem. Int. Ed. 58 (2019) 3162-3166. DOI:10.1002/anie.201900283 |
| [33] |
S. Lee, J. Shin, D.H. Ko, W.S. Han, Chem. Commun. 56 (2020) 12741-12744. DOI:10.1039/D0CC04684A |
| [34] |
K. Nishino, H. Yamamoto, K. Tanaka, Y. Chujo, Asian J. Org. Chem. 6 (2017) 1818-1822. DOI:10.1002/ajoc.201700390 |
| [35] |
J. Ochi, K. Tanaka, Y. Chujo, Eur. J. Org. Chem. (2019) 2984-2988 2019.
|
| [36] |
G.F. Jin, Y.J. Cho, K.R. Wee, et al., Dalton Trans. 44 (2015) 2780-2787. DOI:10.1039/C4DT03123G |
| [37] |
J. Ochi, K. Tanaka, Y. Chujo, Angew. Chem. Int. Ed. 59 (2020) 9841-9855. DOI:10.1002/anie.201916666 |
| [38] |
K. Nishino, H. Yamamoto, J. Ochi, K. Tanaka, Y. Chujo, Chem. Asian J. 14 (2019) 1577-1581. DOI:10.1002/asia.201900396 |
| [39] |
L.A. Smyshliaeva, M.V. Varaksin, E.I. Fomina, et al., Organometallics 39 (2020) 3679-3688. DOI:10.1021/acs.organomet.0c00478 |
| [40] |
G. Tao, Z. Duan, F. Mathey, Org. Lett. 21 (2019) 2273-2276. DOI:10.1021/acs.orglett.9b00562 |
| [41] |
Y. Quan, C. Tang, Z. Xie, Dalton Trans 48 (2019) 7494-7498. DOI:10.1039/C9DT01140D |
| [42] |
C. Tang, J. Zhang, J. Zhang, Z. Xie, J. Am. Chem. Soc. 140 (2018) 16423-16427. DOI:10.1021/jacs.8b10270 |
| [43] |
T.E. Paxson, K.P. Callahan, M.F. Hawthorne, Inorg. Chem. 12 (1973) 708-709. DOI:10.1021/ic50121a050 |
| [44] |
W. Clegg, R. Coult, M.A. Fox, et al., Polyhedron 12 (1993) 2711-2717. DOI:10.1016/S0277-5387(00)80122-9 |
| [45] |
D.M. Murphy, D.M.P. Mingos, J.L. Haggitt, et al., J. Mater. Chem. 3 (1993) 139-148. DOI:10.1039/jm9930300139 |
| [46] |
L.I. Zakharkin, A.I. Kovderov, V.A. Ol'shevskaya, Bull. Acad. Sci. USSR, Div. Chem. Sci. 35 (1986) 1260-1266. DOI:10.1007/BF00956611 |
| [47] |
J. Li, H. Tian, M. Jiang, et al., Chem. Commun. 52 (2016) 8862-8864. DOI:10.1039/C6CC04386K |
| [48] |
T. Hoshikawa, S. Kamijo, M. Inoue, Org. Biomol. Chem. 11 (2013) 164-169. DOI:10.1039/C2OB26785C |
| [49] |
A. Schaffner, V. Darmency, P. Renaud, Angew. Chem. Int. Ed. 45 (2006) 5847-5849. DOI:10.1002/anie.200601206 |
| [50] |
H. Todoroki, M. Iwatsu, D. Urabe, M. Inoue, J. Org. Chem. 79 (2014) 8835-8849. DOI:10.1021/jo501666x |
| [51] |
T. Takeda, M. Ando, T. Sugita, A. Tsubouchi, Org. Lett. 9 (2007) 2875-2878. DOI:10.1021/ol071077w |
| [52] |
P. Chen, C. Zhu, R. Zhu, W. Wu, H. Jiang, Chem. Asian J. 12 (2017) 1875-1878. DOI:10.1002/asia.201700550 |
| [53] |
R.R. Tykwinski, B.L. Williamson, D.R. Fischer, P.J. Stang, A.M. Arif, J. Org. Chem. 58 (1993) 5235-5237. DOI:10.1021/jo00071a037 |
| [54] |
C.C. Chen, J. Waser, Org. Lett. 17 (2015) 736-739. DOI:10.1021/acs.orglett.5b00015 |
| [55] |
D.J. Hamnett, W.J. Moran, Org. Biomol. Chem. 12 (2014) 4156-4162. DOI:10.1039/C4OB00556B |
| [56] |
L. Marzo, I. Pérez, F. Yuste, J. Alemán, J.L.G. Ruano, Chem. Commun. 51 (2015) 346-349. DOI:10.1039/C4CC07574A |
| [57] |
C. Valderas, L. Marzo, M.C. de la Torre, et al., Chem. Eur. J 22 (2016) 15645-15649. DOI:10.1002/chem.201603462 |
| [58] |
N. Shida, S. Owaki, H. Eguchi, et al., Dalton Trans. 49 (2020) 12985-12989. DOI:10.1039/D0DT02205E |
| [59] |
A.V. Marsh, M. Little, N.J. Cheetham, et al., Chem. Eur. J. 27 (2021) 1970-1975. DOI:10.1002/chem.202004517 |
| [60] |
M. Kim, C.H. Ryu, J.H. Hong, et al., Inorg. Chem. Front. 7 (2020) 4180-4189. DOI:10.1039/D0QI00915F |
| [61] |
K.R. Wee, Y.J. Cho, J.K. Song, S.O. Kang, Angew. Chem. Int. Ed. 52 (2013) 9682-9685. DOI:10.1002/anie.201304321 |
 2022, Vol. 33
2022, Vol. 33