b School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China
Substituted indoles exist widely in the structures of numerous biologically active natural products and pharmaceuticals [1-6]. Their synthesis have attracted significant interest among synthetic community. Complementary to the traditional Friedel Crafts reaction, the metal-catalyzed direct C-H functionalization of indole has represented one of the most efficient methods for preparing indole derivatives. While the C-H arylation [7-13], alkenylation [14-18], and 3-alkylation [19-28] of indoles have been well-documented, the 2-alkylation of indoles via direct C-H functionalization is much less studied. In the past ten years, a few metal-catalyzed 2-alkylation of indoles through C-H functionalization with various alkylation reagents including alkenes [29-35], alkyl halides [36-45] and α-diazomalonates [46-47] have been established (Scheme 1). In 2011, Bach [41] and coworkers reported the first direct 2-alkylation of indole with unactivated alkyl bromides through a palladium-catalyzed norbornene-mediated C-H activation cascade at the indole ring (Scheme 1A). By employing a palladium-catalyzed Catellani-type reaction, Liu [44] and Yang [39] realized the 2-trifluoroethylation and 2-methylenephosphorylation of indole from CF3CH2I and diethyl (iodomethyl)phosphonate as the reagents, respectively. Taking advantage of the monodentate-chelation of N-2-Py functionality on indole, Punji [40, 45] developed a nickel-catalyzed 2-alkylation of indoles. This unique alkylation strategy proceeded through a crucial C-H activation process and an alkyl radical intermediate. Stephenson [36, 37] and Melchiorre [38] also described the photochemical 2-alkylation of indoles using bromo malonates and benzyl bromides as the reagents. Despite these progresses, there remain a number of synthetic challenges including harsh reaction conditions, high catalyst loadings, and limited substrate scope. Recent advances on palladium-catalyzed SET processes have significantly expanded the scope of Pd-catalyzed processes [48-57]. By utilizing the Pd-mediated radical pathways, a number of research groups including Ngai [50], Loh [51], Glorius [52], Zhu [53], Zhou [54], and us [49] have contributed to this field. Considering that 2-alkylation of indoles can proceed via a radical pathway [58-63, 38] and α-haloesters can often act as radical precursors under metal catalysis. We envisioned that a Pd-catalyzed 2-alkylation of indoles could be achieved with α-halo esters as the reagents through a radical process. Herein we report a palladium-catalyzed regioselective 2-alkylation of N-protected indoles with α-bromo esters as the reagents, which features mild reaction conditions, good functional group compatibility, and moderate to good yields. The employment of P, P=O ligands [64-69] and 4Å molecular sieve (MS) were essential for the success of the transformation.

|
Download:
|
| Scheme 1. Common alkylation reagents for direct 2-alkylation of indoles. | |
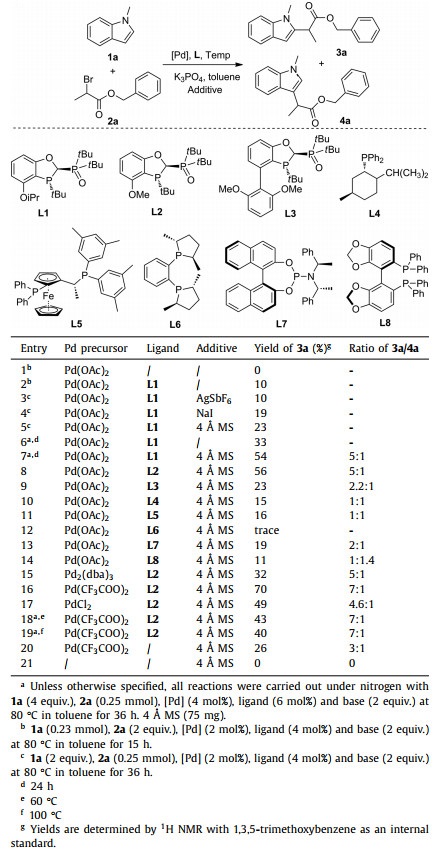
To avoid the N-H deprotonation of indoles in the presence of base, we chose N-methylindole (1a) and benzyl 2-bromo propionate (2a) as the model substrates for cross-coupling. The initial coupling between 1a and 2a was carried out under conditions of Pd(OAc)2 and toluene at 80 ℃, however with no formation of the desired product (Table 1, entry 1). To our surprise, the desired coupling product was obtained in 10% yield when the P, P=O ligand L1 was employed (entry 2). We then examined the additive effect including AgSbF6, NaI, and 4 Å MS (entries 3-5). 4 Å MS provided the highest yield (23%). One major issue with the above conditions was the incomplete conversion, which was responsible for low yields. We thus improved the molar ratio of 1a:2a from 2:1 to 4:1. Consequently, the yield increased from 10% to 33% without additive effect (entries 2 and 6). Encouragingly, the addition of 4 Å MS further enhanced the yield to 54% (entry 7), with a good regioselectivity (3a/4a = 5:1).
|
|
Table 1 Optimization of reaction conditions. |
Screen of various ligands showed that the P, P=O ligand L2 was the optimal ligand in terms of both yield and regioselectivity (entries 7-14). It is noteworthy that all reactions with chiral ligands led to racemic products. Among various palladium precursors employed (entries 15-17), Pd(CF3COO)2 proved to be the best, providing 70% yield (entry 16). The reaction temperature was also important. Use of a lower or higher reaction temperature led to a diminished yield, albeit with a similar ratio of 3a/4a (entries 18 and 19). Control experiments indicated that both the palladium precursor and the ligand were essential for the transformation (entries 20 and 21). Thus, the reaction was performed with K3PO4 as the base, Pd(CF3COO)2 as the Pd source, L2 as the ligand, 4 Å MS as the additive in toluene at 80 ℃ for 36 h as the optimized reaction conditions (see Supporting information for more optimization details).
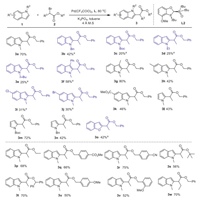
Under the optimized reaction conditions, the scope of the cross-coupling was studied (Scheme 2). In general, the reaction was compatible with a variety of substituted indoles and α-halo esters. However, the reaction was sensitive to N-substituents of the indoles. Substituents such as N-Boc, N-ethyl and N-butyl (3c, 3d, 3e) gave low yields, while N-Bn indoles afforded the desired product in 42% yield. Indole substrates with either electron-withdrawing or -donating substituents at the C3 position led to the desired C2-alkylation product (3f 58%, 3g 80%) in satisfactory yields. In addition, a series of C5-subsitituted indoles was also able to provide the target products in acceptable yields (3h-3k). Other heteroaromatic compounds such as N-substituted pyrroles (3l-3n), and benzofuran 3o were all suitable substrates, providing the 2-alkylation products in 42%-72% isolated yields. As for the α-bromo esters, we were surprise to find that the reaction of various α-bromoesters proceeded well under optimal reaction conditions with moderate to good yield (3p-3w).
|
Download:
|
| Scheme 2. Substrate scope. Unless otherwise specified, all reactions were carried out under nitrogen with 1 (4 equiv.), 2 (0.25 mmol), Pd(CF3COO)2 (4 mol%), L2 (6 mol%) and K3PO4 (2 equiv.) at 80 ℃ in toluene for 36 h. 4 Å MS (75 mg). a Pd(CF3COO)2 (6 mol%), L2 (8 mol%). | |
To gain some mechanistic insight, we conducted the reaction in the presence of a radical inhibitor. As shown in Scheme 3a, the addition of 2, 2, 6, 6, -tetramethylpiperidine-1-oxyl (TEMPO) completely suppressed the transformation and the TEMPO-alkyl adduct 5a was detected by MS. In the presence of a good hydrogen atom donor, 1, 4-cyclohexadiene, benzyl 2-bromopropionate (2a) was significantly reduced to benzyl propionate (6a) (Scheme 3b). These two experiments indicated that the reaction was likely to proceed through an α-carbonyl alkyl radical species. Further support for a radical pathway came from a EPR experiment with the spin-trapping reagent DMPO (5, 5-dimethyl-1-pyrroline N-oxide), a significant signal of alkyl radical was observed (Scheme 3c).

|
Download:
|
| Scheme 3. Preliminary mechanistic studies. | |
On the basis of the above experimental results, a reaction mechanism was proposed shown in Scheme 4. [Pd(0)L] reduces the α-bromoester (2a) through a single electron transfer process to give alkyl radical Ⅲ and [Pd(I)LBr]; subsequent radical addition of intermediate Ⅲ to 1a at C2-position produces a stabilized benzyl radical intermediate Ⅳ, which is responsible for the observed regioselectivity. Oxidation of the benzylic radical by [Pd(I)LBr] followed by deprotonation-aromatization provides the observed product. The role of the P, P=O ligand presumably helps forming the key α-carbonyl radical, therefore allowing the radical cross-coupling to proceed, while the addition of 4 Å MS possibly contributes to provide a weak coordination with the ester substrate.

|
Download:
|
| Scheme 4. A possible catalytic cycle. | |
In summary, we have developed a palladium-catalyzed regioselective 2-alkylation of N-protected indoles with α-bromo esters under mild reaction conditions. The employment of P, P=O ligand as well as 4 Å MS as the additive is crucial for the transformation. Preliminary mechanistic studies have shown that the reaction is likely to proceed through a radical pathway. Further mechanistic studies and applications of palladium-catalyzed cross-coupling is underway in our group.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful to the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), CAS (No. QYZDY-SSW-SLH029), National Natural Science Foundation of China (Nos. 21725205, 21432007, 21572246), STCSM-18520712200, and K.C. Wong Education Foundation.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.091.
| [1] |
J. Bosch, M. Bennasar, Synlett 6 (1995) 587-596. |
| [2] |
A.R. Katritzky, C.W. Rees, in: Comprehensive Heterocyclic Chemistry, 2nd Ed, PergamonPress: Oxford, 1996, pp. 119-206.
|
| [3] |
E.J. Thomas, Science of Synthesis-Houben-Weyl Methods of Molecular Transformations, Thieme: Stuttgart 10 (2000) 361-652.
|
| [4] |
D. Faulkner, J. Nat. Prod. Rep. 19 (2002) 1-48. |
| [5] |
J. Tois, R. Franzen, A. Koskinen, Tetrahedron 59 (2003) 5395-5405. DOI:10.1016/S0040-4020(03)00851-2 |
| [6] |
S. O'Connor, J. Maresh, J. Nat. Prod. Rep. 23 (2006) 532-547. DOI:10.1039/b512615k |
| [7] |
L. Joucla, L. Djakovitch, Adv. Synth. Catal. 351 (2009) 673-714. DOI:10.1002/adsc.200900059 |
| [8] |
R. Jagtap, B. Punji, Asian J. Org. Chem. 9 (2020) 326-342. DOI:10.1002/ajoc.201900554 |
| [9] |
A. Pacheco-Benichou, T. Besson, C. Fruit, Catalysts 10 (2020) 483. DOI:10.3390/catal10050483 |
| [10] |
Y. Yang, Z. Shi, Chem. Commun. 54 (2018) 1676-1685. DOI:10.1039/C7CC08752G |
| [11] |
S. Chen, M. Zhang, R. Su, ACS Catal. 9 (2019) 6372-6379. DOI:10.1021/acscatal.9b01273 |
| [12] |
Y. Yang, X. Qiu, Y. Zhao, Y. Mu, Z. Shi, J. Am. Chem. Soc. 138 (2016) 495-498. DOI:10.1021/jacs.5b11569 |
| [13] |
X. Mu, X. Ge, X. Zhong, L. Han, T. Liu, Mol. Catal. 495 (2020) 111147. DOI:10.1016/j.mcat.2020.111147 |
| [14] |
M. Petrini, Chem. Eur. J. 23 (2017) 16115-16151. DOI:10.1002/chem.201702124 |
| [15] |
S. Pradhan, P. De, T. Shah, T. Punniyamurthy, Chem. Asian J. 15 (2020) 4184-4198. DOI:10.1002/asia.202001159 |
| [16] |
R. Jagtap, B. Punji, Asian J. Org. Chem. 9 (2020) 326-342. DOI:10.1002/ajoc.201900554 |
| [17] |
V. Boyarskiy, D. Ryabukhin, N. Bokach, A. Vasilyev, Chem. Rev. 116 (2016) 5894-5986. DOI:10.1021/acs.chemrev.5b00514 |
| [18] |
J. Leitch, Y. Bhonoah, C. Frost, ACS Catal. 7 (2017) 5618-5627. DOI:10.1021/acscatal.7b01785 |
| [19] |
F. Bartoccini, M. Retini, G. Piersanti, Tetrahedron Lett. 61 (2020) 151875. DOI:10.1016/j.tetlet.2020.151875 |
| [20] |
K. Huang, Q. Ma, X. Shen, L. Gong, E. Meggers, Asian J. Org. Chem. 5 (2016) 1198-1203. DOI:10.1002/ajoc.201600288 |
| [21] |
F. Pu, Y. Li, Y. Song, Adv. Synth. Catal. 358 (2016) 539-542. DOI:10.1002/adsc.201500874 |
| [22] |
J. Liu, L. Gong, E. Meggers, Tetrahedron Lett. 56 (2015) 4653-4656. DOI:10.1016/j.tetlet.2015.06.046 |
| [23] |
S. Mohapatra, P. Mukhi, A. Mohanty, Tetrahedron Lett. 56 (2015) 5709-5713. DOI:10.1016/j.tetlet.2015.08.080 |
| [24] |
C. Zhuo, C. Zheng, S. You, Acc. Chem. Res. 47 (2014) 2558-2573. DOI:10.1021/ar500167f |
| [25] |
A. Putra, K. Takigawa, H. Tanaka, Eur. J. Org. Chem. (2013) 6344-63542013. DOI:10.1002/ejoc.201300744 |
| [26] |
Q. Xu, L. Dai, S. You, Chem. Sci. 4 (2013) 97-102. DOI:10.1039/C2SC21085A |
| [27] |
S. Imm, S. Bähn, A. Dr, Chem. Eur. J. 16 (2010) 2705-2709. DOI:10.1002/chem.200903261 |
| [28] |
M. Wang, C. Zhou, M. Wong, C. Che, Chem. Eur. J. 16 (2010) 5723-5735. DOI:10.1002/chem.200902387 |
| [29] |
T. Shibata, N. Ryu, H. Takano, Adv. Synth. Catal. 357 (2015) 1131-1135. DOI:10.1002/adsc.201401163 |
| [30] |
Y. Nakao, N. Kashihara, K. Kanyiva, T. Hiyama, Angew. Chem. Int. Ed. 49 (2010) 4451-4454. DOI:10.1002/anie.201001470 |
| [31] |
S. Pan, N. Ryu, T. Shibata, J. Am. Chem. Soc. 134 (2012) 17474-17477. DOI:10.1021/ja308742x |
| [32] |
Y. Zhang, J. Zheng, S. Cui, J. Org. Chem. 79 (2014) 6490-6500. DOI:10.1021/jo500902n |
| [33] |
B. Chaudhary, M. Diwaker, S. Sharma, Org. Chem. Front. 5 (2018) 3133-3137. DOI:10.1039/C8QO00932E |
| [34] |
C. Sevov, J. Hartwig, J. Am. Chem. Soc. 135 (2013) 2116-2119. DOI:10.1021/ja312360c |
| [35] |
Y. Schramm, M. Takeuchi, K. Semba, Y. Nakao, J. Hartwig, J. Am. Chem. Soc. 137 (2015) 12215-12218. DOI:10.1021/jacs.5b08039 |
| [36] |
E. Swift, T. Williams, C. Stephenson, Synlett 27 (2016) 754-758. DOI:10.1055/s-0035-1561320 |
| [37] |
L. Furst, B. Matsuura, J. Narayanam, J. Tucker, C. Stephenson, Org. Lett. 12 (2010) 3104-3107. DOI:10.1021/ol101146f |
| [38] |
S. Kandukuri, A. Bahamonde, I. Chatterjee, Angew. Chem. Int. Ed. 54 (2015) 1485-1489. DOI:10.1002/anie.201409529 |
| [39] |
Y. Niu, P. Bai, Q. Lou, S. Yang, ChemCatChem 12 (2020) 3644-3649. DOI:10.1002/cctc.202000415 |
| [40] |
D. Pandey, S. Ankade, A. Ali, C. Vinod, B. Punji, Chem. Sci. 10 (2019) 9493-9500. DOI:10.1039/C9SC01446B |
| [41] |
L. Jiao, T. Bach, J. Am. Chem. Soc. 133 (2011) 12990-12993. DOI:10.1021/ja2055066 |
| [42] |
L. Jiao, E. Herdtweck, T. Bach, J. Am. Chem. Soc. 134 (2012) 14563-14572. DOI:10.1021/ja3058138 |
| [43] |
H. Potukuchi, T. Bach, J. Org. Chem. 78 (2013) 12263-12267. DOI:10.1021/jo402107m |
| [44] |
H. Zhang, H. Wang, Y. Luo, et al., ACS Catal. 8 (2018) 2173-2180. DOI:10.1021/acscatal.7b03220 |
| [45] |
V. Soni, R. Jagtap, R. Gonnade, B. Punji, ACS Catal. 6 (2016) 5666-5672. DOI:10.1021/acscatal.6b02003 |
| [46] |
X. Liu, S. Zhang, J. Wu, Q. Li, H. Wang, Tetrahedron Lett. 56 (2015) 4093-4095. DOI:10.1016/j.tetlet.2015.05.025 |
| [47] |
K. Wan, Z. Li, X. Qu, F. Wang, L. Wang, Catalysts 6 (2016) 89. DOI:10.3390/catal6060089 |
| [48] |
Q. Liu, X. Dong, J. Li, ACS Catal. 5 (2015) 6111-6137. DOI:10.1021/acscatal.5b01469 |
| [49] |
M. Aliyu, B. Li, H. Yang, W. Tang, Org. Chem. Front. 7 (2020) 3505-3508. DOI:10.1039/D0QO01013H |
| [50] |
G. Zhao, W. Yao, J. Mauro, M. Ngai, J. Am. Chem. Soc. 143 (2021) 1728-1734. DOI:10.1021/jacs.0c11209 |
| [51] |
X. Wu, G. Xiao, Y. Ding, et al., ACS Catal 10 (2020) 14107-14116. DOI:10.1021/acscatal.0c04013 |
| [52] |
H. Huang, P. Bellotti, P. Pflüger, J. Am. Chem. Soc. 142 (2020) 10173-10183. DOI:10.1021/jacs.0c03239 |
| [53] |
S. Wang, J. Zhang, L. Kong, Org. Lett. 20 (2018) 5631-5635. DOI:10.1021/acs.orglett.8b02336 |
| [54] |
S. Teng, M. Tessensohn, R. Webster, J. Zhou, ACS Catal. 8 (2018) 7439-7444. DOI:10.1021/acscatal.8b02029 |
| [55] |
C. Wang, G. Dong, J. Am. Chem. Soc. 140 (2018) 6057-6061. DOI:10.1021/jacs.8b03530 |
| [56] |
J. Liao, L. Fan, W. Guo, Org. Lett. 19 (2017) 1008-1011. DOI:10.1021/acs.orglett.6b03865 |
| [57] |
Z. Wu, T. Si, G. Xu, B. Xu, W. Tang, Chin. Chem. Lett. 30 (2018) 597-600. DOI:10.1002/chem.201705533 |
| [58] |
F. Klauck, M. James, F. Glorius, Angew. Chem. Int. Ed. 56 (2017) 12336-12339. DOI:10.1002/anie.201706896 |
| [59] |
M. Guerrero, L. Miranda, Tetrahedron Lett 47 (2006) 2517-2520. DOI:10.1016/j.tetlet.2006.02.052 |
| [60] |
L. Furst, B. Matsuura, J. Narayanam, J. Tucker, C. Stephenson, Org. Lett. 12 (2010) 3104-3107. DOI:10.1021/ol101146f |
| [61] |
C. Theunissen, J. Wang, G. Evano, Chem. Sci. 8 (2017) 3465-3470. DOI:10.1039/C6SC05622A |
| [62] |
X. Wu, J. See, K. Xu, Angew. Chem. Int. Ed. 53 (2014) 13573-13577. DOI:10.1002/anie.201408355 |
| [63] |
A. Nakatani, K. Hirano, T. Satoh, M. Miura, Chem. Eur. J. 19 (2013) 7691-7695. DOI:10.1002/chem.201301350 |
| [64] |
B. Li, M. Aliyu, Z. Gao, Org. Lett. 13 (2020) 4974-4978. |
| [65] |
B. Li, T. Li, M. Aliyu, Z. Li, W. Tang, Angew. Chem. Int. Ed. 58 (2019) 11355-11359. DOI:10.1002/anie.201905174 |
| [66] |
C. Li, T. Chen, B. Li, G. Xiao, W. Tang, Angew. Chem. Int. Ed. 54 (2015) 3792-3796. DOI:10.1002/anie.201411518 |
| [67] |
G. Xu, C. Senanayake, W. Tang, Acc. Chem. Res. 52 (2019) 1101-1112. DOI:10.1021/acs.accounts.9b00029 |
| [68] |
K. Li, M. Nie, W. Tang, Green Synth. Catal. 1 (2020) 171-174. DOI:10.1016/j.gresc.2020.08.003 |
| [69] |
C. Li, D. Chen, W. Tang, Synlett 27 (2016) 2183-2200. DOI:10.1055/s-0035-1562360 |
 2022, Vol. 33
2022, Vol. 33