b School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 210009, China;
c School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
Nowadays, with the fact of resource shortage in the world and the concept of sustainable development in mind, there is an urgent need to develop efficient and green chemical industry through improving the efficiency of synthetic transformations [1-5]. One of the practical ways is to design and prepare highly active catalysts, which can improve the yields of products to reduce production costs while minimizing the impact on environment.
Porous carbon materials with large specific surface area, tunable pore sizes, excellent chemical and mechanical stability, structural diversity and good electric conductivity [6, 7], have been widely applied in the fields such as gas adsorption [8-12], separation [13], batteries [14-21], super-capacitors [22-24], photodynamic therapy [25]. In fact, porous carbon materials can be also considered as efficient catalysts or catalysts supports for many organic transformations, such as hydrogenation [26-28], oxidation reaction [29-32], esterification [33-36], hydrodehalogenation [37, 38]. Carbon materials derived catalysts often exhibit extraordinary advantages over homogeneous catalysts in terms of sustainability, recyclability, robustness, and ease of catalyst separation [39-41]. In addition, porous carbon materials show significant advantages in activity, stability and regenerability compared with other kind of heterogeneous catalysts [42]. Since the tailored carbon materials with the expected catalytic performance can be obtained by tuning the activation, functionalization and carbonization methods, they have been identified to be potential alternatives to conventional catalysts to meet the requirements of green chemistry [43].
Generally, carbon-based materials are prepared by carbonization of organic precursors, such as MOFs [44-49], polymers [50-55], ionic liquid [56, 57], asphaltenes [58, 59] and biomass [60-64] Due to the rapid consumption of chemical fossil fuel resources and increasingly prominent environmental problems [65-67], the development of renewable and environmentally friendly carbon source to replace fossil fuel resources has become another important aspect of green chemistry. Therefore, biomass such as lignin is a better option as the precursors for carbon materials than petroleum-based compounds.
Carbohydrates are the most abundant, renewable and nontoxic biomass with rich variety including cellulose, hemicellulose, chitin, chitosan, starch, alginate, etc. (Fig. 1) [68-71]. Carbohydrates can easily coordinate with metals due to their high content of hydroxyl, carboxyl, amine or other functional groups on the structural skeleton. The pyrolysis of carbohydrates into carbon is beneficial to stabilize and active metal species due to its excellent stability, the graphite coating on metal species and the electron interaction between metal and carbon, thereby preventing the metals from aggregation, corrosion or poisoning and enhancing the catalytic performance. In addition, carbon materials have larger specific surface than carbohydrates, which is favorable for the interaction between substrates and catalytic active sites. Therefore, the carbohydrates should be an ideal precursor for the generation of carbon materials [72], meanwhile, the carbohydrates derived porous carbon materials emerge as excellent and sustainable catalysts for some organic transformations [73-78].

|
Download:
|
| Fig. 1. Sustainable and environmental-friendly carbohydrates. | |
There are several reviews with regard to carbohydrate-derived catalysts. In 2015, the organic transformations that assessed with either chitosan or functionalized chitosan as catalysts were reviewed by Kadib [79]. In 2019, Molnár reviewed chitosan-based metal catalysts mediated organic transformations, which was organized by different reaction types [80]. In 2020, Nasrollahzadeh reviewed the recent literature on the development of carbohydrate-derived catalysts and their application in oxidation reactions [81]. Gläser and co-workers reviewed chitosan-based N-doped carbon materials for electrocatalytic and photocatalytic applications [82]. In addition, there have been several reviews concerning the preparation and application of biomass-derived carbon materials [83-86]. However, limited examples have been mentioned in these reviews with respect to the application of carbohydrate-derived porous carbon materials in organic synthesis. Considering the inherent advantages of carbohydrate-derived carbon materials and their increased application trend in organic reactions, the review on organic transformations over carbohydrates derived carbon materials is of great importance and appealing.
In this review, we aim to provide a brief overview and critical summary of investigations on organic reactions catalyzed by the carbohydrate-derived carbon materials, which is organized according to the types of carbohydrates. Both metal-embedded and metal-free carbon materials are involved in this review. The preparation and characterization methods of these carbon materials are also discussed to propose a concept for designing heterogeneous catalysts that meet catalytic diversity.
2. Cellulose derived carbon materialsAs the most abundant biomass, cellulose is a kind of renewable natural polymer materials [87-91], in which the repeating β-D-glucopyranose molecules are linked through acetal functions between the equatorial OH group of C4 and the C1 carbon atom (Fig. 2) [92]. Therefore, cellulose and its derivatives are a broad linear polymer with a large number of hydroxyl groups, which are conductive to its coordination with metal directly or after functional modification [93, 94]. Nanocellulose and microcrystalline are the two commonly used types of cellulose to prepare carbon materials, which have advantages of better dissolution options, larger surface area and controllable morphology [95-97].

|
Download:
|
| Fig. 2. The chemical structure diagram of cellulose. | |
Chang et al. prepared an amorphous carbon-based sulfonated catalyst by carbonizing cellulose at different temperature and then sulfonated with concentrated sulfuric acid [98]. The Fourier transform infrared (FTIR) spectroscopy shows that the carbon-based sulfonation catalyst contained aromatic structure, hydroxyl, SO3H and carbonyl groups. The as obtained sulfonated catalyst exhibits high activity in the one-step synthesis of hydroxymethyl furfural from inulin in ionic liquid (Fig. 3). Although the sulfonated catalyst is partly deactivated in the process of separating and reusing from the ionic liquid, it can be easily regenerated after treatment with dilute sulfuric acid. Therefore, these carbon-based sulfonation catalysts exhibit better catalytic activity and higher reusability than traditional solid acid catalysts.

|
Download:
|
| Fig. 3. The conversion of inulin to HMF via one-step and two-step pathways. | |
In 2015, Kang and co-workers successfully prepared three-dimensional microporous carbon spheres (3DMPCS) by adding colloidal silica crystal beads into the cellulose or glucose aqueous solution mixture, and then carbonizing at different temperatures (100-900 ℃) under the nitrogen atmosphere. In this procedure, the cellulose or glucose is used as carbon precursor and colloidal silica nanosphere beads as the structural template that can be removed by HF (Fig. 4) [99]. The 3DMPCS is carbonized at 500 ℃ to obtain the final carbon material (donated as C-3DMPCS-500), which shows the highest catalytic efficiency and selectivity in the oxidation of cis-cyclooctene with air as an oxidant (Scheme 1). The excellent catalytic performance of C-3DMPCS-500 is attributed to the fact that carbon sphere in C-3DMPCS-500 is an analog of microreactor, which can promote the diffusion of reaction reagents and further enhance the conversion efficiency and selectivity.

|
Download:
|
| Fig. 4. The procedure for the synthesis of C-3DMPCS. Copied with permission [99]. Copyright 2015, the Royal Society of Chemistry. | |

|
Download:
|
| Scheme 1. The selective oxidation of cis-cyclooctene catalyzed by C-3DMPCS-500. | |
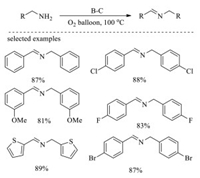
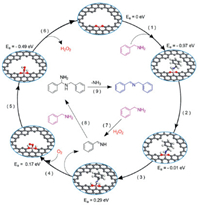
In 2018, Gao and co-workers reported a boron-doped mesoporous carbon material (B-C) by annealing the mixture of boric acid and cellulose at 800 ℃ under inert atmosphere, which exhibited great performance in the aerobic oxidative coupling of amines to imines (Scheme 2) [100]. The results of X-ray photoelectron spectroscopy and X-ray absorption spectrums reveal that boron functions as catalytic active site in the form of CBO2. Based on density functional calculation, the excellent catalytic performance is ascribed to the synergistic effects of B and O atom in CBO2, which promote adsorption and dehydrogenation of benzylamine (Fig. 5). After five runs, the boron-doped mesoporous carbon material can still obtain the desired yield, indicating that this material has good activity and excellent stability.
|
Download:
|
| Scheme 2. The synthesis of imines from aerobic oxidative coupling of amines. | |
|
Download:
|
| Fig. 5. Proposed catalytic mechanism based on DFT calculation. Copied with permission [100]. Copyright 2018, American Chemical Society. | |
A kind of phosphorus and nitrogen co-doped carbon-based metal-free catalysts (NPC) have been fabricated via carbonation of cellulose crystallite and (NH4)2HPO4 [101]. This catalyst shows excellent activity in the reduction of p-nitrophenol with a turnover frequency up to 2 × 10-5 mmol mg-1 min-1, and the apparent kinetic rate constant can reach as high as 0.0394 min-1 at room temperature (Fig. 6). In addition, the rate performance can still maintain over 90% after four cycles, showing the significant durability of this catalyst.
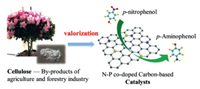
|
Download:
|
| Fig. 6. The reduction of p-nitrophenol catalyzed by NPC prepared from cellulose. Copied with permission [101]. Copyright 2020, Elsevier. | |
Metal embedded carbon materials prepared from the cellulose have also been developed for catalytic organic reactions. Varma et al. have successfully synthesized a carbon-coated magnetic Pd catalyst with 4.81 wt% of Pd (Fe3O4@CPd) (Fig. 7) [102]. This catalyst can be used for oxidation of alcohols, amination of aryl halides and arylation of aryl halides. Due to its inherent magnetic nature, it can be separated by using an external magnet, which prolongs the lifetime of the catalyst and reduces the pollution to the environment.

|
Download:
|
| Fig. 7. Fe3O4@CPd catalyzed oxidation, amination and coupling reactions. | |
In 2019, Nemati et al. immobilized Pd nanoparticles on mesoporous triazine-based carbon (MTC) to prepare a retrievable and efficient heterogeneous catalyst (Pd@MTC) for Heck cross-coupling reaction (Scheme 3). For the preparation of MTC, cellulose modified by ClCH2CN is fully mixed with ZnCl2 and then carbonized at 400 ℃ for 40 h (Fig. 8) [103]. According to the Brunauer-Emmet-Teller analysis, the presence of ZnCl2 and nitrile groups is extremely beneficial for the formation of mesoporous structure during pyrolysis, which can enhance catalytic efficiency.

|
Download:
|
| Scheme 3. The Pd@MTC catalyzed Heck-coupling reactions. | |
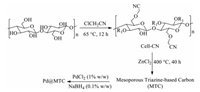
|
Download:
|
| Fig. 8. Scheme illustration for the fabrication of Pd@MTC. | |
Chitin, the second abundant polysaccharide on earth after cellulose, is a sustainable and biocompatible N-containing material that is extensively distributed in the exoskeletons of insects and crustaceans (Fig. 9) [104, 105]. Chitin is an ideal candidate for preparing carbon materials because nitrogen doping is one of the most commonly used methods to improve the performance of carbon materials [106]. On the one hand, the nitrogen in the carbon matrix can adjust the surface polarity and electron-donating ability, which lead to improved electrical properties. On the other hand, the nitrogen exhibits high affinity for metal ions and can optimize the performance of adsorption and catalysis. Therefore, chitin derived carbon materials have been used in many fields such as the chemical industry, food and environment [107-109]. This part mainly covers the great potential of chitin as a precursor of carbon and nitrogen source to prepare carbon materials that applied in catalysis.
|
Download:
|
| Fig. 9. The chemical structure diagram of chitin. | |
In 2015, Yan et al. prepared a series of nitrogen-containing carbon materials by the carbonization of chitin at 400-1000 ℃ under template-free conditions (Fig. 10) [110]. By changing the carbonization temperature, the pore structure, surface area and nitrogen types in the materials can be easily adjusted to make the material suitable for different applications. The N-doped carbon materials prepared at higher temperatures (800-1000 ℃) exhibits excellent catalytic activity towards epoxidation of styrene, because high carbonization temperature increases the content of graphitic nitrogen and carbon. This study reveals the potential application of chitin-derived nitrogen-containing functional carbon materials for organic transformations.
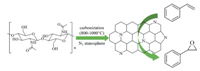
|
Download:
|
| Fig. 10. Preparation of chitin derived nitrogen-containing carbon materials. | |
A novel N-doped carbon supported Rh catalyst (Rh/N-C-700) with well-dispersed Rh nanoparticles (NPs) has been developed via one-pot pyrolysis of chitin and (NH4)3RhCl6 by Wang and co-workers in 2017 [111]. The catalyst exhibits excellent performance in the transformation of benzoic acid to cyclohexane carboxylic acid in 1.5 h at low temperature (Scheme 4). The high catalytic performance of the catalyst is mainly depending on the ration of Rh0/Rh3+ and the diameter of Rh NPs. The valence and particle size of Rh NPs on this N-doped carbon matrix can be adjusted by controlling the pyrolysis temperature. Meanwhile, the catalyst prepared at 700 ℃ (Rh/N-C-700) has better catalytic performance than other catalysts due to the high portion of Rh0 and uniformly dispersed Rh NPs with a diameter of 3.3 nm. Compared with the traditional impregnation and NaBH4-reduction method, this new synthesis strategy significantly increases the interaction between Rh NPs and N-doped carbon, thereby increasing the catalysts durability via preventing the migration, aggregation and leaching of Rh NPs. More importantly, this synthetic method is easy to be scaled up to produce environmentally friendly N-doped carbon supported Rh catalysts, which may hold a broad prospect in industrial application.
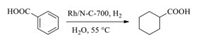
|
Download:
|
| Scheme 4. Rh/NC-700 catalyzed selective hydrogenation of benzoic acid. | |
As we know, metal nanoparticles encapsulated with carbon materials is beneficial to their stability, but it also leads to the block of active sites in catalyst. Therefore, it is of great importance to find a balance between the stability and activity of corresponding catalysts. In 2018, the same group proposed a heat-treat method in which the encapsulated Ru NPs were reduced in H2 atmosphere and calcination in air atmosphere (Fig. 11) [112]. This procedure can destroy the defective carbon-surrounded Ru nanoparticles by achieving the methanation and oxidation in the surface of the carbon material, thus removing the carbon layer without affecting the morphology of Ru NPs, which can enhance the exposure of Ru NPs on the surface and improve their catalytic activity while retaining their stability.

|
Download:
|
| Fig. 11. Schematic illustration of the fabrication of various Ru@CN catalysts. Copied with permission [112]. Copyright 2018, American Chemical Society. | |
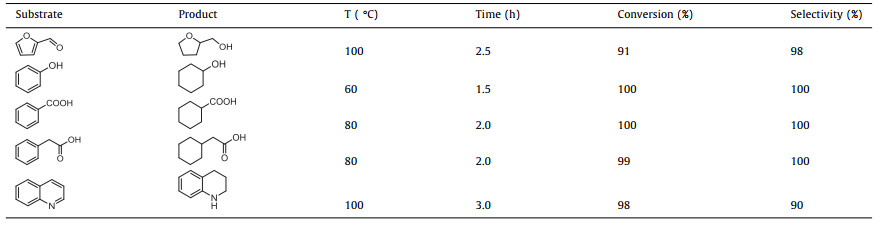
The carbon-surrounded Ru NPs (Ru@CN) after heat treatment is denoted as Ru@CN-800-250A-350H, representing that Ru@CN was calcinated firstly in air atmosphere at 250 ℃ and then reduced in hydrogen atmosphere at 350 ℃. The applicability of Ru@CN-800-250A-350H is tested by the selective hydrogenation of five kinds of organic compounds, and this catalyst shows excellent efficiency and selectivity (Table 1). Therefore, the defective carbon removal method is practical for carbon-encapsulated metal NPs and can be applied for the synthesis of various useful catalysts for organic reactions.
|
|
Table 1 Ru@CN-800-250A-350H catalyzed hydrogenation of various compounds. |
In 2018, Zhang and co-workers successfully fabricated a series of size-controllable palladium nanocatalysts (Pd@chitin) by calcining the mixture of chitin microspheres and different concentrations of palladium salts solution via one-step under an argon atmosphere at 250 ℃ [113]. The prepared nanocatalysts contain well-dispersed and ultrafine Pd NPs with average diameters from about 1 to 3 nm. Importantly, these supported Pd catalysts displayed better catalytic performances and recyclability for the hydrogenation of styrene and benzaldehyde in comparison with commercial Pd/C and unsupported homogeneous Pd(OAc)2 catalysts (Fig. 12). These catalysts still maintain their original catalytic activity after 10 cycles, making them suitable for industrial applications.

|
Download:
|
| Fig. 12. Yields of the desired products as a function of time for styrene (a) and benzaldehyde (b) hydrogenation catalyzed by various Pd@chitin, commercial Pd/C, and unsupported homogeneous Pd(OAc)2 catalysts. Copied with permission [113]. Copyright 2018, Royal Society of Chemistry. | |
Chitosan is a heteropolymer containing both glucosamine units and acetylglucosamine units, which obtains from the deacetylation of chitin in alkaline media (Fig. 13) [114]. Therefore, chitosan has many properties that similar to chitin, including non-toxicity, biodegradability, biocompatibility and availability. Additionally, due to the plenty of amino and hydroxyl groups within its molecule backbone, chitosan owns strong chelating property and electrostatic interaction with metals, which make it considered as one of the most ideal precursors for preparing heterogeneous catalysts [115-117] Recently, several metal@N-doped carbon materials derived from chitosan have been applied in organic synthesis.

|
Download:
|
| Fig. 13. The chemical structure diagram of chitosan. | |
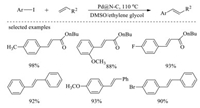
Palladium nanoparticles supported on chitosan-derived carbon (Pd/CS) is a kind of common and high-efficient heterogeneous catalysts that were applied widely in a variety of organic reaction [118-120] For example, a novel Pd@N-C heterogeneous catalyst was reported by Zeng and co-workers in 2016, which was prepared by calcining chitosan/silica/Pd gel beads at high-temperature and then removing silica template [121]. The characterization results indicate that prepared Pd@N-C has a high BET surface area and nitrogen content, which are beneficial for the dispersion and exposure of active sites. The obtained Pd@N-C heterogeneous catalyst is proved to be outstandingly active and stable for Heck reactions (Scheme 5).
|
Download:
|
| Scheme 5. The Heck reactions catalyzed by Pd@NC. | |
In 2017, Xu's group prepared a chitosan/silica derived carrier with controllable and uniform size, high coordination ability and mechanical intensity through microchannel (Figs. 14a and b), which can afford chitosan/silica derived porous microspheres after calcinating and removing silica framework (Figs. 14c and d) [122]. Afterwards, Pd NPs are loaded on these porous microspheres to catalyze Heck-coupling reaction with high efficiency. It is worth noting that the leaching of Pd on chitosan/silica derived porous microspheres is almost negligible and the crystal structure of catalyst could be maintained even after 8 times of recycling.

|
Download:
|
| Fig. 14. (a) Schematic diagram of experimental device. (b) Micrography of chitosan/silica derived carrier. (c, d) SEM images on surface and inner structure of chitosan/silica derived porous microspheres. Copied with permission [122]. Copyright 2017, Elsevier. | |
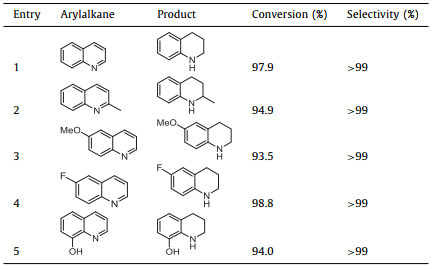
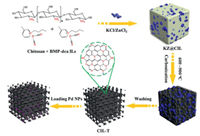
In 2018, Zhang and co-workers prepared an N-doped hierarchical porous carbon anchored tiny Pd NPs catalyst, in which chitosan and nitrogen-rich ionic liquids are used as composite precursors, and the mixture of KCl and ZnCl2 is employed as benign pore-forming agents (Fig. 15) [123]. A series of Pd@CIL-T catalysts (C refers to chitosan, IL refers to ionic liquid, T = 600-900 ℃) are successfully fabricated. The catalyst carbonized at 900 ℃ exhibits the best catalytic performance for selective hydrogenation of quinoline under extremely mild conditions (Table 2). Compared with the traditional catalyst, the Pd@CIL-900 possesses high specific surface area and rich pore structure, and can overcome the problems of nanoparticle aggregation and leaching under relatively harsh reaction conditions owing to graphite carbon layer protection.
|
Download:
|
| Fig. 15. The preparative pathway of Pd@CIL-T (T = 600-900 ℃) catalyst. Copied with permission [123]. Copyright 2018, Elsevier. | |
|
|
Table 2 Chemoselective hydrogenation of quinoline derivatives over Pd@CIL-900. |
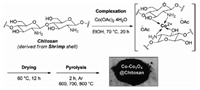
In 2017, Beller et al. synthesized a series of novel sustainable catalysts (Co-Co3O4@Chitosan) by mixing cobalt metal precursors with chitosan following by pyrolysis (Scheme 6) [124]. The obtained Co-Co3O4@Chitosan carbonized at 700 ℃ exhibits the superior activity for the hydrodehalogenation of alkyl and (hetero)aryl halides with wide scope and excellent chemoselectivity using H2 as a green reducing agent (Fig. 16). Furthermore, the Co-Co3O4@Chitosan is also successfully applied in the hydrodehalogenation of pesticides metazachlor and benodanil, and the fire retardant tetrabromobisphenol A (Fig. 16), indicating its potential in efficient detoxification of harmful retardant and pesticides.
|
Download:
|
| Scheme 6. The synthesis of Co-Co3O4@Chitosan materials. Copied with permission [124]. Copyright 2017, Wiley-VCH Verlag. | |
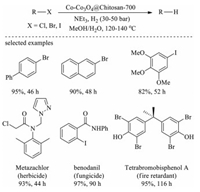
|
Download:
|
| Fig. 16. Hydrodehalogenation of alkyl and aryl halides. | |
Li et al. have developed an efficient catalyst that isolated Co single-site anchored on N-doped porous carbon nanobelt (Co-ISA/CNB) by pyrolysis and acid etching process in 2018 [125]. The porous belt-like nanostructure with large specific surface area and high graphitization degree can be obtained due to the usage of ZnCl2 and CoCl2 salts as effective activation-graphitization agents (Fig. 17). Co species exist as isolated single sites and are stabilized by nitrogen to form the CoN4 structure. It is worth mentioning that the Co-ISA/CNB has an outstanding catalytic performance in the selective oxidation of aromatic alkanes at room temperature, which can achieve 98% ethyl benzene conversion and 99% acetophenone selectivity (Scheme 7), while the Co species in nanoparticles form are nearly inert.
|
Download:
|
| Fig. 17. Preparation of Co-ISA/CNB catalyst. Copied with permission [125]. Copyright 2018, Wiley-VCH Verlag. | |

|
Download:
|
| Scheme 7. Co-ISA/CNB catalyzed selective oxidation of ethyl benzene. | |
Meanwhile, the DFT calculations are performed to explore the advantages of Co-ISA/CNB over Co nanoparticles in catalytic efficiency. The adsorption of TBHP on Co single site metals leads to the heterolytic cleavage of O-O bond in TBHP and the formation of Co=O species, which results in the excellent catalytic efficiency. In comparison, the adsorption of TBHP on the surface of Co nanoparticles leads to the release of tert-butoxyl radical and hydroxyl radical, which can block on metal centers over Co nanoparticles and deactivate Co center.
In 2020, our group introduced a facile and sustainable self-sacrifice template strategy by using zinc and chitosan as sacrificial template and carbon source respectively, which forms a porous Co-embedded nitrogen-doped graphite catalyst (Co@CN-Zn12-5, Fig. 18) [126]. The obtained catalyst shows excellent catalytic efficiency in the oxidative esterification of 5-hydroxymethylfurfural (HMF) to form 2, 5-furandicarboxylicacid dimethyl ester (FDMC), which is an important raw material for polymer synthesis (Fig. 18). Based on the experimental and calculation results, zinc can not only be used as the self-sacrificial template to produce a high specific surface area, but also can optimize the base and acid sites in the catalyst, thereby significantly improving the activity of the catalyst.

|
Download:
|
| Fig. 18. The oxidative esterification of HMF over Co@CN-Zn12-5. Copied with permission [126]. Copyright 2020, Elsevier. | |
Sucrose, a disaccharide consists of glucose and fructose, which is transported from synthesizing (source) organs to sink organs where it is stored (as sucrose or, e.g., as starch) or metabolized, so sucrose is the source of carbon skeletons in plants (Fig. 19) [127]. Most sucrose is a renewable resource extract from sugarcane, a common cash crop that is widely grown, inexpensive and easily available [128]. Furthermore, sucrose can not only act as a carbon precursor, but also as a binder between the carbon particles [129].
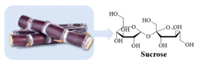
|
Download:
|
| Fig. 19. The chemical structure diagram of sucrose. | |
In 2018, Sels et al. employed sucrose as sustainable carbon source to prepare a silica-carbon nanocomposite acid catalyst with large mesopore interconnectivity via a mild vapor-phase assisted hydrothermal treatment procedure and subsequently sulfonated by sulfuric acid (Fig. 20) [130]. Compared with their counterparts from dry pyrolysis and the commercial strong acid resins, the as-prepared catalyst has a superior catalytic activity in the solvent-free condensation reaction of 2-methylfuran with furfural. The excellent catalytic activity of the prepared catalyst is attributed to the higher SO3H acid density, the larger and better communicating mesopores and the abundant presence of oxygen-containing functional groups on the surface. By discussing the origin of the well-developed large interconnecting mesopores, it is found that the mild hydrothermal treatment causes local corrosion of mesopores in precursor materials, resulting in unexpected interconnection between the pores, while the original micropores are basically eliminated in the process of treatment.

|
Download:
|
| Fig. 20. The preparation of nanocomposite acid catalyst and its catalytic application. Copied with permission [130]. Copyright 2018, American Chemical Society. | |
In 2019, a facile strategy was proposed by Lamei and co-workers for the preparation of tunable N-doped nanospheres using sucrose as carbon source and tris(2-aminoethyl)amine (TAEA) as nitrogen source [131]. As the amount of TAEA increases, the content of doped nitrogen, surface area and pore size of nanosphere can be improved. The nanospheres can be used as a carrier of Ag nanoparticles, and the obtained catalysts (Ag/N-CS-x, x represents the mass of TAEA) could be applied in the formation of aryl nitriles from benzylic alcohols and aldehydes (Fig. 21).
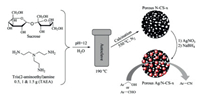
|
Download:
|
| Fig. 21. The preparation process of porous Ag/NCS-x and its catalytic application. Copied with permission [131]. Copyright 2019, John Wiley and Sons Ltd. | |
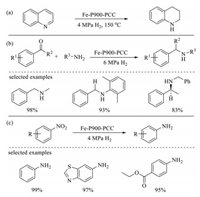
In 2020, Li et al. first prepared graphitic phosphorus species coordinated single-atom Fe on P-doped carbon (Fe-P900-PCC) by carbonizing the mixture of sucrose, phytic acid and silica colloid at 900 ℃, followed by HF-etching to remove the silica colloid (Fig. 22) [132]. The prepared Fe-P900-PCC exhibits outstanding catalytic activity in the heterogeneous hydrogenation of N-heterocycles, functionalized nitroarenes, and reductive amination reactions (Scheme 8), while the corresponding Fe-N900-PCC and Fe-C900-PCC material are inactive for these transformations under the same reaction conditions.

|
Download:
|
| Fig. 22. Schematic illustration of the preparation process of Fe-P900-PCC, Fe-N900-PCC and Fe-C900-PCC. Copied with permission [132]. Copyright 2020, Springer Nature. | |
|
Download:
|
| Scheme 8. The reductive amination and hydrogenation reactions catalyzed by Fe-P900-PCC. | |
The roles of Fe, N, and P in catalysts are studied in detail. According to the experimental and calculation results, it can be concluded that (1) the solely Fe-doped porous carbon material is inactive toward the hydrogenation reactions; (2) the single Fe atom present in Fe-N900-PCC as a planar Fe-N4 structure, which is inert to active H2 to catalyze the hydrogenation reactions; (3) the efficient dissociation of H2 in the hydrogenation is co-catalyzed by Fe species and P species. Furthermore, the reason why Fe-P900-PCC has high activity for hydrogenation reactions is also investigated. A unique distorted O2-Fe-P4 structure is formed by single Fe atom and the surface Pgrap species in Fe-P900-PCC, and it can be reduced by H2 to form Fe-P4 which is the main catalytic sites.
6. Glucose derived carbon materialsGlucose, with five hydroxyl groups and one aldehyde group in structure, is not only the most widely distributed monosaccharide in nature, but also the common units in many carbohydrates (Fig. 23) [92]. Because of its simple structure and single composition, glucose is easy to be carbonized as a carbon precursor. Consequently, glucose is also a promising candidate as the carbon source to synthesis highly efficient, environmentally friendly and mass-produced catalyst due to the abundant source and easily degradable nature [133, 134].

|
Download:
|
| Fig. 23. The chemical structure diagram of glucose. | |
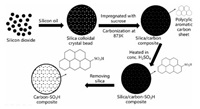
In 2013, Liu and co-workers fabricated highly ordered three-dimensional microporous carbon spheres bearing sulfonic acid groups (MPCS-SO3H) by carbonizing glucose in the crystal bead template, then sulfonating and removing the template (Fig. 24) [135]. MPCS-SO3H has remarkable activity and long-term stability in the esterification reaction of ethanol and acetic acid.
|
Download:
|
| Fig. 24. The preparation pathway for MPCS-SO3H. Copied with permission [135]. Copyright 2013, Elsevier. | |
In 2015, Qi et al. successfully prepared a carbonaceous microsphere which containing -COOH and phenolic -OH groups by hydrothermal carbonization (HTC) of glucose [136]. The obtained carbon material can be directly used for efficient catalytic dehydration of fructose into 5-hydroxymethylfurfural (5-HMF) with 88.1% yield at 100 ℃ in ionic liquid without any post-modification or in situ functionalization (Scheme 9), which can be contributed to the presence of -COOH groups in the catalyst.

|
Download:
|
| Scheme 9. The glucose derived carbonaceous microsphere material catalyzed dehydration of fructose. | |
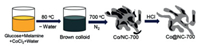
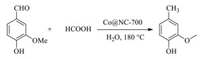
In 2017, Xia et al. synthesized a low-cost and eco-friendly Co embedded N-doped carbon material by an accurate annealing procedure of the mixture of glucose, harmless melamine and CoCl2 (Fig. 25) [137]. Compared with uncovered Co on activated charcoal (Co@AC), the Co@NC pyrolyzed at 700 ℃ (Co@NC-700) exhibits more efficiency in formic acid dehydrogenation and better activity for the hydrodeoxygenation (HDO) of unsaturated hydrocarbons (Scheme 10). According to the results of control experiments, the excellent performance can be ascribed to N-derived defective sites on Co@NC-700, which can enhance FA dehydrogenation and the HDO of vanillin.
|
Download:
|
| Fig. 25. The process for preparing Co@NC-700. Copied with permission [137]. Copyright 2017, Royal Society of Chemistry. | |
|
Download:
|
| Scheme 10. Co@NC-700 catalyzed hydrodeoxygenation of unsaturated hydrocarbons. | |
In the same year, Roy and co-workers prepared monodispersed small and stable copper oxide (CuO) NPs with an average size of 10-20 nm, then embedded these CuO NPs in porous carbon by carbonizing the mixture of CuO NPs and glucose at 850 ℃ (donated as CuO/PCM) (Fig. 26) [138]. The great activity of the obtained catalyst is tested in the synthesis of N-arylamides from several aromatic, aliphatic and heterocyclic aldoxime compounds (Scheme 11). Compared to the example for copper(Ⅱ) salt catalyzed preparation of amides from aldoximes, where ligands and bases, harsh reaction conditions, stoichiometric amount of metal were required, this work has described a mild and efficient method.
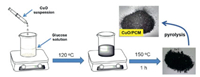
|
Download:
|
| Fig. 26. The preparing process of CuO/PCM. Copied with permission [138]. Copyright 2017, American Chemical Society. | |
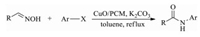
|
Download:
|
| Scheme 11. One-pot synthesis of N-arylamides catalyzed by CuO/PCM. | |
N-Acetyl-D-glucosamine (NAG) is the basic monomeric unit of the biopolymer chitin with 6.3% content of nitrogen atom, which is an ideal precursor for the synthesis of nitrogen-containing carbon materials. In 2017, Gao and co-workers prepared a magnetic nitrogen-containing carbon catalyst (Fe@NC-800) using NAG and iron nitrate as precursors through hydrothermal carbonization and high temperature calcination [139], which showed high catalytic performance in C-H oxidation reactions such as the epoxidation of styrene and the oxidation of ethylbenzene (Scheme 12). From the results of N 1s XPS analysis and density functional theory (DFT) calculations, it can be concluded that the N species in the material acts as the active sites and the iron species function as a promoter in the catalytic performance of the catalyst.

|
Download:
|
| Scheme 12. The oxidation of styrene and ethylbenzene catalyzed by Fe@NC-800. | |
7. General consideration 7.1. The comparison of carbohydrates in preparing carbon materials
Cellulose, chitin, chitosan, sucrose and glucose are the main carbohydrates for the preparation of carbon materials. Cellulose as the most abundant and cheapest carbohydrates, is generally used to prepare metal-free carbon materials [98, 100-101]. Due to the large molecular weight of cellulose and the weak coordination ability of O, cellulose cannot effectively disperse and stabilize metal precursors. Further modifications of cellulose such as N-doping are required for the preparation of cellulose-derived metal NPs embedded carbon catalysts [102-103].
Chitin and chitosan have three dimensional porous structures, which are full of nitrogen atoms and can be used directly as carbon supports of metals without any modification. Metal-nanoparticles embedded carbon and atomically dispersed metal-sites carbon can be obtained from chitin or chitosan, due to their excellent metal dispersion and stabilization ability [110-113, 125-126]. Fructose and glucose have simple structure and component, so it can be easily carbonized as a carbon precursor by hydrothermal treatment under lower temperature, which can be applied to control the fine structure of carbon materials, such as tuning the coordination environment of single metal atoms [130-132, 136]. In addition, sucrose can also be used as a binder between carbon particles, which makes the prepared carbon structure more stable [129].
7.2. Carbohydrates derived metal-free carbon materialsMetal-free heteroatom-doped carbon and functionalized carbon are two main kinds of metal-free carbon materials from carbohydrates.
For metal-free heteroatom-doped carbon, N, P and B are the common doping elements. The heteroatom-doping can be achieved either by calcinating carbohydrates directly or by external addition of heteroatomic dopants. Lots of heteroatomic dopants, such as ethylenediamine, ammonium phosphate, melamine, phytic acid and boric acid are applied for the synthesis of carbohydrate-derived carbon catalysts through chemical bonding, physical adsorption or mixing with carbohydrates [100, 101, 110]. The prepared metal-free heteroatom-doped carbon can be applied in the styrene epoxidation, oxidative coupling and reduction reactions. Taking advantage of the differences of atom radius and electronegativity between heteroatoms and carbon, doping heteroatoms into carbon materials can greatly change the properties of carbon materials, such as surface structure, electron transmission rate, pore structure and specific surface area. In general, the heteroatom sites or defect sites introduced by heteroatom doping are the catalytic active centers of these materials [100, 101, 110].
For functionalized carbon derived from carbohydrates, sulfonic acid group and carboxyl group are the main modifying groups, which can be achieved by in situ functionalization or post-modification [98, 99, 130, 135, 136]. These prepared functionalized carbon materials are common heterogeneous acid catalysts, so dehydration reaction or dehydration involved reactions, such as esterification and condensation, can be achieved by this kind of carbon catalysts [130, 135, 136].
7.3. Carbohydrates derived metal embedded carbon materialsMetal embedded carbon materials can be divided into metal-nanoparticles embedded carbon and atomically dispersed metal-sites carbon. There are mainly two strategies for the synthesis of these materials. One is to get carbon supports by calcinating or hydrothermal methods firstly, followed by trapping metal nanoparticles through deposition or impregnation method [103, 123]. Another one is one-pot pyrolysis of the mixture of carbohydrates and metal precursors, and used directly or followed by further heat treatment procedures [112, 113]. In order to ensure the preparation of atomically dispersed metal-sites carbon, it is necessary to increase the dispersion of metals and heteroatoms, and the contents of heteroatoms with strong coordination ability [125, 132].
In the most cases, the catalytic active sites of these materials are metal sites that in the form of metal nanoparticles or single metal atoms. Heteroatom-doped carbon not only plays a significant role for stabilizing metal species, thereby preventing the metals from corrosion or poisoning, but also can adjust the electronic state of metal sites through electronic interaction to tune their catalytic performance. In addition, some heteroatom-doped carbons can replace base by providing Lewis base sites, and adjust the hydrophilicity of these catalysts to promote the reactions in water [126, 137].
8. Summary and outlookThis review highlights the most recent literature on the design of carbohydrates derived carbon materials and their applications in organic reactions, such as oxidation, hydrogenation, esterification, reduction, cross-couplings and condensation reactions. In spite of the novel and practical strategies that have been developed with regard to the applications of these sustainable materials in organic synthesis, challenges and opportunities still remain:
(1) Carbohydrates are mostly macromolecular chain and polyhydroxy structure, which leads to irregular and weak adsorption of metals, resulting in uneven and unstable metal species after carbonation. Thus, the dispersibility and stability of metal need to be improved, which can affect the catalytic performance and applicability of carbon materials to a certain extent. In addition, the single-atom catalysts as the bridge between homogeneous and heterogeneous catalysts can offer ultimate atom economy and make every active site accessible. Therefore, the development of carbohydrates derived materials with precise functionalities for metal ion binding is necessary, which can be achieved by doping additional heteroatom during pyrolysis or chemical grafting of carbohydrates.
(2) There are limited examples of earth-abundant metal embedded carbon materials catalyzed organic reactions, and almost all of which are single metal catalysts. In fact, the earth-abundant metals such as Fe, Mn and Ni, have been extensively applied in both homogeneous and heterogeneous catalytic reactions, thereby the development of earth-abundant metals embedded carbon materials derived from carbohydrates still possess great potential. Furthermore, bimetal embedded carbon materials, with "ligand effect" and "geometric effect" between the two metals, can promote the surface electronic structure of corresponding catalyst to improve its catalytic activity and selectivity.
(3) The impact mechanism of metal elements and doped heteroatoms on the catalytic performance of carbon materials is not clear, which can be further studied by establishing the structure-activity relationship between catalysts and organic reactions based on some principles (such as Sabatier principle and D-band center theory) and reaction mechanism.
(4) The organic reactions that catalyzed by carbohydrates derived carbon materials are limited to some simple reactions, and the research of complex reactions such as cyclization reactions, tandem reaction and asymmetric reactions still need to be done.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowldgmentsThis work was supported by the National Key Research and Development Project (No. 2021YFC2100100), National Natural Science Foundation of China (Nos. 32001266, 22007049), and Natural Science Foundation of Jiangsu Province (No. BK20200728).
| [1] |
X. Mi, Y. Kong, J. Zhang, et al., Chin. Chem. Lett. 30 (2019) 2295-2298. DOI:10.1016/j.cclet.2019.09.040 |
| [2] |
Q. Huang, L. Zhu, D. Yi, et al., Chin. Chem. Lett. 31 (2020) 373-376. DOI:10.1016/j.cclet.2019.07.049 |
| [3] |
J.Y. Chen, C.T. Zhong, Q.W. Gui, et al., Chin. Chem. Lett. 32 (2021) 373-376. |
| [4] |
J. Jiang, Z. Wang, W.M. He, Chin. Chem. Lett. 32 (2021) 1591-1592. DOI:10.1016/j.cclet.2021.02.067 |
| [5] |
J. Jiang, F. Xiao, W.M. He, L. Wang, Chin. Chem. Lett. 32 (2021) 1637-1644. DOI:10.1016/j.cclet.2021.02.057 |
| [6] |
A. Taguchi, F. Schüth, Micropor. Mesopor. Mat. 77 (2005) 1-45. DOI:10.1016/j.micromeso.2004.06.030 |
| [7] |
Y. Li, J. Fu, S. Deng, X. Lu, J. Colloid Interf. Sci. 424 (2014) 104-112. DOI:10.1016/j.jcis.2014.03.012 |
| [8] |
Y. Shchipunov, Pure Appl. Chem. 84 (2012) 2579-2607. DOI:10.1351/PAC-CON-12-05-04 |
| [9] |
P. Pachfule, B.P. Biswal, R. Banerjee, Chem. Eur. J. 18 (2012) 11399-11408. DOI:10.1002/chem.201200957 |
| [10] |
F. Bai, Y. Xia, B. Chen, et al., Carbon 79 (2014) 213-226. DOI:10.1016/j.carbon.2014.07.062 |
| [11] |
M.I. Kim, Y.S. Lee, J. Nanosci. Nanotechnol. 16 (2016) 4310-4319. DOI:10.1166/jnn.2016.10968 |
| [12] |
H. Elhaes, A. Fakhry, M. Ibrahim, Mater, Mater. Today Proc. 3 (2016) 2483-2492. DOI:10.1016/j.matpr.2016.04.166 |
| [13] |
W. Wang, D. Yuan, Sci. Rep. 4 (2014) 5711. DOI:10.1038/srep05711 |
| [14] |
J. Wu, Y. Song, R. Zhou, et al., J. Mater. Chem. A 3 (2015) 7793-7798. DOI:10.1039/C5TA00805K |
| [15] |
F. Zheng, Y. Yang, Q. Chen, Nat. Commun. 5 (2014) 5261. DOI:10.1038/ncomms6261 |
| [16] |
G. Zhang, S. Hou, H. Zhang, et al., Adv. Mater. 27 (2015) 2400-2405. DOI:10.1002/adma.201405222 |
| [17] |
G. Xu, B. Ding, L. Shen, et al., J. Mater. Chem. A 1 (2013) 4490-4496. DOI:10.1039/c3ta00004d |
| [18] |
W. Bao, Z. Zhang, C. Zhou, et al., J. Power Sources 248 (2014) 570-576. DOI:10.1016/j.jpowsour.2013.09.132 |
| [19] |
K. Xi, S. Cao, X. Peng, et al., Chem. Commun. 49 (2013) 2192-2194. DOI:10.1039/c3cc38009b |
| [20] |
W. Yin, Y. Shen, F. Zou, et al., ACS Appl. Mater. Interf. 7 (2015) 4947-4954. DOI:10.1021/am509143t |
| [21] |
W. Chen, Z. Zhang, W. Bao, et al., Electrochim. Acta 134 (2014) 293-301. DOI:10.1016/j.electacta.2014.04.110 |
| [22] |
R.R. Salunkhe, J. Tang, Y. Kamachi, et al., ACS Nano 9 (2015) 6288-6296. DOI:10.1021/acsnano.5b01790 |
| [23] |
P. Zhang, F. Sun, Z. Shen, D. Cao, J. Mater. Chem. A 2 (2014) 12873-12880. DOI:10.1039/C4TA00475B |
| [24] |
W. Li, F. Zhang, Y. Dou, et al., Adv. Energy Mater. 1 (2011) 382-386. DOI:10.1002/aenm.201000096 |
| [25] |
D. Lu, R. Tao, Z. Wang, Front. Chem. Sci. Eng. 13 (2019) 310-323. DOI:10.1007/s11705-018-1750-7 |
| [26] |
J. Long, Y. Zhou, Y. Li, Chem. Commun. 51 (2015) 2331-2334. DOI:10.1039/C4CC08946D |
| [27] |
K. Sun, H. Shan, G.P. Lu, et al., Angew. Chem. Int. Ed. 60 (2021) 25188-25202. DOI:10.1002/anie.202104979 |
| [28] |
S. Chen, L. Ling, S. Jiang, H. Jiang, Green Chem. 22 (2020) 5730-5741. DOI:10.1039/D0GC01835J |
| [29] |
Z. Qi, C. Hu, Y. Zhong, et al., Org. Chem. Front. 8 (2021) 3137-3149. DOI:10.1039/D1QO00275A |
| [30] |
H. Chen, K. Shen, Q. Mao, et al., ACS Catal. 8 (2018) 1417-1426. DOI:10.1021/acscatal.7b03270 |
| [31] |
T. Rui, G. Lu, X. Zhao, et al., Chin. Chem. Lett. 32 (2021) 685-690. DOI:10.1016/j.cclet.2020.06.027 |
| [32] |
K. Sun, J. Sun, G. Lu, C. Cai, Green Chem. 21 (2019) 4334-4340. DOI:10.1039/C9GC01893J |
| [33] |
K. Sun, S. Chen, Z. Li, et al., Green Chem. 21 (2019) 1602-1608. DOI:10.1039/C8GC03868F |
| [34] |
W. Zhong, H. Liu, C. Bai, et al., ACS Catal. 5 (2015) 1850-1856. DOI:10.1021/cs502101c |
| [35] |
Y.X. Zhou, Y.Z. Chen, L. Cao, et al., Chem. Commun. 51 (2015) 8292-8295. DOI:10.1039/C5CC01588J |
| [36] |
L. Roldán, E. Pires, J.M. Fraile, E. García-Bordejé, Catal. Today 249 (2015) 153-160. DOI:10.1016/j.cattod.2014.10.008 |
| [37] |
Z. Kang, E. Wang, B. Mao, et al., Appl. Catal. A: Gen. 299 (2006) 212-217. DOI:10.1016/j.apcata.2005.10.038 |
| [38] |
J. Zhang, X. Liu, R. Blume, et al., Science 322 (2008) 73-77. DOI:10.1126/science.1161916 |
| [39] |
G.P. Lu, K. Sun, Y. Lin, et al., Nano Res. 15 (2022) 603-611. DOI:10.1007/s12274-021-3526-5 |
| [40] |
C. Copéret, M. Chabanas, R.P. Saint-Arroman, J.M. Basset, Angew. Chem. Int. Ed. 42 (2003) 156-181. DOI:10.1002/anie.200390072 |
| [41] |
S. Kramer, F. Hejjo, K.H. Rasmussen, S. Kegnæs, ACS Catal. 8 (2017) 754-759. |
| [42] |
R. Schlögl, Adv. Catal. 56 (2013) 103-185. |
| [43] |
J.M. Thomas, R. Raja, D.W. Lewis, Angew. Chem. Int. Ed. 44 (2005) 6456-6482. DOI:10.1002/anie.200462473 |
| [44] |
J. Sun, Q. Xu, Energ. Environ. Sci. 7 (2014) 2071-2100. DOI:10.1039/c4ee00517a |
| [45] |
A.J. Amali, H. Hoshino, C. Wu, et al., Chem. Eur. J. 20 (2014) 8279-8282. DOI:10.1002/chem.201402982 |
| [46] |
Y. Zhang, L. Qiu, Y. Yuan, et al., Appl. Catal. B: Environ. 144 (2014) 863-869. DOI:10.1016/j.apcatb.2013.08.019 |
| [47] |
R.H. Palmer, C.W. Kung, J. Liu, et al., Langmuir 34 (2018) 14143-14150. DOI:10.1021/acs.langmuir.8b02166 |
| [48] |
B. Liu, H. Shioyama, T. Akita, Q. Xu, J. Am. Chem. Soc. 130 (2018) 5390. |
| [49] |
H.L. Jiang, B. Liu, Y.Q. Lan, et al., J. Am. Chem. Soc. 133 (2011) 11854-11857. DOI:10.1021/ja203184k |
| [50] |
M. Hu, J. Reboul, S. Furukawa, et al., J. Am. Chem. Soc. 134 (2012) 2864-2867. DOI:10.1021/ja208940u |
| [51] |
L. Radhakrishnan, J. Reboul, S. Furukawa, et al., Chem. Mater. 23 (2011) 1225-1231. DOI:10.1021/cm102921y |
| [52] |
J. Biener, M. Stadermann, M. Suss, et al., Energ. Environ. Sci. 4 (2011) 656-667. DOI:10.1039/c0ee00627k |
| [53] |
G. Wu, K.L. More, C.M. Johnston, P. Zelenay, Science 332 (2011) 443-447. DOI:10.1126/science.1200832 |
| [54] |
Y. Chang, F. Hong, C. He, et al., Adv. Mater. 25 (2013) 4794-4799. DOI:10.1002/adma.201301002 |
| [55] |
Z.L. Yu, G.C. Li, N. Fechler, et al., Angew. Chem. Int. Ed. 55 (2016) 14623-14627. DOI:10.1002/anie.201605510 |
| [56] |
Z. Xie, R.J. White, J. Weber, et al., J. Mater. Chem. 21 (2011) 7434-7442. DOI:10.1039/c1jm00013f |
| [57] |
P. Zhang, Y. Gong, Z. Wei, et al., ACS Appl. Mater. Inter. 6 (2014) 12515-12522. DOI:10.1021/am5023682 |
| [58] |
T.W. Kim, I.S. Park, R. Ryoo, Angew. Chem. Int. Ed. 42 (2003) 4375-4379. DOI:10.1002/anie.200352224 |
| [59] |
Z. Han, S. Kong, J. Cheng, et al., Ind. Eng. Chem. Res. 58 (2019) 14785-14794. DOI:10.1021/acs.iecr.9b02143 |
| [60] |
K. Yoshino, R. Matsuoka, K. Nogami, et al., J. Appl. Phys. 68 (1990) 1720-1725. DOI:10.1063/1.346600 |
| [61] |
M. Nogi, F. Kurosaki, H. Yano, M. Takano, Carbohyd. Polym. 81 (2010) 919-924. DOI:10.1016/j.carbpol.2010.04.006 |
| [62] |
V. Budarin, J.H. Clark, J.J.E. Hardy, et al., Angew. Chem. Int. Ed. 45 (2006) 3782-3786. DOI:10.1002/anie.200600460 |
| [63] |
R.J. White, C. Antonio, V.L. Budarin, et al., Adv. Funct. Mater. 20 (2010) 1834-1841. DOI:10.1002/adfm.201000169 |
| [64] |
A. Primo, P. Atienzar, E. Sanchez, et al., Chem. Commun. 48 (2012) 9254-9256. DOI:10.1039/c2cc34978g |
| [65] |
H. Wang, Int. J. Electrochem. Sci. 13 (2018) 136-146. |
| [66] |
S. Kramer, N.R. Bennedsen, S. Kegnæs, ACS Catal. 8 (2018) 6961-6982. DOI:10.1021/acscatal.8b01167 |
| [67] |
B. List, Chem. Rev. 107 (2007) 5413-5415. DOI:10.1021/cr078412e |
| [68] |
D.N. Phan, M.Q. Khan, N.T. Nguyen, et al., Carbohyd. Polym. 252 (2021) 117175. DOI:10.1016/j.carbpol.2020.117175 |
| [69] |
E. Wiercigroch, E. Szafraniec, K. Czamara, et al., Spectrochim. Acta A 185 (2017) 317-335. DOI:10.1016/j.saa.2017.05.045 |
| [70] |
Z. Wu, S. Xu, Q. Yan, et al., Sci. Adv. 4 (2018) eaat0788. DOI:10.1126/sciadv.aat0788 |
| [71] |
A. Farrán, C. Cai, M. Sandoval, et al., Chem. Rev. 115 (2015) 6811-6853. DOI:10.1021/cr500719h |
| [72] |
C. Xu, M. Nasrollahzadeh, M. Sajjadi, et al., Renew. Sust. Energ. Rev. 112 (2019) 195-252. DOI:10.1016/j.rser.2019.03.062 |
| [73] |
B. Altava, M.I. Burguete, E. Garcia-Verdugo, S.V. Luis, Chem. Soc. Rev. 47 (2018) 2722-2771. DOI:10.1039/C7CS00734E |
| [74] |
M. Benaglia, A. Puglisi, F. Cozzi, Chem. Rev. 103 (2003) 3401-3430. DOI:10.1021/cr010440o |
| [75] |
J. Liang, Y. Huang, R. Cao, Coordin. Chem. Rev. 378 (2019) 32-65. DOI:10.1016/j.ccr.2017.11.013 |
| [76] |
N. Motahharifar, M. Nasrollahzadeh, A. Taheri-Kafrani, et al., Carbohyd. Polym. 232 (2020) 115819. DOI:10.1016/j.carbpol.2019.115819 |
| [77] |
M. Nasrollahzadeh, M. Sajjadi, M. Shokouhimehr, R.S. Varma, Coordin. Chem. Rev. 397 (2019) 54-75. DOI:10.1016/j.ccr.2019.06.010 |
| [78] |
X. Zhang, M. Fevre, G.O. Jones, R.M. Waymouth, Chem. Rev. 118 (2018) 839-885. DOI:10.1021/acs.chemrev.7b00329 |
| [79] |
A. El Kadib, ChemSusChem 8 (2015) 217-244. DOI:10.1002/cssc.201402718 |
| [80] |
Á Molnár, Coordin. Chem. Rev. 388 (2019) 126-171. DOI:10.1016/j.ccr.2019.02.018 |
| [81] |
M. Nasrollahzadeh, N. Shafiei, Z. Nezafat, et al., Carbohyd. Polym. 241 (2020) 116353. DOI:10.1016/j.carbpol.2020.116353 |
| [82] |
A. Khan, M. Goepel, J.C. Colmenares, R. Gläser, A.C.S. Sustain, ACS Sustain. Chem. Eng. 8 (2020) 4708-4727. DOI:10.1021/acssuschemeng.9b07522 |
| [83] |
S. De, A.M. Balu, J.C. van der Waal, R. Luque, ChemCatChem 7 (2015) 1608-1629. DOI:10.1002/cctc.201500081 |
| [84] |
Z. Wang, D. Shen, C. Wu, S. Gu, Green Chem. 20 (2018) 5031-5057. DOI:10.1039/C8GC01748D |
| [85] |
R.S. Varma, A.C.S. Sustain, ACS Sustian. Chem. Eng. 7 (2019) 6458-6470. DOI:10.1021/acssuschemeng.8b06550 |
| [86] |
D.P. Yang, Z. Li, M. Liu, et al., ACS Sustain. Chem. Eng. 7 (2019) 4564-4585. DOI:10.1021/acssuschemeng.8b06030 |
| [87] |
K. Harini, K. Ramya, M. Sukumar, Carbohyd. Polym. 200 (2018) 329-339. DOI:10.1016/j.carbpol.2017.12.072 |
| [88] |
Z. Yuan, J. Zhang, A. Jiang, et al., Carbohyd. Polym. 117 (2015) 414-421. DOI:10.1016/j.carbpol.2014.10.003 |
| [89] |
S.R. Marana, Comput. Struct. Biotec. 2 (2012) e201209006. DOI:10.5936/csbj.201209006 |
| [90] |
M. Kaushik, A. Moores, Green Chem. 18 (2016) 622-637. DOI:10.1039/C5GC02500A |
| [91] |
D. Klemm, F. Kramer, S. Moritz, et al., Angew. Chem. Int. Ed. 50 (2011) 5438-5466. DOI:10.1002/anie.201001273 |
| [92] |
D. Klemm, B. Heublein, H.P. Fink, A. Bohn, Angew. Chem. Int. Ed. 44 (2005) 3358-3393. DOI:10.1002/anie.200460587 |
| [93] |
H.Y. Choi, J.H. Bae, Y. Hasegawa, et al., Carbohyd. Polym. 234 (2020) 115881. DOI:10.1016/j.carbpol.2020.115881 |
| [94] |
F. Rol, M.N. Belgacem, A. Gandini, J. Bras, Prog. Polym. Sci. 88 (2019) 241-264. DOI:10.1016/j.progpolymsci.2018.09.002 |
| [95] |
Q. Wang, Q. Yao, J. Liu, et al., Cellulose 26 (2019) 7585-7617. DOI:10.1007/s10570-019-02642-3 |
| [96] |
H. Xu, T. Bronner, M. Yamamoto, H. Yamane, Carbohyd. Polym. 201 (2018) 182-188. DOI:10.1016/j.carbpol.2018.08.062 |
| [97] |
H. Lee, M. Nishino, D. Sohn, et al., Cellulose 25 (2018) 2829-2837. DOI:10.1007/s10570-018-1744-0 |
| [98] |
S. Kang, J. Ye, Y. Zhang, J. Chang, RSC Adv. 3 (2013) 7360-7366. DOI:10.1039/c3ra23314f |
| [99] |
Y. Han, H. Zhang, Y. Yang, et al., RSC Adv. 5 (2015) 58220-58227. DOI:10.1039/C5RA11408J |
| [100] |
Y. Zhai, M. Chu, C. Xie, et al., ACS Sustain. Chem. Eng. 6 (2018) 17410-17418. DOI:10.1021/acssuschemeng.8b05217 |
| [101] |
X. Xie, J. Shi, Y. Pu, et al., J. Colloid Interf. Sci. 571 (2020) 100-108. DOI:10.1016/j.jcis.2020.03.035 |
| [102] |
R.B.N. Baig, M.N. Nadagouda, R.S. Varma, Green Chem. 16 (2014) 4333-4338. DOI:10.1039/C4GC00748D |
| [103] |
M.S. Mirhosseyni, F. Nemati, A. Elhampour, Carbohyd. Polym. 217 (2019) 199-206. DOI:10.1016/j.carbpol.2019.04.005 |
| [104] |
V.V. Bazhenov, M. Wysokowski, I. Petrenko, et al., Int. J. Biol. Macromol. 76 (2015) 33-38. DOI:10.1016/j.ijbiomac.2015.02.012 |
| [105] |
V.S. Yeul, S.S. Rayalu, J. Polym. Environ. 21 (2012) 606-614. DOI:10.1007/s10924-012-0458-x |
| [106] |
P. Xu, D. Wu, L. Wan, et al., J. Colloid Interf. Sci. 421 (2014) 160-164. DOI:10.1016/j.jcis.2014.02.001 |
| [107] |
Q. Peng, M. Liu, J. Zheng, C. Zhou, Micropor. Mesopor. Mat. 201 (2015) 190-201. DOI:10.1016/j.micromeso.2014.09.003 |
| [108] |
G. Chen, M. Sun, Q. Wei, et al., J. Hazard. Mater. 244- 245 (2013) 86-93. DOI:10.1016/j.jhazmat.2012.11.032 |
| [109] |
X. Luo, S. Liu, J. Zhou, L. Zhang, J. Mater. Chem. 19 (2009) 3538-3545. DOI:10.1039/b900103d |
| [110] |
Y. Gao, X. Chen, J. Zhang, N. Yan, ChemPlusChem 80 (2015) 1556-1564. DOI:10.1002/cplu.201500293 |
| [111] |
Y. Cao, M. Tang, M. Li, et al., ACS Sustain. Chem. Eng. 5 (2017) 9894-9902. DOI:10.1021/acssuschemeng.7b01853 |
| [112] |
Y. Cao, B. Zhao, X. Bao, Y. Wang, ACS Catal. 8 (2018) 7077-7085. DOI:10.1021/acscatal.8b01644 |
| [113] |
X. Pei, Y. Deng, Y. Li, et al., Nanoscale 10 (2018) 14719-14725. DOI:10.1039/C8NR03215G |
| [114] |
M.E.I. Badawy, E.I. Rabea, Inter. J. Carbohyd. Chem. (2011) 1-292011. DOI:10.4324/9781315313535-5 |
| [115] |
R. LogithKumar, A. KeshavNarayan, et al., Carbohyd. Polym. 151 (2016) 172-188. DOI:10.1016/j.carbpol.2016.05.049 |
| [116] |
W.S. Wan Ngah, L.C. Teong, M.A.K.M. Hanafiah, Carbohyd. Polym. 83 (2011) 1446-1456. DOI:10.1016/j.carbpol.2010.11.004 |
| [117] |
H. Zhao, J. Xu, W. Lan, et al., Chem. Eng. J. 229 (2013) 82-89. DOI:10.1016/j.cej.2013.05.093 |
| [118] |
H. Zhong, L. Duan, P. Ye, et al., Res. Chem. Intermediat. 45 (2018) 907-918. |
| [119] |
T. Baran, E. Açıksöz, A. Menteş, J. Mol. Catal. A: Chem. 407 (2015) 47-52. DOI:10.1016/j.molcata.2015.06.008 |
| [120] |
S. Frindy, A. Primo, M. Lahcini, et al., Green Chem. 17 (2015) 1893-1898. DOI:10.1039/C4GC02175D |
| [121] |
M. Zeng, Y. Wang, Q. Liu, et al., Int. J. Biol. Macromol. 89 (2016) 449-455. DOI:10.1016/j.ijbiomac.2016.05.011 |
| [122] |
Y. Su, L. Ma, J. Chen, J. Xu, Carbohyd. Polym. 175 (2017) 113-121. DOI:10.1016/j.carbpol.2017.07.052 |
| [123] |
F. Zhang, C. Ma, S. Chen, et al., Mol. Catal. 452 (2018) 145-153. DOI:10.1016/j.mcat.2018.04.001 |
| [124] |
B. Sahoo, A.E. Surkus, M.M. Pohl, et al., Angew. Chem. Int. Ed. 56 (2017) 11242-11247. DOI:10.1002/anie.201702478 |
| [125] |
Y. Zhu, W. Sun, W. Chen, et al., Adv. Funct. Mater. 28 (2018) 1802167. DOI:10.1002/adfm.201802167 |
| [126] |
Y. Lin, G. Lu, X. Zhao, et al., Mol. Catal. 482 (2020) 110695. DOI:10.1016/j.mcat.2019.110695 |
| [127] |
R. Lemoine, Biochim. Biophys. Acta 1465 (2000) 246-262. DOI:10.1016/S0005-2736(00)00142-5 |
| [128] |
Z. Gao, H. Zhu, Y. Li, et al., J. Appl. Electrochem. 50 (2020) 549-558. DOI:10.1007/s10800-020-01411-6 |
| [129] |
O. Klepel, H. Strauβ, A. Garsuch, K. Böhme, Mater. Lett. 61 (2007) 2037-2039. DOI:10.1016/j.matlet.2006.07.188 |
| [130] |
R. Zhong, Y. Liao, L. Peng, et al., ACS Sustain. Chem. Eng. 6 (2018) 7859-7870. DOI:10.1021/acssuschemeng.8b01003 |
| [131] |
A.N. Hashemi, H. Eshghi, K. Lamei, Appl. Organomet. Chem. 33 (2019) 4835. DOI:10.1002/aoc.4835 |
| [132] |
X. Long, Z. Li, G. Gao, et al., Nat. Commun. 11 (2020) 4074. DOI:10.1038/s41467-020-17903-0 |
| [133] |
C. Du, H. Huang, X. Feng, et al., J. Mater. Chem. A 3 (2015) 7616-7622. DOI:10.1039/C5TA00648A |
| [134] |
X. Liu, L. Li, W. Zhou, et al., ChemElectroChem 2 (2015) 803-810. DOI:10.1002/celc.201500002 |
| [135] |
H. Huang, J. Zhang, Y. Zhang, et al., Solid State Sci. 24 (2015) 115-119. |
| [136] |
X. Qi, N. Liu, Y. Lian, RSC Adv. 5 (2015) 17526-17531. DOI:10.1039/C4RA15296D |
| [137] |
H. Yang, R. Nie, W. Xia, et al., Green Chem. 19 (2017) 5714-5722. DOI:10.1039/C7GC02648J |
| [138] |
M.M. Islam, M. Halder, A. Singha Roy, S.M. Islam, ACS Omega 2 (2017) 8600-8609. DOI:10.1021/acsomega.7b01028 |
| [139] |
Y. Zhang, H. Niu, X. Zhang, et al., RSC Adv. 7 (2017) 51831-51837. DOI:10.1039/C7RA10226G |
 2022, Vol. 33
2022, Vol. 33