b Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, School of Life Sciences, Jilin University, Changchun 130012, China
Solid phase extraction (SPE), a sample pretreatment technique with reliability, high efficiency, and low solvent consumption, has been extensively used for sample separation, purification, and enrichment. SPE adsorbents are applied to adsorb targets from sample, so as to separate them from sample containing interfering substances to achieve the separation and enrichment of targets. At present, SPE is suitable for the enrichment of targets from air, water, and complex biological samples. Notably, the key of SPE technology is the development of adsorbents. In recent years, mesoporous materials, nanomaterials, and magnetic nanomaterials have been successfully used as SPE adsorbents, and adsorbents with great selectivity, high adsorption capacity, and stable performance are needed to be developed [1, 2].
Cyclodextrin (CD), firstly reported by Villiers in 1891 [3], possesses a hydrophilic outer surface and a hydrophobic inner cavity. Depending on the number of glucose units, CDs are commonly divided into α-, β- and γ-CDs containing 6, 7, and 8 glucose units, respectively. CDs contain a large number of hydrophilic hydroxyl groups and cavity that can include other guest molecules, which makes CD become a strong candidate as SPE adsorbent [1, 4]. Although CDs have a series of advantages including low toxicity and excellent chemical reactivity, the low water solubility and instability limit their practical applications. It is necessary to introduce CDs into matrix materials or prepare CDs-based compounds, e.g., CD-functionalized Fe3O4 microspheres [5], CD-modified silica nanoparticles [6], CD polymers (CDPs) [7], and so on.
CD-functionalized Fe3O4 and CD-modified silica materials have been extensively and comprehensively studied, and there have been reviews about their applications in adsorption [8-11]. CDPs containing numerous CD units have abundant affinity sites, high specific surface, stable structure, and good biocompatibility [12]. However, to the best of our knowledge, rare reviews have been reported about the adsorption based on CDPs. Crini's group [13, 14] reviewed the progress of environmental pollutants treatment with epichlorohydrin (EPI)-crosslinked β-CDPs. Karoyo et al. [15] focused on the application of nano-sized β-CD-based molecularly imprinted polymers (MIPs) in the adsorption of perfluorinated compounds. In fact, besides the above two CDPs, other kinds of CDPs have already found their applications in the field of adsorption. However, no review has been comprehensively and systematically reported about the separation and enrichment based on CDPs adsorbents. The objective of this review is to describe the characteristics, adsorption mechanism, and application of different types of CDPs in detail. In addition, the present challenges and future prospects of evolution and employment are also briefly discussed.
2. CDPs adsorbentsThe design of CDPs adsorbents is based on either covalent binding or noncovalent action between CDs and other substances. To be specific, reactive groups on CDs can form stable covalent bonds with certain substances to construct CDPs [16]. In the other case, the formation of CDPs relies on noncovalent action between the hydrophobic cavity of CDs and other substances [17]. The formed CDPs not only maintain inherent macrocyclic structure and functional groups of CDs, but also possess the properties of polymer, thus showing a high degree of "integration" and "synergy" effects.
In previous reports [13, 18], CDPs were generally classified into crosslinked CDPs, grafted CDPs, linear CDPs, and hyperbranched CDPs. So far, linear CDPs have been relatively few and hyperbranched CDPs are rarely applied in the adsorption area. Therefore, the above two types of CDPs are not involved in this review. Notably, no review has been described in detail about the application of CD-based polyrotaxanes or pseudo-polyrotaxanes (CD-PRs/PPRs) and imprinted CDPs in adsorption although they have attracted much attention in the field of adsorption. Herein, CDPs are divided into the following four categories to discuss their respective advantages as adsorbents: 1) crosslinked CDPs; 2) grafted CDPs; 3) CD-PRs/PPRs; and 4) imprinted CDPs.
2.1. Crosslinked CDPsCrosslinked CDPs with stable molecular structure and large crosslinking network are formed by the crosslinking reaction between CD and crosslinker. The unoccupied CD cavity in crosslinked CDPs can form host-guest inclusion with adsorbates [19]. Additionally, the contained functional groups in crosslinked CDPs can act as effective action adsorption sites due to their affinity toward the adsorbates through hydrogen bonding or electrostatic interaction [20]. A variety of chemicals act as crosslinkers, including EPI [21], citric acid [22], 1, 2, 3, 4-butanetetracarboxylic acid (BTCA) [23, 24], tetrafluoroterephthalonitrile (TFN) [25], decafluorobiphenyl (DFB) [26], 4, 4'-difluorodiphenylsulfone [27], ethylene diamine tetraacetic acid (EDTA) [28], and so on. Among the various types of crosslinkers, EPI, citric acid, and TFN are commonly used. EPI is a well-known cheap crosslinker, and EPI-crosslinked CDPs can effectively adsorb Pb(Ⅱ), Cd(Ⅱ) [29], and azo dyes [30]. However, EPI produces carcinogenic byproducts in the crosslinking process, which is considered harmful to humans. Citric acid-crosslinked CDPs are used as efficient adsorbents for the capture of Cu(Ⅱ), cationic dyes, and paraquat [22, 31, 32], but the crosslinking reaction of non-toxic citric acid and CD has the problems of complicated synthesis steps and harsh conditions. Crosslinked CDPs with TFN as crosslinker can remove per- and poly-fluorinated alkyl substances [33], while TFN suffers from the disadvantages of high toxicity and cost. Therefore, it is of continuous interests to find cheap, non-toxic, and green crosslinkers with high activity for the wide development of crosslinked CDPs in the future.
In recent years, hypercrosslinked polymers (HCPs) with high specific surface area, physicochemical stability, and nano-sized pore size have been regarded as promising materials [34-36]. Due to the active groups and hydrophobic cavity of CD, it can be used as a good alternative monomer to construct HCPs. CD-based HCPs also have good adsorption performance toward organic substance and gases. Dai et al. [37] prepared hyper-crosslinked β-CD porous polymer (β-BnCD-HCPP) with high surface area and large pore volume for the adsorption of aromatic pollutants (4-nitrophenol, 4-chlorophenol, phenol) from water. In their another work [38], β-BnCD-HCPP was reported to exhibit high adsorption capacity for CO2 and selectivity toward CO2 over N2. In our previous work [39], we synthesized β-BnCD-HCP for the adsorption of albendazole (ABZ), possessing high surface area (1107 m2/g), high adsorption capacity (181.82 mg/g), and satisfactory reusability (50 adsorption-desorption cycles). The adsorption procedure was driven by host-guest effect, π-π stacking interaction, and encapsulation. In addition, we successfully fabricated viologen-based β-CDP with highly charge-selective adsorption of anionic dyes, exhibiting the adsorption capacity of 323 mg/g and 370 mg/g for congo red (CR) and methyl orange (MO) [40].
2.2. Grafted CDPsIf one or more CD units are connected to the polymer substrates by covalent chemical reaction, the formed CDPs are classified as grafted CDPs [41]. Generally, the polymer substrates grafted with CD are either natural polymers or organic synthetic polymers.
Natural polymers can be used as good substrates to graft with CDs, including chitosan [42, 43] and some cellulose [44], etc. Chitosan has become a very attractive biomaterial with reactive groups which can be regarded as sites for chemical modification of functionalized molecules (such as CDs), e.g., primary amine groups of position C2 and primary alcoholic groups of position C6 [45]. In Fan et al.'s work [43], amino groups on chitosan reacted with carboxyl groups on maleoyl-β-CD to obtain β-CD-grafted magnetic chitosan nanoparticles (CMCN). The presence of free -OH and -NH2 groups on chitosan and the cavity of CD made CMCN exhibit good adsorption performance toward hydroquinol. Cellulose, as an abundant and green natural polymer, is produced by wood, cotton, and some plants. Grafted CDPs with cellulose as substrate have a broad application prospect in water purification, such as the adsorption of Pb(Ⅱ) [46], dyes [47], and bisphenol pollutants [48] from wastewater. For instance, rice husk-based cellulose grafted with CDs [49] and β-CD-grafted bacterial cellulose [50] were prepared for the efficient adsorption of heavy metal ions and organic pollutants to achieve wastewater treatment.
Organic synthetic polymer substrates for CDs grafting mainly include polyethylenimine (PEI) [51], polyvinyl alcohol [52], polydopamine (PDA) [53], poly(glycidyl methacrylate-ethylene dimethacrylate) (poly(GMA-EDMA)) [54], poly(glycidyl methacrylate-pentaerythritol triacrylate) (poly-(GMA-PETA)) [55], and so on. Lee and Kwak [51] grafted β-CD onto branched PEI to prepare grafted β-CDP adsorbent for efficient adsorption of Cu(Ⅱ) through complexation between amine groups of PEI and Cu(Ⅱ). Wu et al. [53] designed and synthesized CD-attached PDA-coated fibers with proper pore structure and high surface area, which showed high adsorption capacity for phenolphthalein (Php) owing to host-guest interactions between β-CD and Php. In our previous study [54], we prepared lactoferrin (LF)-modified β-CD-grafted poly(GMA-EDMA) monolithic materials with strong hydrophilicity and favorable stability for the enrichment of Ga ion in cell line and human body fluids. Compared with other methods, e.g. β-CD incorporated multi-walled carbon nanotubes [56], our method exhibited higher selectivity and lower detection limit (2 ng/L), opening new possibilities for future biological application, chinical diagnostics, and drug discovery. In another work [57], we designed and synthesized a peanut agglutinin-β-CD-functionalized poly(hydroxyethyl methylacrylate-ethyleneglycol dimethacrylate) monolithic support for the selective enrichment of galactosylation glycopeptides, which are regarded as a novel candidate biomarker for pan-cancer screening.
2.3. CD-PRs/PPRsThe formation of CD-PRs/PPRs depends on the noncovalent interaction between multiple CDs and a polymer chain [58]. CD-PRs, a type of supramolecular CDPs possessing an interesting architecture, are usually obtained by threading multiple CDs on a linear polymer and blocking both ends of the polymer chain with bulky end-caps; alternatively, CD-PPRs are obtained without blocking. The matching of size is one of the most basic conditions for the self-assembly of host CD and guest polymer, which means that CD cavity has selectivity to the size and volume of the guest.
Specific linear polymer enters the hydrophobic cavity of CD, while all active hydroxyl groups on CD are not occupied, which can provide many exposed sites for adsorption. On the one hand, unmodified hydroxyl groups on CD have the affinity toward adsorbates, e.g., the chelating effect on metal ions. On the other hand, when the hydroxyl groups on CD are modified with other functional groups, better affinity toward adsorbates can be achieved than unmodified hydroxyl groups. For example, there is strong chelating interactions between Th(Ⅳ) and p-toluensulfonyl groups. Liu et al. [59] modified the hydroxyl groups of position C6 on CD with p-toluensulfonyl groups to obtain sulfated-β-CD (6-OTs-β-CD), after which a PR based on 6-OTs-β-CD was designed for the adsorption of Th(Ⅳ) from low concentration aqueous systems. The above merits guarantee that CD-PRs/PPRs can be considered as good materials for adsorption. Additionally, CD-PRs/PPRs-based materials exhibit satisfactory effect on the separation and enrichment of biomacromolecules. Lin et al. [60] prepared a supramolecular membrane based on CD-PR for the adsorption of bovine serum albumin (BSA). The CD-PR material was obtained by threading many α-CDs onto polyethylene glycol.
2.4. Imprinted CDPs 2.4.1. Molecularly imprinted CDPsMolecularly imprinted technology is a well-established method for the preparation of polymers that possesses specific molecular recognition properties of template species [61, 62]. MIPs have favorable stability, easy preparation, and low-cost potential, and the introduction of CD into MIPs brings extra unique advantages. During the imprinting process, the polymers are formed between CD and the target molecule through many intermolecular interactions such as van der Waals forces, electrostatic affinity, host-guest interaction, and hydrogen bonding, which is conducive to form more affinity binding sites and improve the affinity toward adsorbates. Kyzas and coworkers [63] designed two types of CD-MIPs for the adsorption of toxic and carcinogenic dyes. Three sulfonate groups in dye molecules made dyes form inclusions with CDs in CD-MIP through possible driving forces such as van der Waals forces and host-guest interaction.
Molecularly imprinted CDPs are considered to possess great binding capacity to adsorb organic substances due to the hydrophobic interior cavity of CD. Di(2-ethylhexyl)phthalate (DEHP), one of the most common plasticizers, can be included in the cavity of CD. According to this characteristic, Wang et al. [64] prepared graphene oxide-based MIPs modified with β-CD (GO-MIPs-β-CD) and applied them as SPE column adsorbents to specific recognition of DEHP in water samples. Similarly, MIP with chlorpyrifos as template and β-CD as monomer was developed as solid-phase microextraction (SPME) probe, based on which an efficient and selective MIP-SPME-GC method was established for the determination of chlorpyrifos and other organophosphorus pesticides in food [65].
2.4.2. Ion imprinted CDPsSimilar to MIPs, ion imprinted polymers (IIPs) with ions as templates specifically recognize inorganic ions after imprinting, so they are used for the removal, separation, and extraction of metal ions [66]. Unlike MIPs, IIPs interact with template ions mainly through coordination. The obtained polymer is eluted with an appropriate method to remove the template ions, forming specific identification sites in IIPs with the same shape and size as the template ions. Such specific identification sites allow the formed IIPs to be used to adsorb and separate inorganic ions in complex environments.
Hitherto, various monomers have been utilized to construct IIPs, including methacrylic acid (MAA) [67], 4-vinylpyridine (4-VP) [68], and β-CD [69]. Among them, β-CD as functional monomer has a strong selective recognition toward target ions. The introduction of CD brings many merits for IIPs, e.g., reactive groups of CD enable ions imprinted CDPs to have many recognition sites and strong recognition ability, and hydrophilic surface of CD make ions imprinted CDPs be applied for the removal of target ions in aqueous phase. In recent years, ions imprinted CDPs have been successfully applied for the adsorption of Cr(Ⅵ) [70], Cu(Ⅱ) [71], Ga(Ⅲ) [56], and U(Ⅵ) [69]. The fact that the coordination exists between O atoms of hydroxyl groups on CD and U(Ⅵ) drive the design of ions imprinted CDP for the adsorption of U(Ⅵ). Hao et al. designed ion imprinted β-CD-modified graphitic carbon nitride polymer (IIP-g-C3N4/β-CD) [69] for selective and efficient extraction of U(Ⅵ) from simulated seawater.
3. Adsorption mechanismThe adsorption mechanism of CDPs adsorbents varies with the adsorbates, and there is usually not only one force to realize the capture of adsorbates. CDPs adsorbents achieve the removal and separation of various substances in complex systems due to unique properties and advantages of CDPs. The hydrophobic interior cavity of CD, active groups on CDPs, and specific structure and formed uniform pore size have a great contribution to the adsorption of various adsorbates with CDPs adsorbents.
Firstly, CD cavity acts as a significant part in CDPs adsorbents because of host-guest complexes formed between adsorbates and CD. For example, BPA is known to be encapsulated by β-CD to form a well-defined host-guest complex, so CDPs are generally considered to be efficient and selective adsorbents for BPA. With the exception of BPA, certain dyes and phenolic compounds also have similar interaction with CD cavity [72, 73].
Secondly, in CDP, the active groups on CD or other positions such as groups on crosslinker can react directly with adsorbates. For instance, hydroxyl groups on CD can chelate metal ions, and the hydroxyl groups on EPI of crosslinked CDPs are involved in the adsorption of organic molecules through hydrogen bonding. Furthermore, these active groups are also modified with other functional groups. Active groups modified on CDPs may have chelation [28, 59], hydrogen bonding [74], electrostatic [75, 76] and hydrophilic interactions [77, 78] with certain adsorbates. For example, the PR made of 6-OTs-β-CD was obtained by modifying p-toluenesulfonyl groups on CD, and there was chelation between Th(Ⅳ) and oxygen atoms of p-toluenesulfonyl groups [59]. Similarly, carboxyl groups modified on the crosslinked CDP in an alkaline environment are helpful to adsorb cationic dyes through electrostatic interaction [79].
Thirdly, the structure of the formed CDPs contributes to adsorption capacity, especially crosslinking network structure of crosslinked CDPs and uniform pore size of CDPs [37, 80]. For instance, the peak pore size of β-CD-based HCP [81] of 1.3 nm fitted with the maximum molecular z length of BPA, i.e., 0.86 and 1.24 nm. The accordance between pore size of HCP and the BPA molecular size was conductive to increase the adsorption capacity for BPA.
4. ApplicationCDPs combine the excellent properties of CD and polymers, such as abundant affinity sites provided by active groups and the hydrophobic cavity of CD, high specific surface area, appropriate pore size, stable structure, and good biocompatibility. In addition, CDPs also have chemical stability, good separability, and great recyclability. Due to these advantages, CDPs have gained wide attention in the adsorption realm. Here, the adsorption properties of CDPs adsorbents for the removal of inorganic metal ions, organic pollutants, and biomacromolecules are summarized for reference.
4.1. Adsorption of inorganic metal ionsIn recent years, environmental pollution caused by the accumulation of heavy metals ions has been occurring in all aspects of human life. If the concentration of heavy metal ions exceeds the limits of what the human body can tolerate, acute or chronic poisoning and eventually cancer will be caused [82]. Duan et al. [83] reviewed applications of carbon-based adsorbents in the removal of heavy metals, including the adsorption of Pb(Ⅱ), Cu(Ⅱ), and Cr(Ⅱ) with CD-modified GO adsorbents, which also indicates that CDPs can act as promising adsorbents for the rapid and efficient adsorption of heavy metal ions from the environment [84].
It has been reported that various heavy metal ions such as Cu(Ⅱ) [28], Cd(Ⅱ) [85], and Pb(Ⅱ) [86] have been successfully adsorbed by crosslinked CDPs. The adsorption mechanism of crosslinked CDPs to remove metal ions is mainly through the complexation between the crosslinker and metal ions. Zhao et al. [28] developed EDTA-crosslinked CDP (EDTA-β-CD) with high adsorption performance for Cu(Ⅱ) (1.241 mmol/g) and Cd(Ⅱ) (1.106 mmol/g) from aqueous solution owing to the chelation between EDTA groups and metal ions (Fig. 1A). In another example, Qin et al. [85] synthesized crosslsinked β-CDPs with 1-vinylimidazole (Ⅵ) as crosslinker, featuring that good adsorption capacity for Cd(Ⅱ) (117 mg/g at pH 6.0) mainly due to chelation between N atoms of the imidazole groups on Ⅵ and Cd(Ⅱ) (Fig. 1B). The decreased adsorption performance at pH 7.0 was because of the mild hydrolysis of Cd(Ⅱ). Additionally, the obtained crosslinking network provided easy access to Cd(Ⅱ) to the adsorption sites.

|
Download:
|
| Fig. 1. (A) Adsorption of Cu(Ⅱ) with EDTA-β-CD. Reproduced with permission [28]. Copyright 2015, American Chemical Society. (B) Capture of Cd(Ⅱ) with a variety of crosslsinked β-CDPs with different monomer ratios (Left) and schematic illustration of the proposed adsorption mechanism (Right). Reproduced with permission [85]. Copyright 2019, Elsevier. (C) Adsorption of Pd(Ⅱ) under different pH, inserted adsorption mechanism. Reproduced with permission [41]. Copyright 2017, Elsevier. (D) Adsorption isotherms on IIP-g-C3N4/β-CD at 298 K, 308 K, and 318 K (Left) and hypothetical mechanisms for U(Ⅵ) adsorption (Right). Reproduced with permission [69]. Copyright 2018, Royal Society of Chemistry. | |
In grafted CDPs, large loading capacity of the CD units and functional groups on the substrate can enhance the adsorption capacity for metal ions [87]. Sharma et al. [41] were inspired to design and prepare a β-CD-grafted chitosan adsorbent for the removal of Pd(Ⅱ) by the presence of co-ordination between Pd(Ⅱ) and the lone pairs of oxygen and nitrogen on chitosan and β-CD (Fig. 1C). The maximum adsorption capacity for Pd(Ⅱ) was 202.02 mg/g at pH 6.0. Similarly, Zhao et al. [88] synthesized a porous chitosan-EDTA-β-CD (CS-ED-CD) adsorbent with average pore size of 54.6 nm, showing the high adsorption capacities for two metal ions Pb(Ⅱ) (0.803 mmol/g) and Cd(Ⅱ) (1.258 mmol/g).
Th is a highly toxic radioactive metallic element, which brings serious harm to human body. Notably, CD-PRs/PPRs have a bright application prospect in dealing with Th(Ⅳ) pollution due to the affinity between Th(Ⅳ) and for modified functional groups on CD. Liu et al. [59] prepared a CD-PR with 6-OTs-β-CD as host, poly(propylene glycol)bis(2-aminopropyl ether) (PPG-NH2) as guest, and 3, 5-dinitrobenzoic acid as blocker. The maximum adsorption capacity for Th(Ⅳ) was as high as 13.99 mg/g under the optimum condition thanks to the chelation between Th(Ⅳ) and p-toluensulfonyl groups of 6-OTs-β-CD. In 2018, Liu et al. [89] prepared a CD-PPR with the high capture capacity for Th(Ⅳ) of 15.366 mg/g using 6-OTs-β-CD as host and PPG-NH2 as guest.
Except Th(Ⅳ), U(Ⅵ) is also a radioactive element with high toxicity and tends to cause permanent damage to humans. Hao et al. [69] prepared IIP-g-C3N4/β-CD with a large specific surface area of 34.94 m2/g and a mesoporous structure (pore volume of 0.1601 cm3/g), which played a positive role in the selective adsorption of U(Ⅵ) (Fig. 1D). More importantly, the adsorption process was based on chemical complexation and electrostatic attraction between U(Ⅵ) and IIP-g-C3N4/β-CD and the maximum adsorption capacity for U(Ⅵ) was 859.66 mg/g at 298 K and pH 6.0. In addition, simulating seawater experiments indicated that IIP-g-C3N4/β-CD could be used as a promising extractant in seawater with 90% removal efficiency of U(Ⅵ).
4.2. Adsorption of organic pollutants 4.2.1. Adsorption of dyesDyes play a great role in promoting economic development and creating economic value. However, most dye molecules with stable structures are difficult to degrade and stay in the environment longer. More importantly, some organic dyes have carcinogenicity. Therefore, the removal of dyes is conducive to the establishment of a clean environment and it is crucial to find effective and efficient adsorbents for dyes [90, 91]. The capture of dyes with different types of CDPs is summarized herein.
Various crosslinked CDPs with different crosslinkers were designed to remove dyes. For instance, citric acid-crosslinked β-CDP [92], EDTA-crosslinked β-CDP [28], ethanolamine-modified TFN-crosslinked β-CDP (EA-CDP) [93], and TFN-crosslinked β-CDP containing carboxylic acid groups [94] were applied to adsorb methylene blue (MB). In addition, Xu et al. [95] synthesized two crosslinked CDPs (CDP-DEA and CDP-NH2) by further modification of TFN-crosslinked β-CDP (CDP-CN) with diethanolamine and amide groups. It can be inferred from the zeta potential diagram that the positively charged CDP-DEA and CDP-NH2 have strong electrostatic interaction with anionic dyes (Fig. 2A), CR and orange G (OG). The maximum adsorption capacities of CDP-DEA and CDP-NH2 for CR were 813 and 40 mg/g, while 442 and 446 mg/g for OG. The adsorption mechanism mainly depended on three adsorption contributions: inclusion complexes formed between CD cavity and dyes, crosslinking network capture, and electrostatic interaction between positively charged polymers adsorbents and anionic dyes. Zhou's group made a remarkable contribution to the adsorption of dyes with crosslinked CDPs. They prepared citric acid-crosslinked CDPs-modified PDA adsorbent (CD-CA/PDA) with abundant carboxyl and catechol groups for the adsorption of cationic dyes. CD-CA/PDA exhibited high adsorption capacities of 582.95, 1174.67, and 473.01 mg/g for MB, malachite green (MG), and CV [32].
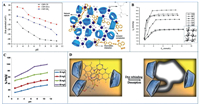
|
Download:
|
| Fig. 2. (A) Zeta potentials of CDP-CN, CDP-DEA, and CDP-NH2 (Left) and the proposed adsorption mechanisms of CDP-DEA (Right). Reproduced with permission [95]. Copyright 2020, Elsevier. (B) Capture of dyes with CD-grafted thiacalix[4]arene netty polymer, inserted the structure of this polymer. Reproduced with permission [97]. Copyright 2016, Springer. (C) Adsorption of MB with SRHG under different pH. Reproduced with permission [98]. Copyright 2018, Elsevier. (D) Adsorption mechanisms of CD-MIP for U(Ⅵ). Reproduced with permission [63]. Copyright 2013, Elsevier. | |
Similarly, the adsorption of dyes with grafted CDPs is also based on electrostatic interaction [96]. Chen and coworkers [97] grafted CDs onto thiacalix[4]arene netty polymer to prepare polymers for the adsorption of both cationic and anionic dyes (Fig. 2B). The adsorption performance for dyes varied with pH as followings. At low pH values, ammonium ions converted from amino groups on the polymer had affinity toward anionic dyes due to electrostatic attraction while no affinity toward cationic dyes because of electrostatic repulsion. In the same way, at high pH values, alkoxy anions changed from hydroxylic groups had affinity toward cationic dyes while no affinity toward anionic dyes. The maximum adsorption capacities for Victoria blue B (VB), Crystal violet (CV), Neutral red (NR), and MB were 5.821, 6.825, 6.135, and 5.381 mmol/g, respectively.
In Soleimani et al.'s work [98], a slide ring hydrogel (SRHG) was synthesized through the reaction of isocyanate-functionalized GO with α-CD-PR. The amide groups on polyacrylamide, hydroxyl groups on α-CD, and oxygen-containing groups on GO sheets provided adsorption sites for MB based on electrostatic interaction, which was favorable for SRHG to have high adsorption capacity of 92.3 mg/g for MB at the optimal pH of 7.0 (Fig. 2C), which enabled SRHG to be used for future wastewater purification applications.
Considering the inclusion between CD cavity and dyes, MIPs with dyes as template molecules are designed. Kyzas et al. [63] prepared a CD-MIP with dye Remazol Red 3BS (RR) as the template molecule. Thanks to CD cavity that could form inclusion complexes with RR (Fig. 2D), the highest adsorption capacity reached 35 mg/g. In another example, Liu et al. [33] designed and synthesized a MIP with β-CD-maleic anhydride (β-CD-MA) and [2-(methacryloyloxy) ethyl] trimethylammonium chloride (DMC) as co-functional monomers and CR as the template. There was the inclusion interaction between β-CD and CR as well as ionic binding effect between DMC and CR, so the MIP was applied for SPE as an effective and selective adsorbent to preconcentrate CR from four food samples (pork, beef, jelly, and hawthorn volumes).
4.2.2. Adsorption of BPABisphenol A (BPA) is used in the production of plastics, epoxy resins and other polymer materials. With the development of modern industry, BPA has appeared in daily life of people. However, once BPA enters the organism of people, endocrine system will be distributed [99]. β-CD is known to encapsulate BPA to form a well-defined host-guest complex, so SPE based on β-CDPs adsorbents is a promising strategy to solve the pollution problem of BPA [100].
β-CD cavity of crosslinked β-CDPs is not occupied, so crosslinked β-CDPs have great potential for BPA adsorption [101]. For example, the maximum adsorption capacities for BPA were 278, 83.01, and 103 mg/g of dichloroxylene-hypercrosslinked β-CDP [81], citric acid-crosslinked β-CDP [92], and EPI-TFN-crosslinked β-CDP [102], respectively. Alsbaiee et al. [25] synthesized TFN-crosslinked porous β-CDPs (P-CDPs) with the high surface area of 263 m2/g and permanent porosity (pores of 1.8–3.5 nm diameter) (Fig. 3A). The CD units in the P-CDP could form stable 1:1 complexes with BPA, but P-CDP achieved BPA: β-CD ratios greater than 1 at higher BPA concentration, demonstrating that the outside of CDs and other non-specific interactions also contributed to the adsorption of BPA. The maximum equilibrium adsorption capacity for BPA reached as high as 88 mg/g. Xu et al. [103] used rigid TFN and common flexible EPI as crosslinkers to prepare macroporous and ultra-microporous β-CDP (T-E-CDP) possessing a fluffy honeycomb structure. T-E-CDP had superior adsorption performance for BPA of 0.56 mmol/g.

|
Download:
|
| Fig. 3. (A) The structure of P-CDPs (Left), N2 adsorption (blue squares) and desorption (grey squares) isotherms (Upper right), and the cumulative pore volume of P-CDPs (Lower right). Reproduced with permission [25]. Copyright 2016, Springer. (B) Absorbance reflecting the adsorption efficiency of different amount of adsorbents toward BPA, inserted TEM images of Fe3O4@SiO2-PGMA-CD nanoparticles. Reproduced with permission [106]. Copyright 2014, Elsevier. (C) Adsorption of BPA with MMIP and MNIP at different initial BPA concentration (Left) and at different contact time (Right). Reproduced with permission [107]. Copyright 2017, Elsevier. (D) Adsorption isotherms on Fe3O4-MIP. Reproduced with permission [108]. Copyright 2016, Elsevier. | |
Similarly, unoccupied CD cavity makes grafted CDPs show significant potential in adsorbing BPA. As grafted β-CDPs, β-CD-carboxymethylcellulose-based (CDCMC) hydrogel beads with a high adsorption capacity for BPA of 167 µmol/g in water were prepared [104]. The adsorption was predominated by host-guest interaction between CD and BPA. Grafted CDPs for the adsorption of BPA are usually combined with magnetic Fe3O4 nanoparticles [105] because the introduction of Fe3O4 nanoparticles facilitates subsequent separation. Wang et al. [106] grafted CDs onto poly(glycidyl methacrylate) (PGMA) and coated on Fe3O4 nanoparticles to prepare Fe3O4@SiO2-PGMA-CD nanoparticles. The significance of the hydrophobic cavity of CD for BPA adsorption was proved by the comparative adsorption experiments of Fe3O4@SiO2-PGMA-CD and Fe3O4@SiO2-PGMA nanoparticles. 10 mg of Fe3O4@SiO2-PGMA-CD with the saturation magnetization of 7.17 emu/g achieved the adsorption of most of BPA (97.2%) (Fig. 3B).
Additionally, Huang et al. [107] successfully synthesized magnetic MIP (MMIP) with β-CD as functional monomer for the selective removal of BPA from wastewater. Active hydroxyl groups and the hydrophobic cavity of CD provided the covalent modification and imprinting effect, which played a vital role in enhancing the adsorption capacity. As shown in Fig. 3C, the adsorption experiments showed that MMIP possessed higher adsorption capacity (105.5 mg/g), higher initial adsorption rate (7.062 mg g−1 min−1) and faster equilibrium rate (~60 min) than MNIP (without the addition of template BPA) at optimal conditions (pH 6, 298 K). Similarly, Yuan et al. [108] prepared MMIP beads with 4-VP and β-CD as binary functional monomers for the adsorption of BPA. This stable and reusable MMIP with adsorption amount for BPA of 17.98 mg/g (Fig. 3D) and fast equilibrium rate (~40 min) can be applied for selective extraction of low levels of BPA in milk.
4.2.3. Adsorption of phenolic compoundsPhenolic compounds widely exist in nature and are commonly found in wastewater from various production processes. However, most of phenolic compounds cause environmental pollution and are even generally regarded as toxic carcinogens that are harmful to human health [109]. Notably, CD can form inclusion complexes with phenolic compounds via host-guest and π-π interactions, and polymers possessing high specific surface area and stable structures have a great contribution to the capture of phenolic compounds [110].
Among CDPs adsorbents, crosslinked CDPs account for the majority and exhibit good adsorption performance toward phenolic compounds. It is worth mentioning that the crosslinking networks and consistent pore structure of crosslinked CDPs also contribute to the adsorption of phenolic compounds. Garcia-Zubiri et al. [111] studied the adsorption of phenol and 1-naphthol with a set of crosslinked CDPs with four different crosslinkers, confirming the importance of CD cavity and crosslinking network. The adsorption of other phenolic compounds with crosslinked CDPs also shows good performance similar to that of phenols [112, 113]. The removal of cresols [112], 2, 4-dinitrophenol (2, 4-DNP) [114], 2, 4, 6-trichlorophenyl (2, 4, 6-TCP) [115], hydroquinone [116], and several volatile phenols [117] with CDPs adsorbents has been achieved. Huang et al. [115] prepared phenyl-rich crosslinked β-CDPs with high crosslinking density (PCD-PCP(H)), featuring abundant underlying porous network and dense surface pores. As shown in Fig. 4A, the ultrahigh adsorption capacity of PCD-PCP(H) for 2, 4, 6-TCP (378.18 mg/g) was ascribed to CD cavity and crosslinking units. Specifically, CD can form inclusions with 2, 4, 6-TCP through hydrogen bonding and van der Waals interactions, and crosslinking units can affinity 2, 4, 6-TCP through hydrogen bonding, van der Waals, and π-π interactions.

|
Download:
|
| Fig. 4. (A) Adsorption isotherms of 2, 4, 6-TCP on PCD-PCP(H) at 25, 35 and 45 ℃ (Left) and optimized configurations of 2, 4, 6-TCP adsorbed on two active adsorption sites (Right). Reproduced with permission [115]. Copyright 2020, Elsevier. (B) Adsorption of phenol (Left), p-nitrophenol (Middle), and p-chlorophenol (Right). Reproduced with permission [73]. Copyright 2009, Elsevier. | |
The adsorption of phenolic compounds with grafted CDPs containing abundant CD units has also been reported. Li et al. [73] found that β-CD-functionalized chitosan (CS-CD) exhibited higher adsorption amount of 34.93, 179.73, 20.56 mg/g for phenol, p-nitrophenol (PNP), and p-chlorophenol from aqueous solution compared with chitosan (Fig. 4B). Hydroxyl bonding, host-guest and π-π interactions dominated the adsorption process. Guo et al. [74] grafted β-CD onto poly(acrylic acid) (PAA) to prepare microsphere β-CD-PAA copolymers with great β-CD content for the adsorption of PNP based on host-guest interaction, hydrogen bonding, and van der Waals forces. The adsorption capacities of these copolymers for PNP were 0.359−2.20 mmol/g at pH 4.6 and 0.070−0.191 mmol/g at pH 10.3.
The adsorption of phenolic compounds with molecularly imprinted CDPs has been rarely reported. Surikumaran et al. [118] used 2, 4-DCP as the template molecule and MAA-functionalized β-CD as functional monomer to prepare MIP MAA-β-CD with unique properties of β-CD and the imprinted cavities, which was used in molecular imprinted-solid phase extraction (MISPE) to extract and remove 2, 4-DCP and five other several phenolic compounds from environmental water samples.
4.3. Enrichment of biomacromoleculesBiomacromolecules refer to organic molecules with molecular weights of tens of thousands or more as the main active components in living organisms. Common biomacromolecules include proteins, nucleic acids, lipids, and carbohydrates. Efficient enrichment strategies are often needed since the direct analysis of biomacromolecules in complex biological systems is usually difficult [119]. The capture of enzymes and proteins with CDPs materials has gain great efforts, while the enrichment of other biomacormolecules is still on its way [120].
4.3.1. Enrichment of LysozymeLysozyme (Lys) is widely distributed in various tissues of human body, secretions of mammals, and egg white of poultry. Due to anti-bacterial, anti-viral, and anti-tumor effects of Lys, it acts as a natural food preservative and is used as an essential tool enzyme for cell fusion [121]. Hence, the separation and enrichment of Lys with CDPs adsorbents have received widespread attention recently.
CD contained in CDPs can form many intermolecular interactions (i.e., van der Waals forces, electrostatic affinity, hydrogen bonding) with Lys [122]. Among the various CDPs, crosslinked CDPs have attracted much attention for Lys enrichment. Duan et al. [123] synthesized carboxyl-functionalized porous CDPs (P-CDP-COO−) with average pore size of 12.7 nm and external surface area of 18.7 m2/g for the selective extraction of Lys from egg white (Fig. 5A). The porous structure, abundant carboxylic functional groups, and preventing aggregation provided by CDs make the maximum saturated adsorption capacity of P-CDP-COO− as high as 1520 mg/g. Notably, electrostatic interaction between carboxylic groups and Lys dominated the adsorption process.

|
Download:
|
| Fig. 5. (A) Adsorption of Lys and other proteins with P-CDP-COO−, inserted TEM image of P-CDP-COO−. Reproduced with permission [123]. Copyright 2019, Elsevier. (B) Adsorption of Lys with ILs-Fe3O4@DA/GO/β-CD-SMIP and ILs-Fe3O4@DA/GO/β-CD-SNIP at different initial BPA concentration (Left) and at different contact time (Right). Reproduced with permission [124]. Copyright 2016, Elsevier. | |
CD-based MIPs [124-126] with modified CD as functional monomer and Lys as the template molecule have been prepared. The high capacity was raised from various interactions between Lys and CD-based MIPs, such as hydrophobic interaction and hydrogen bonding. Duan et al. [124] prepared surface molecularly imprinted polymer (SMIP) with ionic liquid modified Fe3O4@dopamine/GO/β-CD (ILs-Fe3O4@DA/GO/β-CD) as the supporting material, featuring high adsorption amount for Lys of 101 mg/g and fast equilibrium rate (~10 min) (Fig. 5B). Due to imprinting effect, ILs-Fe3O4@DA/GO/β-CD-SMIP exhibited good adsorption sensitivity to Lys under the interference of other proteins and amino acids.
4.3.2. Enrichment of proteinsAs one kind of biomacromolecules, proteins play an essential role in biochemical and biomedical processes. It is possible to analyze and detect proteins directly in complex biological samples, but direct analysis is not commonly used due to the low protein content in real complex samples [127-129]. CDPs can be one of the strong candidates to effectively enrich and purify target proteins from real samples.
Until now, a wide variety of strategies for enriching glycopeptides have been studied [130, 131], including lectin affinity chromatography, hydrazide chemistry, hydrophilic interaction liquid chromatography (HILIC) [132, 133], and boronic acid affinity chromatography [134-136]. Over the past five years, our group has been devoted to design diverse grafted CDPs as adsorbents for HILIC. CDs modified with various hydrophilic substances (such as Glu [77], lysine [137], and glycocluster [78]) were grafted onto polymer monolith to construct grafted CDPs to enrich glycopeptides based on hydrophilic partitioning. In 2018, we grafted glu-functionalized CD molecular tube (gluCDMT) [77] onto monolith materials to prepare hydrophilic poly(hydroxyethyl methylacry-pentaerythritol triacrylate-gluCDMT) (poly(HEMA-PETA-gluCDMT)) monolith with stable structure and good acidic/alkalic resistance. The hydrophilicity (the water contact angle of 59o ± 1.1o), high surface area (199.1 m2/g), and pore structure (the average pore size of 120−180 nm) contributed to the enrichment of glycoproteins and glycopeptides (Fig. 6A). Poly(HEMA-PETA-gluCDMT) monolith exhibited high binding capacities for glycoproteins (49.57 and 47.26 mg/g for horseradish peroxidase (HRP) and immunoglobulin G (IgG)), great sensitivity (LOD of 0.5 fmol), and high selelectivity (HRP/BSA = 1:10000).

|
Download:
|
| Fig. 6. (A) Mass spectra of tryptic digests of HRP (Left) and IgG (Right) after enrichment by poly(HEMA-PETA-gluCDMT) monolith, inserted TEM (Left) and water contact angle (Right) of poly(HEMA-PETA-gluCDMT) monolith. Reproduced with permission [77]. Copyright 2018, American Chemical Society. (B) Schematic illustration of recognition of target proteins with MIP. Reproduced with permission [139]. Copyright 2013, Elsevier. (C) Adsorption isotherm of BHb with MIP (Left) and selective adsorption of BHb, BSA, Lyz or Cyt with MIP and NIP (Right). Reproduced with permission [140]. Copyright 2010, Elsevier. | |
The application of CD-PRs in protein enrichment is usually combined with molecular imprinting technique [138]. In 2012, Guo et al. [139] prepared protein MIPs with CD-PPRs as pseudo-supports and BSA as the template (Fig. 6B). The spatial configuration created by CD-PPR and Cu2+ was helpful to increase the recognition specificity of MIPs toward BSA. The highest adsorption capacity for BSA of 5.11 mg/g was obtained and high selective specificity toward BSA was proved by the separation BSA from the protein mixtures.
There is no doubt that molecularly imprinted CDPs have aroused great interest in the separation of proteins. Zhang et al. [140] prepared a protein MIP with acryloyl-β-CD and acrylamide as monomers for enrichment of bovine hemoglobin (BHb) based on hydrogen bonding and hydrophobic interaction between β-CD and BHb. This MIP possessed the imprinted cavities with a special shape and functional groups, exhibiting high adsorption capacity (37.1 mg/g) and high selectivity toward BHb from mixtures of BSA, Lys, and cytochrome c (Cyt) (selectivity factor of 3.81, 3.51, and 4.71) (Fig. 6C).
At present, CDPs for the capture of other biomacromolecules have not been reported. However, from the example of Lys and proteins adsorption, it can be concluded that CDPs are expected to be modified with groups owing affinity to the adsorbates. From this, we can infer that CDPs have the potential to separate and enrich other biomacromolecules by virtue of various functional groups.
Typical applications of CDPs for various targets adsorption are listed in Table 1.
|
|
Table 1 Summary of CDPs adsorbents toward various adsorbates including inorganic metal ions, organic pollutants, and biomacromolecules. |
Until now, taking advantages of many adsorption sites and high specific surface area of CDPs adsorbents, CDPs adsorbents have attracted wide attention in the field of adsorption and various CDPs adsorbents with good adsorption performance have been reported. In this review, a wide range of CDPs adsorbents are presented, including crosslinked CDPs, grafted CDPs, CD-PRs/PPRs, and imprinted CDPs. The structural characteristics, chemical properties, and advantages of the four types of CDPs are also introduced. Subsequently, the adsorption mechanism of CDPs toward different targets is briefly described. Finally, examples of different types CDPs for inorganic metal ions, organic pollutants, and biomacromolecules are listed.
Although CDPs can be effective adsorbents for the separation and enrichment of various substances, many problems have not been solved definitely. For example, certain adsorption mechanisms have not been clearly explained up to now, and more importantly, CDPs adsorbents are still far from practical applications, in which more and higher requirements are put forward for the performance of adsorbents. In order to make CDPs adsorbents be applied in practice, the following problems and challenges need to be solved.
Firstly, as for the preparation of CDPs adsorbents, it is very essential to choose a green and environmentally friendly synthetic approach to achieve sustainable development. Therefore, on the premise of ensuring high adsorption performance of adsorbents, non-toxic solvents and raw materials should be selected; facile and green synthetic routes should be designed; and emerging methods and technologies should be considered as far as possible. Considering the requirement of sustainable development, green and eco-friendly adsorbents with high affinity and specificity toward adsorbates urgently need to be developed.
Secondly, there is no doubt that the cost of CDPs adsorbents is worthy of great attention and the development of low-cost adsorbents with high performance has always been a hot topic in research. In Alsbaiee et al.'s work [25], a detailed analysis of the yield of P-CDPs and the cost of raw materials, pilot-scale, and dedicated manufacturing was made. A comparison of the cost of CDPs adsorbents and commercial activated carbons proved that the prepared P-CDPs were economically competitive. But few articles have mentioned the cost of the prepared adsorbents and the comparison to that of traditional adsorbents. Cost savings will help CDPs adsorbents achieve sustainable development in the future, so the cost of CDPs adsorbents need to be saved to the maximum extent in future studies.
Thirdly, the stability of CDPs adsorbents is an important factor to determine whether the adsorbent is suitable for practical application. Thermostability, chemical stability, and reusability of CDPs adsorbents need to be investigated in detail under actual environmental conditions. Regrettably, the research of CDPs adsorbents in these aspects has not received the attention they deserve.
Fourthly, it is vital to consider the effect of actual environmental conditions on CDPs adsorbents such as pH, temperature, ionic strength, and existence of competing compounds in solution. Since most adsorption and separation experiments are carried out under environmental conditions, it is necessary to conduct a systematic study on environmental parameters.
In summary, similar to other adsorbents, concerns such as green and eco-friendly methods, low cost, long-term stability, reusability, practicality under environmental conditions should be solved in order to enable CDPs adsorbents to be applied on an industrial scale. In future studies, more attention should be paid to the development of environmentally friendly, inexpensive, stable, reusable, and highly practical adsorbents. Additionally, CDPs are expected to have a broad application prospect in the catalytic degradation of pollutants due to catalytic degradation effect of CDs [141, 142]. Kim et al. [143] embedded crosslinked CDPs into TiO2 as a catalytic support, which showed high photocatalytic capability toward aromatic pollutants. Therefore, the application of CDPs in catalytic degradation should be also considered in the future development. Although the route for adsorption and separation of various substances with CDPs adsorbents is full of challenges, it is worth devoting our best efforts to attain the target and we also believe that CDPs will be of great value in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe acknowledge the financial support of Open Project of State Key Laboratory of Supramolecular Structure and Materials, Jilin University, China (No. sklssm2021014) and the Fundamental Research Funds for the Central Universities, JLU.
| [1] |
J. Ma, Y. Zhang, B. Zhao, Q. Jia, Anal. Chim. Acta 1122 (2020) 97-113. DOI:10.1016/j.aca.2020.04.054 |
| [2] |
X. Pan, J. Ji, N. Zhang, M. Xing, Chin. Chem. Lett. 31 (2020) 1462-1473. DOI:10.1016/j.cclet.2019.10.002 |
| [3] |
S.D. Eastburn, B.Y. Tao, Biotechnol. Adv. 12 (1994) 325-339. DOI:10.1016/0734-9750(94)90015-9 |
| [4] |
A. Harada, Y. Takashima, H. Yamaguchi, Chem. Soc. Rev. 38 (2009) 875-882. DOI:10.1039/b705458k |
| [5] |
A.Z.M. Badruddoza, A.S.H. Tay, P.Y. Tan, K. Hidajat, M.S. Uddin, J. Hazard. Mater. 185 (2011) 1177-1186. DOI:10.1016/j.jhazmat.2010.10.029 |
| [6] |
A. Bibby, L. Mercier, Green Chem. 5 (2003) 15-19. DOI:10.1039/b209251b |
| [7] |
F. van de Manakker, T. Vermonden, C.F. van Nostrum, W.E. Hennink, Biomacromolecules 10 (2009) 3157-3175. DOI:10.1021/bm901065f |
| [8] |
H. Shen, H. Wu, H. Ji, H. Shi, Chin. J. Org. Chem. 34 (2014) 630-646. DOI:10.6023/cjoc201311016 |
| [9] |
R.M. Kakhki, J. Incl. Phenom. Macrocycl. Chem. 82 (2015) 301-310. DOI:10.1007/s10847-015-0512-0 |
| [10] |
A. Gentili, J. Chromatogr. A 1609 (2020) 460654. DOI:10.1016/j.chroma.2019.460654 |
| [11] |
Q. Liu, Y. Zhou, J. Lu, Y. Zhou, Chemosphere 241 (2020) 125043. DOI:10.1016/j.chemosphere.2019.125043 |
| [12] |
F. Seidi, Y. Jin, H. Xiao, Carbohyd. Polym. 242 (2020) 116277. DOI:10.1016/j.carbpol.2020.116277 |
| [13] |
N. Morin-Crini, G. Crini, Prog. Polym. Sci. 38 (2013) 344-368. DOI:10.1016/j.progpolymsci.2012.06.005 |
| [14] |
N. Morin-Crini, P. Winterton, S. Fourmentin, et al., Prog. Polym. Sci. 78 (2018) 1-23. DOI:10.1016/j.progpolymsci.2017.07.004 |
| [15] |
A.H. Karoyo, L.D. Wilson, Nanomaterials 5 (2015) 981-1003. DOI:10.3390/nano5020981 |
| [16] |
B. Gidwani, A. Vyas, Colloid Surf. B 114 (2014) 130-137. DOI:10.1016/j.colsurfb.2013.09.035 |
| [17] |
A. Harada, Y. Takashima, M. Nakahata, Accounts Chem. Res. 47 (2014) 2128-2140. DOI:10.1021/ar500109h |
| [18] |
S. Qie, Y. Hao, Z. Liu, J. Wang, J. Xi, Acta Chim. Sin. 78 (2020) 232-244. DOI:10.6023/A20010006 |
| [19] |
Z. Wang, S. Guo, B. Zhang, J. Fang, L. Zhu, J. Hazard. Mater. 384 (2020) 121187. DOI:10.1016/j.jhazmat.2019.121187 |
| [20] |
M.J. Klemes, Y. Ling, C. Ching, et al., Angew. Chem. Int. Ed. 58 (2019) 12049-12053. DOI:10.1002/anie.201905142 |
| [21] |
H. Liu, X. Cai, Y. Wang, J. Chen, Water Res. 45 (2011) 3499-3511. DOI:10.1016/j.watres.2011.04.004 |
| [22] |
J. Junthip, J. Macromol. Sci. A 56 (2019) 555-563. DOI:10.1080/10601325.2019.1586444 |
| [23] |
J. Junthip, Fiber. Polym. 19 (2018) 2335-2343. DOI:10.1007/s12221-018-8557-5 |
| [24] |
J. Junthip, W. Promma, S. Sonsupap, C. Boonyanusith, Iran. Polym. J. 28 (2019) 213-223. DOI:10.1007/s13726-019-00692-9 |
| [25] |
A. Alsbaiee, B.J. Smith, L. Xiao, et al., Nature 529 (2016) 190-194. DOI:10.1038/nature16185 |
| [26] |
L. Xiao, Y. Ling, A. Alsbaiee, et al., J. Am. Chem. Soc. 139 (2017) 7689-7692. DOI:10.1021/jacs.7b02381 |
| [27] |
Z. Wang, P. Zhang, F. Hu, Y. Zhao, L. Zhu, Carbohydr. Polym. 177 (2017) 224-231. DOI:10.1016/j.carbpol.2017.08.059 |
| [28] |
F. Zhao, E. Repo, D. Yin, et al., Environ. Sci. Technol. 49 (2015) 10570-10580. DOI:10.1021/acs.est.5b02227 |
| [29] |
M. Nejad, H. Nejad, H. Sheibani, A. Heydari, J. Polym. Environ. 28 (2020) 1626-1636. DOI:10.1007/s10924-020-01701-2 |
| [30] |
K.D. Zhang, F.C. Tsai, N. Ma, et al., Desalin. Water Treat. 86 (2017) 174-182. DOI:10.5004/dwt.2017.21187 |
| [31] |
J. Junthip, N. Jumrernsuk, P. Klongklaw, W. Promma, S. Sonsupap, SN Appl. Sci. 1 (2019) 106. DOI:10.1007/s42452-018-0102-z |
| [32] |
H. Chen, Y. Zhou, J. Wang, et al., J. Hazard. Mater. 389 (2020) 121897. DOI:10.1016/j.jhazmat.2019.121897 |
| [33] |
F. Liu, S. Zhang, G. Wang, J. Zhao, Z. Guo, RSC Adv. 5 (2015) 22811-22817. DOI:10.1039/C4RA14719G |
| [34] |
L. Tan, B. Tan, Chem. Soc. Rev. 46 (2017) 3322-3356. DOI:10.1039/C6CS00851H |
| [35] |
S. Wang, M. Xu, T. Peng, et al., Nat. Commun. 10 (2019) 676. DOI:10.1038/s41467-019-08651-x |
| [36] |
D. Wang, X. Li, X. Jin, Q. Jia, Sep. Purif. Technol. 216 (2019) 9-15. DOI:10.1016/j.seppur.2019.01.065 |
| [37] |
H. Li, B. Meng, S.H. Chai, H. Liu, S. Dai, Chem. Sci. 7 (2016) 905-909. DOI:10.1039/C5SC04034E |
| [38] |
B. Meng, H. Li, S.M. Mahurin, H. Liu, S. Dai, RSC Adv. 6 (2016) 110307-110311. DOI:10.1039/C6RA18307G |
| [39] |
D. Wang, G. Chen, X. Li, Q. Jia, Sep. Purif. Technol. 227 (2019) 115720. DOI:10.1016/j.seppur.2019.115720 |
| [40] |
X. Li, M. Zhou, J. Jia, Q. Jia, React. Funct. Polym. 126 (2018) 20-26. DOI:10.1016/j.reactfunctpolym.2018.03.004 |
| [41] |
S. Sharma, N. Rajesh, J. Environ. Chem. Eng. 5 (2017) 1927-1935. DOI:10.1016/j.jece.2017.03.015 |
| [42] |
N. Aoki, M. Nishikawa, K. Hattori, Carbohydr. Polym. 52 (2003) 219-223. DOI:10.1016/S0144-8617(02)00308-9 |
| [43] |
L. Fan, M. Li, Z. Lv, et al., Colloid Surf. B 95 (2012) 42-49. DOI:10.1016/j.colsurfb.2012.02.007 |
| [44] |
A. Celebioglu, S. Demirci, T. Uyar, Appl. Surf. Sci. 305 (2014) 581-588. DOI:10.1016/j.apsusc.2014.03.138 |
| [45] |
M. Prabaharan, J.F. Mano, Carbohydr. Polym. 63 (2006) 153-166. DOI:10.1016/j.carbpol.2005.08.051 |
| [46] |
J. Qu, Q. Meng, X. Lin, et al., Sci. Total Environ. 752 (2021) 141854. DOI:10.1016/j.scitotenv.2020.141854 |
| [47] |
X. Yue, J. Huang, F. Jiang, H. Lin, Y. Chen, J. Eng. Fiber Fabr. 14 (2019) 1-10. |
| [48] |
Y. Lv, J. Ma, K. Liu, et al., J. Hazard. Mater. 403 (2021) 123666. DOI:10.1016/j.jhazmat.2020.123666 |
| [49] |
J. Qu, Y. Yuan, Q. Meng, et al., J. Hazard. Mater. 400 (2020) 123142. DOI:10.1016/j.jhazmat.2020.123142 |
| [50] |
F. Liu, C. Chen, J. Qian, J. Hazard. Mater. 405 (2021) 124122. DOI:10.1016/j.jhazmat.2020.124122 |
| [51] |
J.H. Lee, S.Y. Kwak, J. Appl. Polym. Sci. (2019) 48475. |
| [52] |
D. Ghemati, D. Aliouche, J. Appl. Spectrosc. 81 (2014) 257-263. DOI:10.1007/s10812-014-9919-4 |
| [53] |
H. Wu, J. Kong, X. Yao, et al., Chem. Eng. J. 270 (2015) 101-109. DOI:10.1016/j.cej.2015.02.019 |
| [54] |
H. Zheng, T. Zhu, X. Li, G. Wang, Q. Jia, Biomacromolecules 18 (2017) 3971-3977. DOI:10.1021/acs.biomac.7b01003 |
| [55] |
H. Zheng, X. Li, Q. Jia, ACS Appl. Mater. Interfaces 10 (2018) 5909-5917. DOI:10.1021/acsami.7b18999 |
| [56] |
Y. Hu, Z. Zhang, H. Zhang, et al., Chin. J. Chem. 30 (2012) 377-385. DOI:10.1002/cjoc.201180474 |
| [57] |
H. Zheng, T. Zhu, X. Li, J. Ma, Q. Jia, Anal. Chim. Acta 983 (2017) 141-148. DOI:10.1016/j.aca.2017.06.034 |
| [58] |
G. Wenz, B.H. Han, A. Muller, Chem. Rev. 106 (2006) 782-817. DOI:10.1021/cr970027+ |
| [59] |
H. Liu, C. Qi, Z. Feng, L. Lei, S. Deng, J. Radioanal. Nucl. Chem. 314 (2017) 1607-1618. DOI:10.1007/s10967-017-5518-1 |
| [60] |
L. Lin, M. Dong, C. Liu, et al., Macromol. Rapid Commun. 35 (2014) 1587-1591. DOI:10.1002/marc.201400289 |
| [61] |
P. Zhang, L.Y. Jiang, J.T. Ma, Q. Jia, Chin. J. Anal. Chem. 49 (2020) 24-33. |
| [62] |
L. Li, Y. Lu, Z. Bie, H.Y. Chen, Z. Liu, Angew. Chem. Int. Ed. 52 (2013) 7451-7454. DOI:10.1002/anie.201207950 |
| [63] |
G.Z. Kyzas, N.K. Lazaridis, D.N. Bikiaris, Carbohydr. Polym. 91 (2013) 198-208. DOI:10.1016/j.carbpol.2012.08.016 |
| [64] |
C. Wang, L. Cheng, L. Zhang, Y. Zuo, J. Sep. Sci. 42 (2019) 1248-1256. DOI:10.1002/jssc.201801171 |
| [65] |
J.K. Ma, X.C. Huang, S.L. Wei, J. Sep. Sci. 41 (2018) 3152-3162. DOI:10.1002/jssc.201800385 |
| [66] |
T.P. Rao, R. Kala, S. Daniel, Anal. Chim. Acta 578 (2006) 105-116. DOI:10.1016/j.aca.2006.06.065 |
| [67] |
Z. Zhou, D. Kong, H. Zhu, et al., J. Hazard. Mater. 341 (2018) 355-364. DOI:10.1016/j.jhazmat.2017.06.010 |
| [68] |
X. Cai, J. Li, Z. Zhang, et al., ACS Appl. Mater. Interfaces 6 (2014) 305-313. DOI:10.1021/am4042405 |
| [69] |
X. Hao, R. Chen, Q. Liu, et al., Inorg. Chem. Front. 5 (2018) 2218-2226. DOI:10.1039/C8QI00522B |
| [70] |
O.B. Nchoe, M.J. Klink, F.M. Mtunzi, V.E. Pakade, J. Mol. Liq. 298 (2020) 111991. DOI:10.1016/j.molliq.2019.111991 |
| [71] |
Y. Cao, J. Li, J. Liu, et al., Polym. Int. 68 (2019) 694-699. DOI:10.1002/pi.5752 |
| [72] |
Y. Li, P. Lu, J. Cheng, et al., Talanta 187 (2018) 207-215. DOI:10.1016/j.talanta.2018.05.030 |
| [73] |
J.M. Li, X.G. Meng, C.W. Hu, J. Du, Bioresour. Technol. 100 (2009) 1168-1173. DOI:10.1016/j.biortech.2008.09.015 |
| [74] |
R. Guo, L.D. Wilson, J. Appl. Polym. Sci. 125 (2012) 1841-1854. DOI:10.1002/app.36272 |
| [75] |
L. Fan, Y. Zhang, C. Luo, et al., Int. J. Biol. Macromol. 50 (2012) 444-450. DOI:10.1016/j.ijbiomac.2011.12.016 |
| [76] |
J. He, Y. Li, C. Wang, et al., Appl. Surf. Sci. 426 (2017) 29-39. DOI:10.1016/j.apsusc.2017.07.103 |
| [77] |
H. Zheng, X. Li, Q. Jia, ACS Appl. Mater. Interfaces 10 (2018) 19914-19921. DOI:10.1021/acsami.8b01445 |
| [78] |
H.J. Zheng, J.T. Ma, W. Feng, Q. Jia, J. Chromatogr. A 1512 (2017) 88-97. DOI:10.1016/j.chroma.2017.07.032 |
| [79] |
G. Crini, H. Peindy, Dyes Pigments 70 (2006) 204-211. DOI:10.1016/j.dyepig.2005.05.004 |
| [80] |
Q. Hu, D.W. Gao, H. Pan, L. Hao, P. Wang, RSC Adv. 4 (2014) 40071-40077. DOI:10.1039/C4RA05653A |
| [81] |
X. Li, M. Zhou, J. Jia, J. Ma, Q. Jia, Sep. Purif. Technol. 195 (2018) 130-137. DOI:10.1016/j.seppur.2017.12.007 |
| [82] |
W.S.W. Ngah, M.A.K.M. Hanafiah, Bioresour. Technol. 99 (2008) 3935-3948. DOI:10.1016/j.biortech.2007.06.011 |
| [83] |
C. Duan, T. Ma, J. Wang, Y. Zhou, J. Water Process Eng. 37 (2020) 101339. DOI:10.1016/j.jwpe.2020.101339 |
| [84] |
A.R. Pedrazzo, A. Smarra, F. Caldera, et al., Polymers 11 (2019) 1658. DOI:10.3390/polym11101658 |
| [85] |
X. Qin, L. Bai, Y. Tan, et al., Chem. Eng. J. 372 (2019) 1007-1018. DOI:10.1016/j.cej.2019.05.006 |
| [86] |
Z. Duan, M. Song, T. Li, et al., RSC Adv. 8 (2018) 31542-31554. DOI:10.1039/C8RA06171H |
| [87] |
J.T. Tsiepe, B.B. Mamba, Inamuddin, A.S. Abd-El-Aziz, A.K. Mishra, J. Inorg. Organomet. Polym. Mater. 28 (2018) 467-480. DOI:10.1007/s10904-017-0741-3 |
| [88] |
F. Zhao, E. Repo, D. Yin, et al., Sci. Rep. 7 (2017) 15811. DOI:10.1038/s41598-017-16222-7 |
| [89] |
H. Liu, S. Deng, L. Lei, et al., Radiochim. Acta 106 (2018) 373-381. DOI:10.1515/ract-2016-2746 |
| [90] |
X. Li, Y.Y. Cui, Y.J. Chen, C.X. Yang, X.P. Yan, Microporus Mesoporius Mater. 296 (2020) 110013. DOI:10.1016/j.micromeso.2020.110013 |
| [91] |
P. Li, D.W. Zhang, Q. Jia, Chin. J. Chromatogr. 38 (2020) 297-306. |
| [92] |
W. Huang, Y. Hu, Y. Li, et al., J. Taiwan Inst. Chem. Eng. 82 (2018) 189-197. DOI:10.1016/j.jtice.2017.11.021 |
| [93] |
H.L. Jiang, M.Y. Xu, Z.W. Xie, et al., J. Mol. Struct. 1203 (2020) 127373. DOI:10.1016/j.molstruc.2019.127373 |
| [94] |
H.L. Jiang, J.C. Lin, W. Hai, et al., Colloid Surf. A 560 (2019) 59-68. DOI:10.1016/j.colsurfa.2018.10.004 |
| [95] |
M.Y. Xu, H.L. Jiang, Z.W. Xie, et al., J. Taiwan Inst. Chem. Eng. 108 (2020) 114-128. DOI:10.1016/j.jtice.2020.01.005 |
| [96] |
X. Yue, F. Jiang, D. Zhang, H. Lin, Y. Chen, Fiber. Polym. 18 (2017) 2102-2110. DOI:10.1007/s12221-017-1211-9 |
| [97] |
S. Chen, H. Guo, F. Yang, X. Di, J. Polym. Res. 23 (2016) 28. DOI:10.1007/s10965-016-0920-x |
| [98] |
K. Soleimani, A.D. Tehrani, M. Adeli, Carbohydr. Polym. 187 (2018) 94-101. DOI:10.1016/j.carbpol.2018.01.084 |
| [99] |
P. Hu, Z.H. Xiong, J.M. Liu, Chem. J. Chin. Univ. 33 (2012) 2111-2116. |
| [100] |
D.M. Alzate-Sanchez, Y. Ling, C. Li, et al., ACS Appl. Mater. Interfaces 11 (2019) 8089-8096. DOI:10.1021/acsami.8b22100 |
| [101] |
C. Yang, H. Huang, T. Ji, et al., Polym. Int. 68 (2019) 805-811. |
| [102] |
X. Hu, G. Xu, H. Zhang, et al., ACS Appl. Mater. Interfaces 12 (2020) 12165-12175. DOI:10.1021/acsami.0c00597 |
| [103] |
G. Xu, X. Xie, L. Qin, et al., Green Chem. 21 (2019) 6062-6072. DOI:10.1039/C9GC02422K |
| [104] |
H. Kono, K. Onishi, T. Nakamura, Carbohydr. Polym. 98 (2013) 784-792. DOI:10.1016/j.carbpol.2013.06.065 |
| [105] |
Y. Kang, L. Zhou, X. Li, J. Yuan, J. Mater. Chem. 21 (2011) 3704-3710. DOI:10.1039/c0jm03513k |
| [106] |
N. Wang, L. Zhou, J. Guo, et al., Appl. Surf. Sci. 305 (2014) 267-273. DOI:10.1016/j.apsusc.2014.03.054 |
| [107] |
D. Huang, Z. Tang, Z. Peng, et al., J. Taiwan Inst. Chem. Eng. 77 (2017) 113-121. DOI:10.1016/j.jtice.2017.04.030 |
| [108] |
Y. Yuan, Y. Liu, W. Teng, et al., J. Chromatogr. A 1462 (2016) 2-7. DOI:10.1016/j.chroma.2016.06.045 |
| [109] |
X. Li, Y.Y. Cui, C.X. Yang, X.P. Yan, Talanta 208 (2020) 120434. DOI:10.1016/j.talanta.2019.120434 |
| [110] |
X. Xue, Q. Gu, G. Pan, et al., Inorg. Chem. 53 (2014) 1521-1529. DOI:10.1021/ic402494m |
| [111] |
I.X. Garcia-Zubiri, G. Gonzalez-Gaitano, J.R. Isasi, J. Colloid Interface Sci. 307 (2007) 64-70. DOI:10.1016/j.jcis.2006.10.076 |
| [112] |
A. Romo, F.J. Peñas, J.R. Isasi, I.X. García-Zubiri, G. González-Gaitano, React. Funct. Polym. 68 (2008) 406-413. DOI:10.1016/j.reactfunctpolym.2007.07.005 |
| [113] |
H. Huang, Y. Fan, J. Wang, H. Gao, S. Tao, Macromol. Res. 21 (2013) 726-731. DOI:10.1007/s13233-013-1086-6 |
| [114] |
J.M. Anne, Y.H. Boon, B. Saad, et al., R. Soc. Open Sci. 5 (2018) 180942. DOI:10.1098/rsos.180942 |
| [115] |
Q. Huang, K. Chai, L. Zhou, H. Ji, Chem. Eng. J. 387 (2020) 124020. DOI:10.1016/j.cej.2020.124020 |
| [116] |
F. Zha, S. Li, Y. Chang, J. Yan, J. Membr. Sci. 321 (2008) 316-323. DOI:10.1016/j.memsci.2008.05.007 |
| [117] |
C. Dang, V. Jiranek, D.K. Taylor, K.L. Wilkinson, Molecules 25 (2020) 910. DOI:10.3390/molecules25040910 |
| [118] |
H. Surikumaran, S. Mohamad, N.M. Sarih, M. Raoov, Sep. Sci. Technol. 50 (2015) 2342-2351. |
| [119] |
H. Chen, C. Deng, X. Zhang, Angew. Chem. Int. Ed. 49 (2010) 607-611. DOI:10.1002/anie.200904885 |
| [120] |
X. Li, H. Zhang, N. Zhang, et al., ACS Sustain. Chem. Eng. 7 (2019) 11511-11520. DOI:10.1021/acssuschemeng.9b01382 |
| [121] |
S. Yamaguchi, C. Hong, T. Mannen, S. Tsukiji, T. Nagamune, Biotechnol. Lett. 26 (2004) 1787-1791. DOI:10.1007/s10529-004-4609-6 |
| [122] |
K. Ishimura, K. Fukunaga, T. Irie, et al., J. Chromatogr. A 769 (1997) 209-214. DOI:10.1016/S0021-9673(97)00088-5 |
| [123] |
H.L. Duan, Q.L. Niu, J. Wang, et al., J. Chromatogr. A 1600 (2019) 80-86. DOI:10.1016/j.chroma.2019.04.056 |
| [124] |
H. Duan, X. Wang, Y. Wang, et al., Anal. Chim. Acta 918 (2016) 89-96. DOI:10.1016/j.aca.2016.03.008 |
| [125] |
J. Zhang, Y. Tian, L. Wang, Y. Han, Biomed. Chromatogr. 28 (2014) 534-540. DOI:10.1002/bmc.3065 |
| [126] |
W. Zhang, L. Qin, X.W. He, W.Y. Li, Y.K. Zhang, J. Chromatogr. A 1216 (2009) 4560-4567. DOI:10.1016/j.chroma.2009.03.056 |
| [127] |
P.F. Guo, X.M. Wang, X.W. Chen, et al., TrAC Trends Anal. Chem. 120 (2019) 115650. DOI:10.1016/j.trac.2019.115650 |
| [128] |
X.Y. Long, Z.J. Zhang, J.Y. Li, D. Sheng, H.Z. Lian, Anal. Chem. 89 (2017) 10446-10453. DOI:10.1021/acs.analchem.7b02476 |
| [129] |
M. Zhang, X. Zhang, X. He, L. Chen, Y. Zhang, Nanoscale 4 (2012) 3141-3147. DOI:10.1039/c2nr30316g |
| [130] |
H. Zheng, J. Jia, Z. Li, Q. Jia, Anal. Chem. 92 (2020) 2680-2689. DOI:10.1021/acs.analchem.9b04691 |
| [131] |
H. Qi, Z. Li, H. Zheng, L. Fu, Q. Jia, Chin. Chem. Lett. 30 (2019) 2181-2185. DOI:10.1016/j.cclet.2019.06.046 |
| [132] |
G. Qing, J. Yan, X. He, X. Li, X. Liang, TrAC Trends Anal. Chem. 124 (2020) 115570. DOI:10.1016/j.trac.2019.06.020 |
| [133] |
B. Jiang, Q. Wu, N. Deng, et al., Nanoscale 8 (2016) 4894-4897. DOI:10.1039/C5NR08126B |
| [134] |
Y. Xu, Z. Wu, L. Zhang, et al., Anal. Chem. 81 (2009) 503-508. DOI:10.1021/ac801912t |
| [135] |
Y. Qu, J. Liu, K. Yang, et al., Chem. Eur. J. 18 (2012) 9056-9062. DOI:10.1002/chem.201103514 |
| [136] |
L. Zhang, Y. Xu, H. Yao, et al., Chem. Eur. J. 15 (2009) 10158-10166. DOI:10.1002/chem.200901347 |
| [137] |
H. Zheng, N. Song, X. Li, Q. Jia, Analyst 142 (2017) 4773-4781. DOI:10.1039/C7AN01436H |
| [138] |
Y. Zhang, M.J. Guo, J.X. Yao, et al., Chin. J. Anal. Chem. 42 (2014) 186-191. DOI:10.1016/S1872-2040(13)60709-4 |
| [139] |
M. Guo, X. Hu, Z. Fan, et al., Talanta 105 (2013) 409-416. DOI:10.1016/j.talanta.2012.10.061 |
| [140] |
W. Zhang, L. Qin, R.R. Chen, et al., Appl. Surf. Sci. 256 (2010) 3000-3005. DOI:10.1016/j.apsusc.2009.11.064 |
| [141] |
Y. Zhou, J. Lu, Q. Liu, et al., J. Hazard. Mater. 384 (2020) 121267. DOI:10.1016/j.jhazmat.2019.121267 |
| [142] |
Y. Zhou, J. He, J. Lu, Y. Liu, Y. Zhou, Chin. Chem. Lett. 31 (2020) 2623-2626. DOI:10.1016/j.cclet.2020.02.008 |
| [143] |
D. Kim, H. Kim, J.Y. Chang, Small 16 (2020) 1907555. DOI:10.1002/smll.201907555 |
 2022, Vol. 33
2022, Vol. 33