b School of Pharmacy, Second Military Medical University, Shanghai 200433, China;
c Institute of Human Virology and Department of Microbiology and Immunology, University of Maryland School of Medicine, Baltimore MD 21201, United States;
d Department of Molecular Medicine, University of Padova, Padova 35121, Italy;
e Institute of Translational Medicine, Shanghai University, Shanghai 200444, China
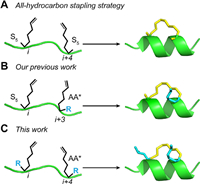
Stapled helical peptides with preferable biophysical properties (stabilized α-helix topology and enhanced membrane permeability) have been widely applied to target various "undruggable" protein–protein interactions (PPIs) [1-3]. Among them, emerging all-hydrocarbon stapling chemistry pioneered by Verdine and co-workers is regarded as one of most promising stapling strategies to promote clinical translation of peptide drugs [4, 5]. However, the lacks of the native side chains in the stapling position have been described as potential limitations of hydrocarbon stapled peptides [6]. To explore whether the retention of the side chain of the stapling residue offered beneficial advantages, we previously developed a new series of stapling amino acids with native amino acid side chains, and then incorporated them combined with one α-methyl, α-alkenyl amino acid (S5 or R5) into the β-catenin-binding domain in i, i + 3 or i, i + 4 space (Fig. 1). Biochemical experiments proved that, compared to hydrocarbon stapled peptides, stapled peptides with the retention of important peripheral residues are very critical for Wnt/β-catenin signaling pathway [7].
|
Download:
|
| Fig. 1. The difference between the previous strategies and the new strategy in this work. i = stapling position; S5 = Fmoc-S5-OH; AA* = amino acids with modifications of pentene groups on α-carbon; R = amino acid side chain. | |
In that case, only one stapling amino acid with native side-chain was introduced into the peptide sequence and thus there still existed one missing native side-chain for another stapling position of the stapled peptides. Hence it is challenging to reasonably install the staple, especially in lengthy peptides (the number of amino acids is greater than twenty) [8, 9]. To balance multiple biophysical parameters like charge, solubility, key peripheral residues and intramolecular steric hindrance of peptide, we have to design a panel of stapled peptides in different stapling positions and analyze the structure–activity relationships [8]. It is undoubtedly a tedious trial-and-error process at the cost of considerable amount of time and energy [10, 11]. We envision that peptide stapling strategy characterized as the retention of double native side-chains, with the assistance of our previously reported stapling amino acids, could facilitate the design of the stapled peptides regardless of the stapling sites (Fig. 1). Here we described the first synthesis of stapled lengthy peptides with the retention of double native side-chains. Such stapled variants displayed superiority relative to the traditional stapled peptides on sequence design, hydrophilicity and bioactivity.
To undertake this proof of concept, we used SC34EK, a helical potent human immunodeficiency virus-1 (HIV-1) fusion peptide inhibitor with 34 residues [12], as template for modification by stapling with the retention of two native side chains. SC34EK was developed via structure modification of the peptide derived from C-terminal heptad repeat region (CHR) 628–661 of gp41 (HIV-1 surface envelope glycoprotein, regulating virus and cell fusion processes), it can bind to gp41 N-terminal heptad repeat region (NHR) and prevent the formation of the 6-helix bundle, the key fusion process mediated by HIV-1 with host cells [13]. The unique structural feature of SC34EK are X-EE-XX-KK units formed by changing all the amino acids exposed to the solvent surface in the peptide sequence to glutamic acid (Glu) and lysine (Lys), those effective units can provide more salt bridges to constrain helical conformation of SC34EK [14]. Even Though, intramolecular electrostatic stability is not enough to resist enzymatic hydrolysis in vivo, which hinder its clinical translation as therapeutic. To overcome the proteolytic shortcomings of SC34EK, Guo et al. [15] incorporated 1–2 all-hydrocarbon staples into SC34EK at two X-EE-XX-KK units for a stable α-helical structure. However, stapled SC34EK peptides did not exert more significant α-helicity (1.1–1.3-fold increase) and protease stability (1.8–1.9-fold increase) compared to prototype. In addition, doubly stapled peptide had no more extra advantages than singly stapled peptide both in structure and anti-HIV-1 activity. This observation may suggest that salt bridges are not the best sites for stapling, and it is a very heavy workload to perform a 'staple scan' for this so long peptide sequence [16]. Under the circumstances, stapling strategy with the retention of double native side-chains can provide an attractive alternative to constrain α-helix with minimum structural change.
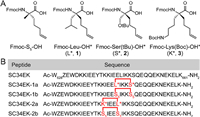
To choose the positions for stapling, we found two pairs of appropriate (i, i + 4) substitutions near the amino acids (Leu-645 & Ser-649 and Lys-641 & Leu-645) to insert one staple (Fig. 2). We designed the insertion of a single staple at those positions using our Leu* (L*, 1), Ser* (S*, 2) and Lys* (K*, 3) to generate SC34EK-1a and -2a, respectively. Meanwhile, the substitutions (SC34EK-1b and -2b) made by S5 in the same sites are set to as controls. Peptides were prepared by using standard Fmoc solid-phase peptide synthesis (SPPS) on the rink amide resin [17]. The synthetic route for preparing novel stapled SC34EKs as SC34EK-1a for example was shown in Fig. 3B, Amino acids were introduced into the peptide backbone using 5‑chloro-1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate (HCTU) and N, N-diisopropylethylamine (DIPEA) as the coupling reagents to provide Fmoc-protected on-resin peptide. After Fmoc deprotection and N-terminal acetylation, intramolecular ring-closing metathesis (RCM) of on-resin peptide was accomplished with the first-generation Grubbs' reagent. The peptides were cleaved off from the resin and concomitant global deprotected with reagent K (82.5% TFA, 5% H2O, 2.5% EDT, 5% thioanisole and 5% phenol). Cold ether precipitation gave the crude stapled SC34EKs, followed by reverse-phase high performance liquid chromatography (RP-HPLC) and high resolution mass spectrometry (HR-MS) analysis. Final target compounds were obtained by semi-preparative RP-HPLC with more than 95% purity. It is worth noting that, for synthetic convenience, previous Fmoc-Lys(6-azide)-OH* was converted to the 3 by reduction of side-chain azide in a zinc/acetic acid solution and further protection via Boc group with a satisfactory yield (> 90%) over two steps, which can be directly used to SPPS without purification (Fig. 3A). All the designed peptides were successfully synthesized, suggesting a good residue compatibility of our new stapling amino acids in SPPS.
|
Download:
|
| Fig. 2. (A) Structure of Fmoc-S5-OH and amino acid derivatives Fmoc-AA*-OH used in this work. (B) Amino acid sequences of stapled SC34EKs. | |

|
Download:
|
| Fig. 3. (A) Synthetic route of 3. (B) Synthetic route for the preparation of stapled SC34EK-1a. (C) CD spectra of the peptides. (D) HPLC retention time and α-helicity of the peptides. | |
With SC34EK and its novel variants in hand, the impact of two staples on peptide biophysical parameters (charge, HPLC retention time and α-helicity) were analyzed. The introduction of severely hydrophobic hydrocarbon stapling usually lead to poor aqueous solubility and undesired hemolytic toxicity for stapled peptides, especially in those cases where the native hydrophilic side chains of Lys and Arg [18]. More importantly, cationic charge charges on Arg and Lys are invoked as critical contributing factors for cell uptake propensity [8, 19]. Owing to the retention of Lys-641, SC34EK-2a has extra positive charge and increased hydrophilicity when compared to SC34EK-2b (Fig. 3D), suggesting that SC34EK-2a may exhibited higher potent activity than control SC34EK-2b. Secondary structure of these peptides was further compared by circular dichroism (CD) in H2O. As shown in Fig. 3C, all stapled peptides displayed representative helical characteristics with a positive absorption peak at 195 nm, and two strong negative absorption peaks at 208 and 222 nm in the CD spectra [20], indicating that they formed stable α-helical structure. The helicity of linear template (469–482) was 6.8%, while the helicity of stapled derivatives ranges from 7.6% to 13.7% corresponding to a 1.1- to 2.0-fold increase. Among them, SC34EK-1a and -2a showed the similar α-helical content value compared to the corresponding regular stapled peptides SC34EK-1b and -2b. These results demonstrate that our novel double side-chain-retention stapling strategy can maintain multiple biophysical determinants for pharmacological activity when compared with electrostatically constrain and the regular stapling strategy.
We then sought to confirm whether two types of stapled peptides retained at least equivalent biological activity to the parent wild-type peptide (Fig. 4). Their anti-HIV-1 activities were evaluated using HIV inoculum (HIVIIB, X4-tropic). From the non-linear regression analysis and half maximal inhibitory concentration (IC50) values of the peptides against HIV-1 infecting cells (Figs. 4A and F). We found that only SC34EK-1a exhibited slightly superior efficacy at sub-nanomolar IC50 value compared to SC34EK, whereas the anti-HIV-1 effect of SC34EK-1b showed slightly decrease, highlighting the influence of the side chain Leu-645 and Ser-649. Another both two substitutions (Lys-641 and Leu-645), SC34EK-2a and -2b, did not maintain inhibitory activity against HIVIIB as efficiently as SC34EK. Insertion of huge hydrocarbon stapling residue might hinder interaction between SC34EK-2a/2b and N36, which would explain their dramatically declined anti-HIV-1 activity. To understand why SC34EK-1a was more potent than parent peptide, we analyzed the crystal structure of the complex between HIV-1 gp41 fragment N36 and SC34EK. As anticipated, the side chains of Leu-645 and Ser-649 in SC34EK are closer to the core of helix bundle structure (Fig. 4B), particularly the Asn-554 and Arg-557 of N36 (Fig. 4C), implying that strong interaction between SC34EK-1a and N36 is relied on key residue Leu-645 and Ser-649 on SC34EK-1a. These results clearly demonstrate the inherent superiority of the double side-chain-retention stapled peptides over regular stapled peptides.

|
Download:
|
| Fig. 4. (A) Anti-HIV-1 activity of SC34EK and stapled derivatives. (B) Crystal structure of SC34EK (green, PDB code: 2Z2T) and N36 (gray) viewed from the C-terminus of SC34EK. (C) Crystal structure of SC34EK (green) and N36 (gray) viewed from side. Interacted residues were shown as stick models. (D) The Analytical HPLC chromatogram (λ = 214 nm) of peptides (SC34EK and SC34EK-1a) following a-chymotrypsin solution. (E) Proteolytic stability of the peptides. (F) The IC50 values and half-life of peptides. | |
To demonstrate the proteolytic challenges faced by SC34EK and its functionally optimized stapled derivatives, we subjected a sampling of peptides to chymotrypsin digestion in vitro and monitored the kinetics of degradation by HPLC. There are several cleavage sites for chymotrypsin on SC34EK like Tyr, Trp and Leu. The typical dynamic process and all the degradation curves of peptides at different time intervals were shown in Figs. 4D and E. As expected, the half-life (T1/2) of SC34EK was calculated to be only 39 min, while stapled SC34EK-1a to -2b exhibited the significantly longer T1/2 that ranged from 105 min to 331 min, reflecting a 2.7- to 8.5-fold enhancement compared to the parent peptide (Fig. 4F). In addition, the higher the helical content, the higher the enzyme stability. It's worth noting that this degree of enhanced protease resistance dramatically surpassed stapled substitutions on salt bridges of SC34EK [15]. These data indicated that our novel strategy can confer the protease-resistance ability of long peptide due to appropriate stapling position.
To summarize, we demonstrated the proof of concept for the design and synthesis of stapled SC34EK peptides with the retention of two native side chains. Owing to our own stapling amino acids, we choose non-solvent surface residues to replace, and those stapled SC34EK peptides possessed higher promotion with α-helicity and protease resistance comparing to previous work [15]. More importantly, two side-chain-retention stapling strategy once again proved the significance of native side chains on stapling position, such as hydrophilicity, charge and effective interaction for target. In addition to PPIs, all-hydrocarbon stapling has also been applied to maximize the utility of natural bioactive helices for targeting membrane of tumor cells and pathogens. However, hydrophobic stapler generally been viewed as contributing to nonspecific hemolysis [21, 22]. Therefore, the retention of hydrophilic residues on stapling position is vital for the safety of natural peptides. We expect that the stapling strategy with the retention of two native side chains for more peptide drug discovery.
Declaration of competing interestWe further confirm that the authors declare no competing interest.
AcknowledgmentsThis work was supported by the National Key R & D Program of China (No. 2019YFC1711000, to X. Li), the National Nature Science Foundation of China (No. 21807112, to X. Li; No. 91849129, to H. Hu; No. 22077078, to H. Hu) and Shanghai Rising-Star Program (to X. Li).
Appendix Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.030.
| [1] |
X. Li, S. Chen, W.D. Zhang, et al., Chem. Rev. 120 (2020) 10079-10144. DOI:10.1021/acs.chemrev.0c00532 |
| [2] |
D.A. Guarracino, J.A. Riordan, G.M. Barreto, et al., Chem. Rev. 119 (2019) 9915-9949. DOI:10.1021/acs.chemrev.8b00623 |
| [3] |
R.R. Araghi, A.E. Keating, Curr. Opin. Struct. Biol. 39 (2016) 27-38. DOI:10.1016/j.sbi.2016.04.001 |
| [4] |
L.A. Carvajal, D.B. Neriah, A. Senecal, et al., Sci. Transl. Med. 10 (2018) eaao3003. DOI:10.1126/scitranslmed.aao3003 |
| [5] |
L.D. Walensky, G.H. Bird, J. Med. Chem. 57 (2014) 6275-6288. DOI:10.1021/jm4011675 |
| [6] |
X. Li, W.D. Tolbert, H.G. Hu, et al., Chem. Sci. 10 (2019) 1522-1530. DOI:10.1039/c8sc03275k |
| [7] |
Y. Wu, Y.H. Li, X. Li, et al., Chem. Sci. 8 (2017) 7368-7373. DOI:10.1039/C7SC02420G |
| [8] |
G.H. Bird, E. Mazzola, K. Opoku-Nsiah, et al., Nat. Chem. Biol. 12 (2016) 845-852. DOI:10.1038/nchembio.2153 |
| [9] |
R. Mourtada, H.D. Herce, D.J. Yin, et al., Nat. Biotechnol. 37 (2019) 1186-1197. DOI:10.1038/s41587-019-0222-z |
| [10] |
Y.L. Li, M.H. Wu, Q. Chang, et al., RSC Adv. 8 (2018) 22268-22275. DOI:10.1039/C8RA03446J |
| [11] |
Y.L. Li, Y.H. Zhang, M.H. Wu, et al., ACS Chem. Biol. 14 (2019) 516-525. DOI:10.1021/acschembio.9b00046 |
| [12] |
W. Nomura, C. Hashimoto, T. Suzuki, et al., Bioorg. Med. Chem. 21 (2013) 4452-4458. DOI:10.1016/j.bmc.2013.05.060 |
| [13] |
H. Nishikawa, S. Nakamura, E. Kodama, et al., Int. J. Biochem. Cell. Biol. 41 (2009) 891-899. DOI:10.1016/j.biocel.2008.08.039 |
| [14] |
A. Otaka, M. Nakamura, D. Nameki, et al., Angew. Chem. Int. Ed. 41 (2002) 2937-2940. DOI:10.1002/1521-3773(20020816)41:16<2937::AID-ANIE2937>3.0.CO;2-J |
| [15] |
Y. Guo, L.L. Fu, X. Fan, et al., Chin. Chem. Lett. 29 (2018) 1167-1170. DOI:10.1016/j.cclet.2018.03.024 |
| [16] |
G.H. Bird, N. Madani, A.F. Perry, et al., Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 14093-14098. DOI:10.1073/pnas.1002713107 |
| [17] |
Y. Wu, M.F. Han, C. Liu, et al., RSC Adv. 7 (2017) 17514-17518. DOI:10.1039/C6RA26427A |
| [18] |
Y. Tian, J.X. Li, H. Zhao, et al., Chem. Sci. 7 (2016) 3325-3330. DOI:10.1039/C6SC00106H |
| [19] |
L. Dietrich, B. Rathmer, K. Ewan, et al., Cell. Chem. Biol. 24 (2017) 958-968.e5. DOI:10.1016/j.chembiol.2017.06.013 |
| [20] |
Y. Guo, P.P. Zhou, S.Y. Zhang, et al., Medchemcomm 9 (2018) 1226-1231. DOI:10.1039/C8MD00124C |
| [21] |
H. Chapuis, J. Slaninová, L. Bednárová, et al., Amino Acids 43 (2012) 2047-2058. DOI:10.1007/s00726-012-1283-1 |
| [22] |
D. Migoń, D. Neubauer, W. Kamysz, Protein J. 37 (2018) 2-12. DOI:10.1007/s10930-018-9755-0 |
 2021, Vol. 32
2021, Vol. 32 

