Tumor-associated carbohydrate antigens (TACAs) that are abundantly expressed on the surface of cancer cells are attractive biological targets for the development of cancer vaccines or cancer immunotherapies [1, 2]. However, carbohydrate antigens are typically T cell-independent and poorly immunogenic, which impedes the success of TACA-based vaccines [3]. In addition, as self-antigens, most TACAs are tolerated by the immune system, which further creates difficulties in vaccine design and development [4]. Over the past few decades, many novel vaccine strategies have been developed to overcome these problems [5, 6]. Among them, the non-natural TACA strategy that aims to promote the immunogenicity and mitigate the immune-tolerance of vaccines shows great potential. For example, artificial TACA-based vaccines, including Tn [7], sTn [8, 9], GM3 [10], TF [11], Globo H [12] and MUC1 analogs [13], successfully provoked robust immune responses against these carbohydrate antigens. However, one potential issue of these strategies is that the antibodies generated by non-natural TACAs had limited cross-reactivity with the natural glycans expressed on the surface of cancer cells.
To address this dilemma, a novel immunotherapy strategy that combined the non-natural TACA strategy and the glycoengineering of cancer cells was developed [9]. Previous work demonstrated that an unnatural NeuNPhAc-containing TACA-based vaccine elicited a strong immune response but could not recognize the natural TACAs present on the surface of cancer cells. However, after the cancer cells were glycoengineered with the corresponding precursor N-phenylacetyl-d-mannosamine to express NeuNPhAc-modified TACAs on their surfaces, the pre-elicited antibodies were able to recognize and kill the engineered cancer cells effectively [14]. Nevertheless, a primary requisite for the success of the above-mentioned immunotherapies is to provide an effective immunization that could generate a strong and antigen-specific humoral and cellular immunity by a non-natural TACAs-based cancer vaccine.
Endogenous antibodies are naturally occurring antibodies that exist in human sera. Redirecting endogenous antibodies, such as anti-galactose-α-(1, 3)-galactose (α-Gal), anti-2, 4-ditrophenyl (DNP), and anti-rhamnose (Rha) antibodies, to target cancer cells can activate the immune system to selectively kill the cancer cells [15, 16]. Recent studies demonstrated that the metabolic incorporation of DNP or Rha into the cell-surface glycocalyx redirected and enhanced the immune response for targeting cancer cells [17-22]. Moreover, these studies validated that endogenous antibodies were able to mediate the targeted delivery of TACAs-based vaccines to antigen-processing cells (APCs), resulting in an antibody-dependent enhancement (ADE) of immune responses [23-26]. In our previous research, we demonstrated that the incorporation of the Rha antigen into bovine serum albumin (BSA) as a carrier protein led to a significant improvement in the immunogenicity of sTn in the presence of anti-Rha antibodies [25]. These results illustrated the potential collaborative role of endogenous antibodies in vaccine development.
Ganglioside GM3 is a common TACA that is highly expressed by several types of malignancies, such as lung, brain and breast cancers, as well as melanomas [27]. The expression of GM3 influences the development and proliferation of cancer cells and is positively correlated with tumor malignancy. GM3 is one of the 50 most investigated cancer antigens for the development of cancer immunotherapies and has been widely targeted in vaccine development [28, 29]. Based on previous work related to non-natural TACA-based cancer vaccines and cell-surface glycoengineering, we designed and synthesized a new 5ʹ-N-DNP-modified GM3 antigen (GM3-NHDNP) for use in cancer immunotherapy (Fig. 1). The incorporation of the DNP hapten into the structure of GM3 had multiple purposes. First, the artificial GM3-NHDNP antigen could elicit strong immune responses and overcome immune tolerance because of its unnatural structure. Second, the targeted delivery of the GM3-NHDNP antigen to APCs could be achieved in an ADE manner to further enhance the antigen-presenting process in the presence of endogenous anti-DNP antibodies. Finally, the surface of cancer cells could be glycoengineered to express the GM3-NHDNP antigen using established metabolic approaches. Thus, it was expected that the endogenous anti-DNP antibodies, together with the elicited endogenous anti-GM3-NHDNP antibodies, would synergistically promote tumor binding and phagocytosis after effective vaccinations. In this context, we communicate the chemoenzymatic synthesis of 5ʹ-N-DNP-modified GM3 and the evaluation of the immune activity of the GM3-NHDNP glycoconjugate in the presence of anti-DNP antibodies as a potential carbohydrate-based cancer vaccine.

|
Download:
|
| Fig. 1. Design and structure of the GM3-NHDNP glycoconjugate vaccine construct. | |
The synthesis of the DNP-modified GM3 analog required a key intermediate: 5ʹ-N-DNP-substituted sialic acid containing an aminocaproic acid linker (8). The intermediate was prepared by a chemoenzymatic method, as shown in Scheme 1. First, aminocaproic acid was reacted with 1-fluoro-2, 4-dinitrobenzene in the presence of sodium bicarbonate to afford intermediate 6 in 83% yield, after which 6 was coupled to d-mannosamine using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDCI) as the condensation reagent in the presence of triethylamine, affording the DNP-modified mannosamine derivative (Man-NHDNP) 7, albeit in only 30% yield. Bacterial sialic acid aldolase typically has a broad substrate specificity [30], so it was able to recognize Man-NHDNP (7) as a substrate in our experiment. Therefore, Man-NHDNP (7) was enzymatically transformed into 5ʹ-N-DNP-substituted sialic acid 8 in the presence of sodium pyruvate in excellent yield (91%).
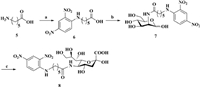
|
Download:
|
| Scheme 1. a) 1-Fluoro-2, 4-dinitrobenzene, acetone, 2 mol/L Na2CO3, r.t., 1 h, 83%; b) d-Mannosamine, EDCI, Et3N, MeOH, r.t., 18 h, 30%; c) Sialic acid aldolase, MgCl2 (20 mmol/L), Tris-HCl buffer (100 mmol/L, pH 7.5), sodium pyruvate, DTT, 37 ℃, 24 h, 91%. | |
The synthesis of the DNP-modified GM3 is provided in Scheme 2. An efficient chemoenzymatic "1+2" approach was applied to assemble the trisaccharide because the glycosyltransferases required for this biochemical transformation could be easily obtained either by recombinant expression in Escherichia coli (E. coli) or commercially. Accordingly, the lactose acceptor 10 was prepared with an azidoethyl group as a linker at the reducing end following a reported procedure [31]. The GM3-NHDNP analog 11a was assembled by two-step, one-pot enzymatic transformation. First, the sialic acid derivatives 8 were converted to the CMP-Neu5R derivatives 9a by Neisseria meningitidis CMP-sialic acid synthetase (NmCSS) according to a previously published procedure [32]. Then, the lactose acceptor 10 and the recombinant enzyme Pasteurella multocida α-(2→3)-sialyltransferase (PmST1) were added into the reaction mixture, which was incubated for 12 h at 37 ℃. After quenching the reaction and a brief purification using a Bio-P2 column, the azido group on the reducing end of the 5ʹ-N-DNP-substituted GM3 analog 11a was reduced to the corresponding aminoethyl group, affording GM3-NHDNP 12a in 77% yield. Following a similar procedure, the natural GM3-NHAc 12b was prepared, as well. All of the intermediates and final compounds were characterized by 1H and 13C NMR and HRMS.

|
Download:
|
| Scheme 2. a) MgCl2 (20 mmol/L), Tris-HCl buffer (100 mmol/L, pH 8.8), CTP, NmCSS, 37 ℃, 5 h; b) Compound 10, PmST1, 37 ℃, 12 h, 77%; c) Ph3P, H2O: THF (1:1), 60 ℃, overnight; d) disuccinimidyl glutarate, DMF: PBS (4:1), 5 h, r.t.; e) KLH/HSA, PBS, r.t., 3 days. | |
The GM3 analogs were conjugated to Keyhole limpet hemocyanin (KLH) or human serum albumin (HSA) via the bifunctional glutaryl ester method, which is an established conjugation method that was not expected to affect the immunological properties of the conjugates [33]. Briefly, the GM3 analogs 12a or 12b were reacted with a large excess (15.0 equiv.) of disuccinimidyl glutarate (DSG) in a solution of DMF and PBS buffer (4:1) to afford the corresponding monoesters 13a or 13b, respectively. The monoesters were then mixed with KLH or HSA in phosphate buffer saline (PBS) to generate conjugates 1–4, which were purified by centrifugal filter devices (10 kDa for HSA conjugates and 30 kDa for KLH conjugates). The antigen loading percentages of the resulting glycoconjugates 1–4 were determined by the Svennerholm method (described in the Supporting information), which were calculated to be 3%, 6%, 6.1% and 4.9%, respectively.
To evaluate the immunological activities of our glycoconjugate vaccine candidates, we created three groups of mice. Group 1 (control group), which consisted of five mice, were immunized with natural GM3-NHAc-KLH (3). The mice in the second group were immunized with GM3-NHDNP-KLH (1) only, while the mice in the third group were first pre-immunized with DNP-ovalbumin (OVA) to establish a high level of anti-DNP antibodies (Fig. S2 in Supporting information) and then immunized with conjugate 1. All of these conjugates were premixed with Freund's complete adjuvant to activate the vaccine. Mouse blood samples were collected on days 0, 21 and 28, and the specific antibodies were determined by enzyme-linked immunosorbent assays (ELISA). The ELISA results from the antisera of the three groups on Day 28 are summarized in Fig. 2. As expected, the mice in group 1 produced the lowest number of GM3-specific antibodies (approx. 9784, Fig. 2A), while the mice in group 2 generated a significantly higher number of antibodies specifically against GM3-NHDNP (approx. 164502, Fig. 2B). Therefore, the specific antibody production increased nearly 17-fold compared to the mice in group 1. This result revealed that the artificial DNP-modified GM3 demonstrated a significantly improved immunogenicity compared to GM3, which was in consistent with a previously published report [34]. For the mice in group 3 that were pre-immunized with the DNP-OVA conjugate, the highest production of GM3-NHDNP-specific antibodies was observed (approx. 424294, Fig. 2C), which was approximately 2.6-fold higher than the mice in group 2. This result indicated that the endogenous anti-DNP antibodies further promoted delivery of the vaccine to the APCs, which resulted in better internalization, antigen-presenting efficiency and specific antibody production.

|
Download:
|
| Fig. 2. Immunological evaluation of GM3 analog-KLH vaccine construct: (A) GM3-NHAc-specific antibodies in group 1; (B) GM3-NHDNP-specific antibodies in group 2; (C) GM3-NHDNP-specific antibodies in group 3 pre-immunized with DNP-OVA. (D) The average titers of GM3-NHDNP-specific total antibodies in pooled antisera collected on Days 0, 21 and 27 from mice of groups 2 and 3. Error bars represent the standard deviation (SD) of five parallel experiments. *P < 0.05. | |
Antibody cross-reaction analysis revealed that antibodies elicited by the GM3-NHDNP conjugate recognized the natural GM3 structure. In addition, three conjugates produced IgG antibodies, but not IgM antibodies, as the major isotype, indicating that T cell immunity was involved. We also compared the kinetic immune response in the mice in groups 2 and 3 (Fig. 2D). For the mice in group 2, the total antibodies in the pooled antisera collected on Days 21 and 28 were 146782 and 165056, respectively, compared to 324068 and 403431, respectively, in the mice of group 3. This result suggested that a much faster and stronger immune response was produced in the group 3 mice, and it demonstrated that the DNP-specific antibodies mediated a faster immune response compared to group 2, which can help to reduce injection frequency in the vaccine protocol.
After establishing a successful immunization strategy, we then investigated the capability of the elicited GM3-NHDNP-specific antibodies and DNP-specific antibodies to recognize cancer cells that express the GM3-NHDNP antigen, which is crucial for any glycoengineering-based vaccine immunotherapy. For this purpose, we chose B16F10, a mouse melanoma cell line that abundantly expresses GM3 [14], to carry out the in vitro cell surface glycoengineering. First, the B16F10 cells were treated with the precursor (Man4Ac-DNP, compound S6 in Supporting information) formulated in liposomes. Then, they were detected for GM3-NHDNP expression by flow cytometry using either the Day 0 sera from group 3 (containing DNP-specific antibodies after DNP-OVA immunization) or the Day 28 antisera of groups 2 and 3. The serum collected from the mice without any immunization was used as the blank, and the FITC-conjugated goat anti-mouse antibody was used as secondary antibody. As shown in Fig. 3A, the cells treated with precursor had a noticeably higher fluorescein isothiocyanate (FITC) intensity than the untreated cells, which indicated that the cancer cells metabolized Man4Ac-DNP and successfully expressed artificial GM3-NHDNP on the cell surface after a series of carbohydrate biosynthetic enzymes and the antibodies elicited by GM3-NHDNP-KLH vaccine could recognize them efficiently. Moreover, the anti-DNP antibodies elicited by the DNP-OVA conjugate recognized the precursor-treated cells. Therefore, both anti-DNP and anti-GM3-NHDNP antibodies recognized and bound to the glycoengineered cancer cells, suggesting that they have potential for mediating cancer cell phagocytosis by immune cells.
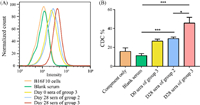
|
Download:
|
| Fig. 3. (A) FACS assays of the binding between B16F10 cancer cells treated with precursor and mouse sera. (B) CDC activities of antisera from each group were measured using the CCK-8 assay. Error bars represent the SD of three parallel experiments. *P < 0.05, ***P < 0.001. | |
We further analyzed the amount of interleukin-6 (IL-6)-representing Th2 cells and interferon gamma (IFN-γ)-representing Th1 cells present in the antisera of the immunized mice by ELISA. As shown in Fig. S4 (Supporting information), IFN-γ and IL-6 were both provoked in groups 1, 2 and 3, which indicated that T cell immune responses were involved.
Finally, the potential antitumor activities of the antibodies elicited by the GM3-NHDNP vaccine were evaluated by antibody-mediated, complement-dependent cytotoxicity (CDC) analysis in vitro. For the CDC measurements, mouse melanoma B16F10 cells were glycoengineered to express the GM3-NHDNP carbohydrate on the cell surface, after which they were incubated with the Day 28 sera from groups 2 and 3 or Day 0 sera from group 3 (containing anti-DNP antibodies) and diluted rabbit complement at 37 ℃ for 4 h, respectively. Cell lysis was conducted using a commercial cell counting kit-8 (CCK-8). As presented in Fig. 3B, the antibodies generated by DNP-OVA or GM3-NHDNP-KLH in groups 2 and 3 were able to trigger a significant cytotoxicity in the cancer cells. Comparable cytotoxicity to group 2 was only observed in the sera containing anti-DNP antibodies. Notably, the antibodies from the mice in group 3 exhibited the highest cytotoxicities compared to both DNP-OVA and group 2. The most potent cytotoxicity was attributed to the fact that group 3 had the highest antibody production, and the enhanced immune-triggered phagocytosis might have been synergistically mediated by anti-DNP antibodies and anti-GM3-NHDNP antibodies, indicating that both antibodies played important roles in this CDC.
In summary, we described herein the synthesis of a new DNP-modified GM3 antigen for the development of a carbohydrate-based cancer vaccine. In vivo immunological studies demonstrated that the GM3-NHDNP conjugate provoked robust and rapid immune responses in the presence of endogenous anti-DNP antibodies, which mediated a targeted delivery of the vaccine construct to the APCs. We validated that this strategy has the potential to utilize the metabolic glycoengineering of cancer cells for manifesting immunotherapy. In vitro CDC studies revealed that an enhanced cancer cell death was triggered by both pre-immunized anti-DNP- and anti-GM3-NHDNP-specific antibodies. In future work, we aim to further optimize the structure of the GM3-NHDNP conjugate, including the linker and the position of DNP modification, to improve the glycoengineering efficiency of the surface of the cancer cells. Considering the abundance of anti-DNP antibodies in human sera and the success of non-natural TACAs-based cancer vaccine, this strategy could potentially be applied to the development of other TACAs-based vaccines.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21907038, 32000904), the Natural Science Foundation of Jiangsu Province (No. BK20200601, China), the National Postdoctoral Program for Innovative Talents of China (No. BX20200153), the Health and Family Planning Commission of Wuxi, China (No. Z202005), and the Social Development Key Project of Jiangsu Province (No. BE2019632, China). This research was partly supported by the 111 Project (No. 111-2-06, China) and the National First-class Discipline Program of Food Science and Technology (No. JUFSTR20180101, China). We appreciate Prof. Hongzhi Cao from Shandong University for providing us the enzymes Neisseria meningitidis CMP-sialic acid synthetase (NmCSS) and Pasteurella multocida α-(2→3)-sialyltransferase (PmST1)[32].
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.034.
| [1] |
Z. Yin, X. Huang, J. carbohyr. Chem. 31 (2012) 143-186. DOI:10.1080/07328303.2012.659364 |
| [2] |
Z. Zhou, H. Lin, C. Li, Z. Wu, Chin. Chem. Lett. 29 (2018) 19-26. DOI:10.1016/j.cclet.2017.09.047 |
| [3] |
J. Heimburg-Molinaro, M. Lum, G. Vijay, et al., Vaccine 29 (2011) 8802-8826. DOI:10.1016/j.vaccine.2011.09.009 |
| [4] |
M.M. Wei, Y.S. Wang, X.S. Ye, Med. Res. Rev. 38 (2018) 1003-1026. DOI:10.1002/med.21493 |
| [5] |
F. Liao, H. Wang, Y. Dao, et al., Chin. Chem. Lett. 32 (2021) 1577-1581. |
| [6] |
L.M. Zeng, Z.L. Liao, W.W. Li, et al., Chin. Chem. Lett. 31 (2020) 1162-1164. DOI:10.1016/j.cclet.2019.10.015 |
| [7] |
C. Song, S. Sun, C.X. Huo, et al., Bioorg. Med. Chem. 24 (2016) 915-920. DOI:10.1016/j.bmc.2016.01.015 |
| [8] |
F. Yang, X.J. Zheng, C.X. Huo, et al., ACS Chem. Biol. 6 (2011) 252-259. DOI:10.1021/cb100287q |
| [9] |
Z. Zhou, M. Mondal, G. Liao, Z. Guo, Org. Biomol. Chem. 12 (2014) 3238-3245. DOI:10.1039/C4OB00390J |
| [10] |
X.J. Zheng, F. Yang, M. Zheng, et al., Org. Biomol. Chem. 13 (2015) 6399-6406. DOI:10.1039/C5OB00405E |
| [11] |
M. Johannes, M. Reindl, B. Gerlitzki, E. Schmitt, A. Hoffmann-Roder, Beilstein J. Org. Chem. 11 (2015) 155-161. DOI:10.3762/bjoc.11.15 |
| [12] |
H.Y. Lee, C.Y. Chen, T.I. Tsai, et al., J. Am. Chem. Soc. 136 (2014) 16844-16853. DOI:10.1021/ja508040d |
| [13] |
Z. Wang, Q. Chen, H. Zhu, et al., Chin. Chem. Lett. 32 (2021) 1888-1892. DOI:10.1016/j.cclet.2021.01.036 |
| [14] |
Q. Wang, Z. Zhou, S. Tang, Z. Guo, ACS Chem. Biol. 7 (2012) 235-240. DOI:10.1021/cb200358r |
| [15] |
R.T. Sheridan, J. Hudon, J.A. Hank, P.M. Sondel, L.L. Kiessling, ChemBioChem 15 (2014) 1393-1398. DOI:10.1002/cbic.201402019 |
| [16] |
C.E. Jakobsche, C.G. Parker, R.N. Tao, et al., ACS Chem. Biol. 8 (2013) 2404-2411. DOI:10.1021/cb4004942 |
| [17] |
C.G. Parker, M.K. Dahlgren, R.N. Tao, et al., Chem. Sci. 5 (2014) 2311-2317. DOI:10.1039/C4SC00484A |
| [18] |
A.F. Rullo, K.J. Fitzgerald, V. Muthusamy, et al., Angew. Chem. Int. Ed. 55 (2016) 3642-3646. DOI:10.1002/anie.201510866 |
| [19] |
A. Uvyn, R. De Coen, O. De Wever, et al., Chem. Commun. (Camb.) 55 (2019) 10952-10955. DOI:10.1039/c9cc03379c |
| [20] |
R. De Coen, L. Nuhn, C. Perera, et al., Biomacromolecules 21 (2020) 793-802. DOI:10.1021/acs.biomac.9b01483 |
| [21] |
H. Hong, Z. Zhou, K. Zhou, et al., Chem. Sci. 10 (2019) 9331-9338. DOI:10.1039/c9sc03840j |
| [22] |
B.J. Lin, X.J. Wu, H. Zhao, et al., Chem. Sci. 7 (2016) 3737-3741. DOI:10.1039/C5SC04133C |
| [23] |
J. Abraham, Nat. Rev. Immunol. 20 (2020) 401-403. DOI:10.1038/s41577-020-0365-7 |
| [24] |
P. Karmakar, K. Lee, S. Sarkar, et al., Bioconjug. Chem. 27 (2016) 110-120. DOI:10.1021/acs.bioconjchem.5b00528 |
| [25] |
H. Lin, H.F. Hong, J.F. Wang, et al., Chem. Commun. (Camb.) 56 (2020) 13959-13962. DOI:10.1039/d0cc05263a |
| [26] |
U. Galili, Vaccine 38 (2020) 6487-6499. DOI:10.1016/j.vaccine.2020.08.032 |
| [27] |
C. Zheng, M. Terreni, M. Sollogoub, Y. Zhang, Curr. Med. Chem. 26 (2019) 2933-2947. DOI:10.2174/0929867325666180129100619 |
| [28] |
M.A. Cheever, J.P. Allison, A.S. Ferris, et al., Clin. Cancer. Res. 15 (2009) 5323-5337. DOI:10.1158/1078-0432.CCR-09-0737 |
| [29] |
X.G. Yin, J. Lu, J. Wang, et al., J. Med. Chem. 64 (2021) 1951-1965. DOI:10.1021/acs.jmedchem.0c01186 |
| [30] |
P. Chefalo, Y. Pan, N. Nagy, Z. Guo, C.V. Harding, Biochemistry 45 (2006) 3733-3739. DOI:10.1021/bi052161r |
| [31] |
B. Sun, A.V. Pukin, G.M. Visser, H. Zuilhof, Tetrahedron Lett. 47 (2006) 7371-7374. DOI:10.1016/j.tetlet.2006.08.008 |
| [32] |
X. Meng, W. Yao, J. Cheng, et al., J. Am. Chem. Soc. 136 (2014) 5205-5208. DOI:10.1021/ja5000609 |
| [33] |
Z. Zhou, S.S. Mandal, G. Liao, J. Guo, Z. Guo, Sci. Rep. 7 (2017) 11403. DOI:10.1038/s41598-017-11500-w |
| [34] |
Y. Pan, P. Chefalo, N. Nagy, C. Harding, Z. Guo, J. Med. Chem. 48 (2005) 875-883. DOI:10.1021/jm0494422 |
 2021, Vol. 32
2021, Vol. 32 

