b Advanced Research Institute and Department of Chemistry, Taizhou University, Taizhou 318000, China;
c Anhui Laboratory of Clean Catalytic Engineering, Anhui Laboratory of Functional Complexes for Materials Chemistry and Application, College of Chemical and Environmental Engineering, Anhui Polytechnic University, Wuhu 241000, China
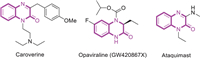
Quinoxalin-2(1H)-one derivatives represent a privileged class of nitrogen containing heterocycle that are extensively found in pharmaceutical molecules, materials, agrochemical industries and synthetic chemistry [1, 2]. In particular, C3-substituted quinoxalin-2(1H)-ones have attracted increasingly attention because of their significant biological and pharmaceutical properties [3-5]. For instance as shown in Fig. 1, Caroverine is known to be muscle relaxant agent [6], Opaviraline (GW-420867X) is endowed with anti-HIV activity [7], and Ataquimast can be used to treat chronic obstructive bronchopulmonary disease [8]. Owing to their unique properties, numerous efforts have been devoted to the preparation of C3-substituted quinoxalin-2(1H)-ones. In recent years, the direct C−H functionalization of available quinoxalin-2(1H)-ones has emerged as an efficient approach to C3-substituted quinoxalin-2(1H)-ones and remarkable achievements have been accomplished, including C3-selective alkylation [9-16], arylation [17-23], trifluoromethylation [24-27], amination [28-31], phosphonation [32-34], acylation [35-39] and so on [40-43]. Despite these significant advances, to the best of our knowledge, the application of quinoxaline-2(1H)-ones as starting materials for divergent synthesis by simply switching between anaerobic and aerobic conditions has not been reported [44]. Considering of the relevance of alkyl groups in life and materials sciences, the development of an efficient and mild method to construct C3-alkylated quinoxalin-2(1H)-ones is still highly desirable.
|
Download:
|
| Fig. 1. Bioactive molecules containing quinoxalin-2(1H)-one motif. | |
Visible-light-mediated photocatalysis has witnessed dramatic developments over the past decade [45-51], and a number of efficient visible-light-mediated C−H functionalization protocols have been explored [52-59]. In the recent years, a few examples on the use of visible light to realize C3-selective alkylation of quinoxalin-2(1H)-ones have been developed. In 2018, Wei and co-workers reported metal-free visible-light-promoted alkylation of quinoxalin-2(1H)-ones with simple ethers [13]. He et al. disclosed a decarboxylative alkylation of quinoxalin-2(1H)-ones with phenyliodine(Ⅲ) dicarboxylates catalysed by Ru(Ⅱ) under photoinduced conditions in 2019 [14]. Yang's group reported lately a practical protocol for alkylation of quinoxalin-2(1H)-ones with hydrazines under visible light irradiation [15].
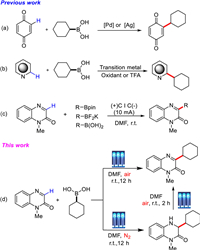
After Frankland's report in 1860, organoboranes were widely introduced into the studies on the free-radical reactions [60-63]. It is well known that alkylboronic acids have been gradually used as low-toxicity and stable alkylation reagents, which were introduced into quinone moieties [64-65] or N-heteroarenes [66-69] (Schemes 1a and b). However, transition metal, oxidant or strong acid are usually required in the reported examples. Very recently, Wang's group accomplished a mild electro-oxidative method for the C3-alkylation of quinoxalin-2(1H)-ones with alkylboronic acids and esters, and alkyl trifluoroborates (Scheme 1c) [70]. As part of our continuing efforts in development of visible-light-promoted green synthesis [71-76], we report herein a transition-metal-free photoinduced divergent addition/C3-selective alkylation reaction of quinoxalin-2(1H)-ones with alkylboronic acids under air (O2) and N2 atmosphere, which generated the corresponding 3-alkylquinoxalin-2(1H)-ones and 3, 4-dihydroquinoxalin-2(1H)-ones in high yields, respectively (Scheme 1d).
|
Download:
|
| Scheme 1. The reaction of alkylboronic acids as alkylation reagent. | |
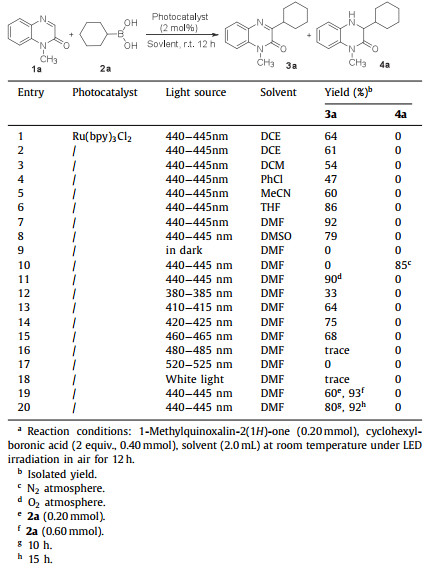
Initial attempts at C3-selective alkylation were carried out by using 1-methylquinoxalin-2(1H)-one (1a) and cyclohexylboronic acid (2a) as a model reaction under various conditions (Table 1). When the reaction was conducted in the presence of photocatalyst Ru(bpy)3Cl2 under blue light irradiation for 12 h in 1, 2-dichloroethane (DCE) at room temperature, the C3-alkylated product 3a was obtained in 64% isolated yield (entry 1). We were delighted to find that the reaction performed well without any photocatalyst (entry 2). Then, different solvents were screened and the results suggested that N, N-dimethylformamide (DMF) is critical for obtaining high yield (entries 3–8). When the reaction was carried out in dark (entry 9), no product 3a was observed. When the reaction was conducted under a nitrogen atmosphere, the yield of 3a was 0% (entry 10), but afford another addition product 4a in 85% yield. The structure of 4a has been characterized by 1H NMR and 13C NMR, and further confirmed by X-ray single crystal analysis (Scheme 4). A 90% yield of 3a was achieved when an oxygen balloon was used instead of air atmosphere (entry 11). Further screening of light sources showed that the C3 alkylation of quinoxalin-2(1H)-ones was drove by visible light, and blue LED (440−445 nm, 1.5 W) was the most effective light source for the reaction (entries 7 and 12–18). Finally, the ratio of 1a to 2a, and the reaction time were optimized and presented in Table 1 (entries 19–20).
|
|
Table 1 Optimization of the reaction conditions.a |
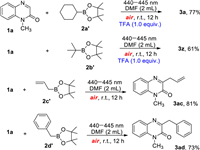
Having established the optimized reaction conditions, the generality of direct C3-alkylation of different substituted quinoxalin-2(1H)-ones with alkylboronic acids was examined. First, as shown in Scheme 2, a series of N-protected quinoxalin-2(1H)-ones, including N-methyl, N-ethyl, N-benzyl, N-acetyate, N-2-oxo-2-phenylethyl, N-allylic, and N-propynyl, could react with cyclohexylboronic acid (2a) and the corresponding products (3a–3g) were obtained in good to excellent yields. Then, a wide range of substituted 1-methylquinoxalin-2(1H)-ones were explored, and to our pleasure, the substrates with electron-donating groups such as MeO, Me or electron-withdrawing groups such as F, Cl, CF3, CN, CO2Me, NO2, reacted smoothly to generate the anticipated products (3h–3r) in high yields. In addition, quinoxalin-2(1H)-one without N-protected group was also matched with the reaction, providing the product (3s) in 72% yield. It is worth noting that 2H-benzo[b][1, 4]oxazin-2-one also underwent the alkylation reaction to generate the product 3t in 75% yield. Encouraged by these results, reactions of various alkylboronic acids (2) with 1a were carried out, and the results were also outlined in Scheme 2. When cyclopropylboronic acid, cyclobutylboronic acid, cyclopentylboronic acid, isopropylboronic acid, sec-butylboronic acid and tert-butylboronic acid were used as the alkyl precursors, all reactions proceeded smoothly to produce the expected products (3u−3z) in 56%–80% yields. Employing tert-butylboronic acid as one of the starting material, the reaction occurred in moderate yield, steric hindrance has no obvious effect on the reaction. However, propylboronic acid and phenylboronic acid could not deliver the corresponding products 3aa and 3ab, and the staring material was recovered. Allyl and benzyl boronic acids were used to react with 1a, the corresponding products, 3ac and 3ad were obtained in 86% and 79% yields, respectively. To extend the substrate scope, alkylboronic esters, such as 2-cyclohexyl-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolane (2a′) and 2-(tert-butyl)−4, 4-dimethyl-1, 3, 2-dioxaborolane (2b′) were used to react with 1a under the optimized reaction conditions, and no desired product was observed. When trifluoroacetic acid (1.0 equiv.) was added, the products 3a and 3z were obtained in 77% and 61% yields, owing to the hydrolysis of esters. In addition, the reaction of 1a with 2-allyl-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolane (2c′) and 2-benzyl-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolane (2d′) generated the products 3ac and 3ad in 81% and 73% yields (Scheme 3).

|
Download:
|
| Scheme 2. The reaction of 1 with 2 under air. Quinoxalin-2(1H)-one (1, 0.20 mmol), alkylboronic acid (2, 0.40 mmol), DMF (2.0 mL), at room temperature under 1.5 W blue LED (440–450 nm) irradiation for 12 h; isolate yield of the product. | |
|
Download:
|
| Scheme 3. The reaction of 1a with alkylboronic esters under air. | |
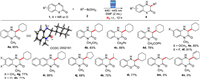
Subsequently, the substrate scope of the addition reaction in nitrogen atmosphere under the optimized reaction conditions (Table 1, entry 10) was investigated (Scheme 4). Most quinoxalin-2(1H)-ones could react with alkylboronic acids to give the desired addition products in moderate to good yields.
|
Download:
|
| Scheme 4. The reaction of 1 with 2 under N2 atmosphere. Quinoxalin-2(1H)-one (1, 0.20 mmol), alkylboronic acid (2, 0.40 mmol), DMF (2.0 mL), at room temperature under 1.5 W blue LED (440–450 nm) irradiation for 12 h; isolate yield of the product. | |
The established reaction conditions can accommodate N-methyl, N-ethyl, N-benzyl and N-2-oxo-2-phenylethyl quinoxalin-2(1H)-ones, furnishing the products (4a–4d) in good yields. Quinoxalin-2-ones with substituents at the phenyl ring underwent smoothly and delivered the products (4e–4i) in 71%–86% yields. Remarkably, disubstituted derivates were also suitable for the transformation (4g–4i). The generality of alkylboronic acids was also examined. The results were indicated that cyclopentylboronic acid and isopropylboronic acid were compatible under the optimal conditions, providing the products 4j and 4k in 68% and 72% yield, respectively. The further study found that the current reaction condition was also suitable for the addition reaction of 2H-benzo[b][1, 4]oxazin-2-one with cyclohexylboronic acid, and the product (4l) was obtained in 77% yield. However, propylboronic acid and phenylboronic acid failed to give the addition products 4m and 4n.
Considering the synthetic application of this method, the scalability of the reaction was performed. A gram-scale experiment was conducted using 1a (5.0 mmol) with 2a (10.0 mmol), providing 3a in 78% yield (Scheme 5), which shows great potential of this photo-induced reaction.

|
Download:
|
| Scheme 5. Gram-scale experiment. | |
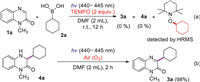
To further clarify the mechanism of this transformation, several control experiments were conducted, as shown in Scheme 6. When the model reaction was carried out in the presence of a radical scavenger 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO, 2.0 equiv.) under the standard conditions, the addition or substitution reaction was completely inhibited, no 3a or 4a was observed and the corresponding adduct of cyclohexyl radical and TEMPO was detected by HRMS analysis, suggesting that a radical process might be involved in the reaction (Scheme 6a). Additionally, 4a can be converted to 3a completely under blue LED (440–445 nm) irradiation in presence of air (Scheme 6b). In order to understand the role of oxygen in the reaction, the electron paramagnetic resonances (EPR) were recorded using 2, 2, 6, 6-tetramethylpiperidine (TEMP) and 5, 5-dimethyl-pyrroline-N-oxide (DMPO) to capture 1O2 and O2•−, respectively, and the resulting EPR spectra are presented in Fig. S1 (Supporting information). A strong characteristic signal of an O2•− adduct with DMPO was observed when DMPO was added into a solution of 4a in air-saturated DMF was irradiated with LED 440–445 nm. While no signal of the 1O2 adduct with TEMP was found when the above solution was irradiated with a LED (440–445 nm), illustrating that O2•− might be a key intermediate in this transformation.
|
Download:
|
| Scheme 6. The control experiments. | |
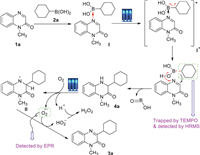
Based on the experimental results and literature reports [77-79], a plausible mechanism is proposed as shown in Scheme 7. First, 1a reacts with 2a to produce the complex Ⅰ, which was excited by visible-light irradiation (blue LED) to generate the excited-state (Ⅰ*). Subsequently, a homolytic cleavage of the excited-state Ⅰ* generates a pair of radicals, and the cyclohexyl radical added to C=N bond, generates a N-centred free radical, which then capture a hydrogen atom from hydroxyl group, produces the addition product 4a. Next, 4a acts as an electron donor to undergo SET to oxygen, producing a new N-centred free radical Ⅱ and a hydroperoxyl radical along with the deprotonation. Finally, capture of the hydrogen radical by hydroperoxyl radical from Ⅱ produces 3a as the final product.
|
Download:
|
| Scheme 7. The proposed mechanism. | |
In summary, we have developed a visible light-promoted switchable synthetic procedure for the synthesis of 3-alkylquinoxalin-2(1H)-ones and 3, 4-dihydroquinoxalin-2(1H)-ones under photoredox catalyst free and transition-metal-free conditions, using the readily available quinoxalin-2(1H)-ones and alkylboronic acids as starting materials. The selectivity of the reaction could be easily tuned by the aerobic or anaerobic conditions. When the reaction was performed in air, that is aerobic condition, a wide range of 3-alkylquinoxalin-2(1H)-ones can be obtained with good to excellent yields. When the reaction was performed in nitrogen atmosphere, that is anaerobic condition, the products 3, 4-dihydroquinoxalin-2(1H)-ones were obtained in high yields. Further investigation on the reaction mechanism and applications of photocatalyst-free system are underway in our laboratory.
Declaration of competing interestAll authors declare that no conflict of interest exists.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 22071171, 21901081), the Young Talent Key Project of Anhui Province (No. 170808J02), the Natural Science Foundation of Anhui Province (No. 2008085QB90) and the University Synergy Innovation Program of Anhui Province (No. GXXT-2020–-078) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.005.
| [1] |
X. Li, K. Yang, W. Li, et al., Drugs Future 31 (2006) 979-989. DOI:10.1358/dof.2006.031.11.1037128 |
| [2] |
J.A. Pereira, A.M. Pessoa, M.N.D.S. Cordeiro, et al., Eur. J. Med. Chem. 7 (2015) 664-672. |
| [3] |
R. Liu, Z.H. Huang, M.G. Murray, et al., J. Med. Chem. 54 (2011) 5747-5768. DOI:10.1021/jm200394x |
| [4] |
X. Qin, X. Hao, H. Han, et al., J. Med. Chem. 58 (2015) 1254-1267. DOI:10.1021/jm501484b |
| [5] |
Y.F. Si, K. Sun, X.L. Chen, et al., Org. Lett. 22 (2020) 6960-6965. DOI:10.1021/acs.orglett.0c02518 |
| [6] |
Y. Kudo, S. Shibata, Brist. J. Pharmacol. 83 (1984) 813-820. DOI:10.1111/j.1476-5381.1984.tb16237.x |
| [7] |
L. Wang, H. Liu, F. Li, et al., Adv. Synth. Catal. 361 (2019) 2354-2359. DOI:10.1002/adsc.201900066 |
| [8] |
L. Shi, W. Hu, J. Wu, et al., Mini-Rev. Med. Chem. 18 (2018) 392-413. DOI:10.2174/1389557517666171101111134 |
| [9] |
L. Yang, P. Gao, X.H. Duan, et al., Org. Lett. 20 (2018) 1034-1037. DOI:10.1021/acs.orglett.7b03984 |
| [10] |
J. Yuan, J. Fu, J. Yin, et al., Org. Chem. Front. 5 (2018) 2820-2828. DOI:10.1039/c8qo00731d |
| [11] |
L. Hu, J. Yuan, J. Fu, et al., Eur. J. Org. Chem. 2018 (2018) 4113-4120. DOI:10.1002/ejoc.201800697 |
| [12] |
J. Fu, J. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390. DOI:10.1039/c8qo00979a |
| [13] |
W. Wei, L. Wang, H. Yue, et al., ACS Sustainable Chem. Eng. 6 (2018) 17252-17257. DOI:10.1021/acssuschemeng.8b04652 |
| [14] |
L.Y. Xie, L.L. Jiang, J.X. Tan, et al., ACS Sustainable Chem. Eng. 7 (2019) 14153-14160. DOI:10.1021/acssuschemeng.9b02822 |
| [15] |
M. Tian, S. Liu, X. Bu, et al., Chem. Eur. J. 26 (2020) 369-373. DOI:10.1002/chem.201903523 |
| [16] |
X.K. He, J. Lu, A.J. Zhang, et al., Org. Lett. 22 (2020) 5984-5989. DOI:10.1021/acs.orglett.0c02080 |
| [17] |
A. Carrër, J.D. Brion, S. Messaoudi, et al., Org. Lett. 15 (2013) 5606-5609. DOI:10.1021/ol4028946 |
| [18] |
J. Yuan, S. Liu, L. Qu, Adv. Synth. Catal. 359 (2017) 4197-4207. DOI:10.1002/adsc.201701058 |
| [19] |
K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533. DOI:10.1021/acs.orglett.7b00310 |
| [20] |
L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464. DOI:10.1007/s11426-018-9446-1 |
| [21] |
L. Wang, P. Bao, W. Liu, et al., Chin. J. Org. Chem. 38 (2018) 3189-3196. DOI:10.6023/cjoc201807014 |
| [22] |
J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042. DOI:10.1039/d0qo00872a |
| [23] |
L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378. DOI:10.1039/d0gc02844d |
| [24] |
L. Wang, Y. Zhang, F. Li, et al., Adv. Synth. Catal. 360 (2018) 3969-3977. DOI:10.1002/adsc.201800863 |
| [25] |
J. Wang, B. Sun, L. Zhang, et al., Asian J. Org. Chem. 8 (2019) 1942-1946. DOI:10.1002/ajoc.201900414 |
| [26] |
W. Xue, Y. Su, K.H. Wang, et al., Asian J. Org. Chem. 8 (2019) 887-892. DOI:10.1002/ajoc.201900118 |
| [27] |
G.Y. Dou, Y.Y. Jiang, K. Xu, et al., Org. Chem. Front. 6 (2019) 2392-2397. DOI:10.1039/c9qo00552h |
| [28] |
A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792. DOI:10.1021/acs.joc.7b00464 |
| [29] |
J. Yuan, S. Liu, Y. Xiao, et al., Org. Biomol. Chem. 17 (2019) 876-884. DOI:10.1039/c8ob03061h |
| [30] |
W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130. DOI:10.1021/acs.orglett.8b03079 |
| [31] |
K.J. Li, K. Xu, Y.G. Liu, et al., Adv. Synth. Catal. 361 (2019) 1033-1041. DOI:10.1002/adsc.201800989 |
| [32] |
M. Gao, Y. Li, L. Xie, et al., Chem. Commun. 52 (2016) 2846-2849. DOI:10.1039/C5CC08049E |
| [33] |
K.J. Li, Y.Y. Jiang, K. Xu, et al., Green Chem. 21 (2019) 4412-4421. DOI:10.1039/C9GC01474H |
| [34] |
C. Hu, G. Hong, C. Zhou, et al., Asian J. Org. Chem. 8 (2019) 2092-2096. DOI:10.1002/ajoc.201900484 |
| [35] |
X. Zeng, C. Liu, X. Wang, et al., Org. Biomol. Chem. 15 (2017) 8929-8935. DOI:10.1039/C7OB02187A |
| [36] |
H. Zhang, J. Shen, Z. Yang, et al., RSC Adv. 9 (2019) 7718-7722. DOI:10.1039/C9RA01200A |
| [37] |
P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498. DOI:10.1039/c9qo01334b |
| [38] |
H. Ni, X. Shi, Y. Li, et al., Org. Biomol. Chem. 18 (2020) 6558-6563. DOI:10.1039/d0ob01423k |
| [39] |
J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182. DOI:10.1002/adsc.202000116 |
| [40] |
J. Wang, B. Sun, L. Zhang, et al., Org. Chem. Front. 7 (2020) 113-118. DOI:10.1039/C9QO01055F |
| [41] |
J. Xu, H. Yang, H. Cai, et al., Org. Lett. 21 (2019) 4698-4702. DOI:10.1021/acs.orglett.9b01578 |
| [42] |
Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245. DOI:10.1039/c9gc03045j |
| [43] |
L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955. DOI:10.1039/c9qo01240k |
| [44] |
Y.F. Si, X.L. Chen, X.Y. Fu, et al., ACS Sustainable Chem. Eng. 8 (2020) 10740-10746. |
| [45] |
X. Mi, Y. Kong, J. Zhang, et al., Chin. Chem. Lett. 30 (2019) 2295-2298. DOI:10.1016/j.cclet.2019.09.040 |
| [46] |
L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70. DOI:10.1016/j.cclet.2019.05.041 |
| [47] |
S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867. DOI:10.1016/j.cclet.2019.12.031 |
| [48] |
W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1895-1898. DOI:10.1016/j.cclet.2020.02.011 |
| [49] |
G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258. DOI:10.1016/j.cclet.2020.03.007 |
| [50] |
Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021) 1907-1910. DOI:10.1016/j.cclet.2021.01.021 |
| [51] |
K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500. DOI:10.1039/d0gc02663h |
| [52] |
I. Ghosh, L. Marzo, A. Das, et al., Acc. Chem. Res. 49 (2016) 1566-1577. DOI:10.1021/acs.accounts.6b00229 |
| [53] |
D. Ravelli, M. Fagnoni, A. Albini, Chem. Soc. Rev. 42 (2013) 97-113. DOI:10.1039/C2CS35250H |
| [54] |
D.R. Heitz, J.C. Tellis, G.A. Molander, J. Am. Chem. Soc. 138 (2016) 12715-12718. DOI:10.1021/jacs.6b04789 |
| [55] |
S. Paul, J. Guin, Green Chem. 19 (2017) 2530-2534. DOI:10.1039/C7GC00840F |
| [56] |
N.A. Romero, D.A. Nicewicz, Chem. Rev. 116 (2016) 10075-10166. DOI:10.1021/acs.chemrev.6b00057 |
| [57] |
Y. Zhao, B. Huang, C. Yang, et al., Org. Lett. 18 (2016) 3326-3329. DOI:10.1021/acs.orglett.6b01371 |
| [58] |
J.P. Barham, G. Coulthard, K.J. Emery, et al., J. Am. Chem. Soc. 138 (2016) 7402-7410. DOI:10.1021/jacs.6b03282 |
| [59] |
J.J. Murphy, D. Bastida, S. Paria, et al., Nature 532 (2016) 218-222. DOI:10.1038/nature17438 |
| [60] |
C. Ollivier, P. Renaud, Chem. Rev. 101 (2001) 3415-3434. DOI:10.1021/cr010001p |
| [61] |
F. Brucelle, P. Renaud, Org. Lett. 14 (2012) 3048-3051. DOI:10.1021/ol301120w |
| [62] |
G. Povie, L. Ford, D. Pozzi, et al., Angew. Chem. Int. Ed. 55 (2016) 11221-11225. DOI:10.1002/anie.201604950 |
| [63] |
B. Wyler, F. Brucelle, P. Renaud, Org. Lett. 18 (2016) 1370-1373. DOI:10.1021/acs.orglett.6b00306 |
| [64] |
S.E. Walker, J.A. Jordan-Hore, D.G. Johnson, et al., Angew. Chem. Int. Ed. 53 (2014) 13876-13879. DOI:10.1002/anie.201408054 |
| [65] |
Y. Fujiwara, V. Domingo, I.B. Seiple, et al., J. Am. Chem. Soc. 133 (2011) 3292-3295. DOI:10.1021/ja111152z |
| [66] |
J. Dong, F. Yue, H. Song, et al., Chem. Commun. 56 (2020) 12652-12655. DOI:10.1039/d0cc05946c |
| [67] |
G.X. Li, C.A. Morales-Rivera, Y. Wang, et al., Chem. Sci. 7 (2016) 6407-6412. DOI:10.1039/C6SC02653B |
| [68] |
A. Ling, L. Zhang, R.X. Tan, et al., J. Org. Chem. 83 (2018) 14489-14497. DOI:10.1021/acs.joc.8b02277 |
| [69] |
L. Zhang, Z.Q. Liu, Org. Lett. 19 (2017) 6594-6597. DOI:10.1021/acs.orglett.7b03297 |
| [70] |
K. Niu, Y. Hao, L. Song, et al., Green Chem. 23 (2021) 302-306. DOI:10.1039/d0gc03892j |
| [71] |
L. Zou, L. Wang, L. Sun, et al., Chem. Commun. 56 (2020) 7933-7936. DOI:10.1039/d0cc02471f |
| [72] |
Y. Mei, L. Zhao, Q. Liu, et al., Green Chem. 22 (2020) 2270-2278. DOI:10.1039/d0gc00009d |
| [73] |
Y. Zhang, W. Chen, X. Jia, et al., Chem. Commun. 55 (2019) 2785-2788. DOI:10.1039/c8cc10235j |
| [74] |
L. Zou, P. Li, B. Wang, et al., Green Chem. 21 (2019) 3362-3369. DOI:10.1039/c9gc00938h |
| [75] |
Y. Zhang, W. Chen, L. Wang, et al., Org. Chem. Front. 5 (2018) 3562-3566. DOI:10.1039/c8qo00980e |
| [76] |
L. Zhao, P. Li, X. Xie, et al., Org. Chem. Front. 5 (2018) 1689-1697. DOI:10.1039/C8QO00229K |
| [77] |
L. Candish, M. Teders, F. Glorius, J. Am. Chem. Soc. 139 (2017) 7440-7443. DOI:10.1021/jacs.7b03127 |
| [78] |
L. Zhang, L. Jiao, J. Am. Chem. Soc. 139 (2017) 607-610. DOI:10.1021/jacs.6b11813 |
| [79] |
S. Xie, D. Li, H. Huang, et al., J. Am. Chem. Soc. 141 (2019) 16237-16242. DOI:10.1021/jacs.9b09099 |
 2021, Vol. 32
2021, Vol. 32