b Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China;
c Suzhou Institute of Wuhan University, Suzhou 215123, China
Chiral pyrrolidines have been regarded as an important class of five-membered nitrogen-containing heterocycles, which frequently appear as the key frameworks in a great many agrochemicals, natural products, chiral organocatalysts and ligands [1, 2]. In addition, they are also ranked as the prevailing chiral nitrogen-containing heterocycles with diversified biological activities [2]. On the other hand, compared with the conventional sulfonyl chlorides, sulfonyl fluorides have some distinctive properties, such as controllable reactivity, relatively good stability and strong hydrogen-bonding interaction [3]. Therefore, they have been received great attraction in recent years, which not only widely applied as powerful probes and inhibitors against protein or nucleic acid targets, but also worked as versatile organic intermediates [3a, 4]. Sharpless and co-workers have developed a few examples of sulfur(Ⅵ) fluoride exchange (SuFEx) reactions to easily access a variety of sulfur(Ⅵ)-based functional groups [3a]. Among the well available synthetic methods, the transformations involving the interesting difunctional ethylene sulfonyl fluorides (ESFs) are valuable strategies to introduce a sulfonyl fluoride group, which always served as preferential reaction partners. In 1954, Truce and Hoerger developed Diels-Alder reaction of 2-para-nitrophen ylethylene sulfonyl fluoride as the dienophile with cyclopentadiene [5]. Lupton and co-workers developed double electrophile α,β-unsaturated sulfonyl azoliums intermediates through the combination of substituted ESFs and N-heterocyclic carbene (NHC) catalyst to prepare a range of unsaturated δ-sultones [6]. Qin and co-workers also developed some methodologies involving ESFs as the reaction substrates to access some other important sulfonyl derivatives [7]. Moreover, the ESFs could be served as excellent Michael acceptors, especially for the asymmetric exploration [3b, 8]. Recently, Leung reported Pd-catalyzed enantioselective hydrophosphination of ESFs derivatives, affording chiral sulfonyl fluoride-derived phosphines in high yields with good to excellent enantioselecitivites [8a]. Qin and co-workers described Rh-catalyzed asymmetric conjugate addition of arylboronic acids to β-aryl ESFs and dienesulfonyl fluorides with excellent results [8b]. Yan and co-workers developed quinine-derived squaramide-catalyzed asymmetric conjugate addition of 3-amido-2-oxindoles to ESFs, and the desired products spirocyclic oxindole sultams were obtained with excellent yields and enantioselectivities [3b].
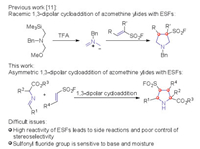
Viewing the structural significance of both pyrrolidines and sulfonyl fluorides, it is important to develop efficient methods for the simultaneous introduction of both pyrrolidines and sulfonyl fluorides. The 1,3-dipolar cycloadditions of azomethine ylides have been regarded as the general and reliable methods for the construction of highly substituted chiral pyrrolidines and derivatives [9, 10]. In 2018, Grygorenko and coworkers developed a racemic 1,3-dipolar cycloaddition of substituted ESFs with in situ generated azomethine ylides, and this protocol provided a practical approach to afford a series of pyrrolidine-3-sulfonyl fluorides [11]. However, to the best of our knowledge, there is no example involving the asymmetric catalytic version to access these valuable moieties. The vinyl phenyl sulfones have been applied in the transition metal-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides to deliver pyrrolidine-3-sulfones by Carretero [12], Fukuzawa [13] and our group [14]. Encouraged by these early achievements, we envision that the electron-deficient ESFs could be employed in the asymmetric 1,3-dipolar cycloaddition, however, there are two difficult issues that need to be addressed (Scheme 1): (1) ESFs owned high reactivity because of the strong electron-withdrawing sulfonyl fluoride group, which could lead to some side reactions and the difficult control of stereoselectivity. (2) The sulfonyl fluorides are more sensitive to base and moisture than common α,β-unsaturated sulfur(Ⅵ) sulfones, sulfonic esters, sulfonamides and derivatives, but the general 1,3-dipolar cycloaddition always required base, which would result in their instability in the reaction system with poor yields. In the course of our research to pursue these valuable motifs containing both functionalized pyrrolidines and sulfonyl fluorides, we herein developed Cu-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with ESFs, which provided an efficient entry to diverse structurally unique chiral pyrrolidine-3-sulfonyl fluorides with high yields, excellent diastereoselectivities, and moderate to excellent enantioselectivities (up to 87% yield, > 20:1 dr, 94% ee).
|
Download:
|
| Scheme 1. 1,3-Dipolar cycloaddition of azomethine ylides with ethenesulfonyl fluorides (ESFs). | |
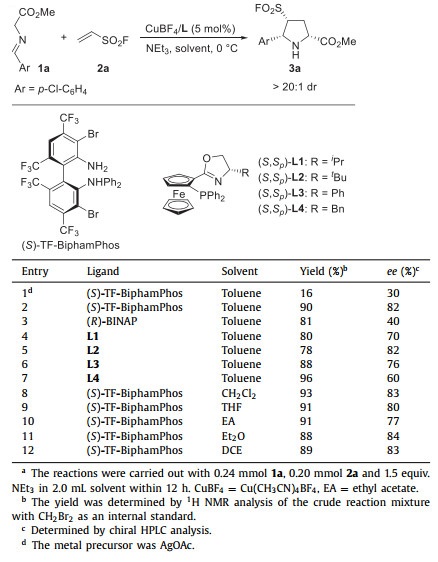
The initial investigation of the asymmetric 1,3-dipolar cycloaddition of model substrates azomethine ylide 1a with ESF 2a was started in AgOAc/(S)-TF-BiphamPhos catalytic system, and very poor conversion was observed (Table 1, entry 1). To our delight, the desired cycloaddition product 3a could be easily obtained in high yield and good enantioselectivity through the replacement of AgOAc with CuBF4 (90% yield, > 20:1 dr, 82% ee, Table 1, entry 2). The Cu-catalyzed 1,3-dipolar cycloaddition was then continued to examine other chiral ligands, such as (R)-BINAP and ferrocene-based P,N-ligands L1-L4 bearing different substituted groups on the oxazoline ring, which provided product 3a in good to high yields and with moderate to good enantioselectivities (78%−96% yields, > 20:1 dr, 40%−82% ee, Table 1, entries 3–7). A variety of solvents were then screened in the presence of the privileged ligand (S)-TF-BiphamPhos. Generally, this 1,3-dipolar cycloaddition was proceeded smoothly in nonprotic solvents, 88%−93% yields and 77%−84% ee were obtained (Table 1, entries 8–12). CH2Cl2 was found as the best reaction solvent in 93% yield, > 20:1 dr and 83% ee (Table 1, entry 8).
|
|
Table 1 Optimization reaction conditions for the 1,3-dipolar cycloaddition of azomethine ylide 1a with ESF 2a.a |
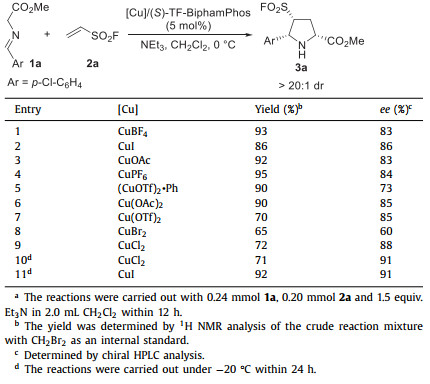
Subsequently, this 1,3-dipolar cycloaddition was continued to investigate with a number of common copper salts in combination of (S)-TF-BiphamPhos ligand. As shown in Table 2, these [Cu]/(S)-TF-BiphamPhos catalytic systems are efficient to afford the desired product 3a in moderate to excellent results. In these attempts, CuI and CuCl2 could provide satisfactory yields and enantioselectivities (Table 2, entries 2 and 9). In order to achieve higher enantioselectivity, this transformation was conducted at −20 ℃ within 24 h catalyzed by CuI and CuCl2/(S)-TF-BiphamPhos system, respectively. It should be noted that the remarkable improvement in enantioselectivity was observed in the CuI/(S)-TF-BiphamPhos catalytic system (92% yield, 91% ee, Table 2, entry 11).
|
|
Table 2 Screening metal precursors for the 1,3-dipolar cycloaddition of azomethine ylide 1a with ESF 2a.a |
After establishing the optimal reaction conditions for CuI/(S)-TF-BiphamPhos-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with ESFs, we then focused on the investigation of substrate scope generality. These reaction results were summarized in Scheme 2. A variety of azomethine ylides 1 derived from glycinates and aldehydes proceeded efficiently with ESFs 2 to offer the corresponding cycloaddition products 3. A wide range of azomethine ylides containing electron-withdrawing (1a-1g), electron-neutral (1h) or electron-donating (1i-1k) groups on the phenyl ring worked well as excellent partners, affording the desired endo-cycloaddition products (3a-3k) exclusively in moderate to good yields and good to excellent enantioselectivities (64%−87% yields, > 20:1 dr, 84%−94% ee). It was found that the position of the substituents on the phenyl ring did not seem to greatly affect the reaction results, the substrates containing 2-Cl (1c), 2-Br (1d), 2-Me (1k) groups with steric hindrance were well-tolerated to provide the corresponding products in 64%−87% yields with 85%−89% ee. In addition, the azomethine ylides bearing large 1- and 2-naphthyl group also performed smoothly to give the corresponding products 3l-3m with moderate to good results (78%−86% yields, 78%−87% ee). We also attempted the heterocyclic pyridinyl substituted azomethine ylide (1n) under the optimized reaction conditions, the expected product (3n) was obtained with acceptable result (73% yield, 64% ee). Furthermore, we also examined the effect of ester group on the azomethine ylide, switching the methyl ester (1a) to ethyl ester (1o), which did not affect the reactivity and enantioselectivity with comparable results (81% yield, 90% ee). The trans-2-phenylethene-1-sulfonyl fluoride could be also employed into this transformation, the desired product 3p was afforded with good stereochemical control, albeit in moderate yield (56% yield, 83% ee). Remarkably, the azomethine ylide 1q generated from the α-methyl substituted amino acid alanine reacted with ESF 2a to result in endo-3q bearing a quaternary stereogenic center with limitation yield and enantioselectivity, presumably due to the steric effect (54% yield, 54% ee). The less reactive alkyl substituted azomethine ylide was not well compatible in this transformation. In addition, the absolute configuration of product 3a was determined to be (2R, 4R, 5R) by X-ray diffraction analysis.

|
Download:
|
| Scheme 2. Substrate scope study for the 1,3-dipolar cycloaddition of azomethine ylides with ESFs. The reactions were carried out with 0.24 mmol 1, 0.20 mmol 2 and 1.5 equiv. NEt3 in 2.0 mL CH2Cl2 within 24 h. The yield is isolated yield and the ee value was determined by chiral HPLC analysis. | |
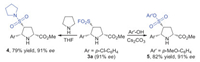
To demonstrate the great application of the cycloaddition products in the SuFEx chemistry, they could be easily converted to other useful sulfonyl derivatives, such as sulfonamides and sulfonates, which occupied important position in the fields of pharmaceuticals, agrochemicals and synthetic chemistry. As depicted in Scheme 3, the amine pyrrolidine could be worked as effective nucleophile to react with cycloaddition product pyrrolidine-3-sulfonyl fluoride 3a, providing the corresponding sulfonamide 4 in 79% yield without erosion of ee value [15, 3b]. Moreover, compound 3a could react with aryl phenol smoothly under basic condition, the expected sulfonate 5 could be obtained in good yield and with maintained ee value (82% yield, 91% ee) [16].
|
Download:
|
| Scheme 3. The application of product 3a in SuFEx reactions. | |
In conclusion, we developed a facile and efficient synthetic method to access a variety of chiral pyrrolidine-3-sulfonyl fluorides through Cu-catalyzed endo-selective asymmetric 1,3-dipolar cycloaddition of azomethine ylides with ethenesulfonyl fluorides in high reactivities, excellent diastereoselectivities, and moderate to excellent enantioselectivities (up to 87% yield, > 20:1 dr, 94% ee). The cycloaddition products pyrrolidine-3-sulfonyl fluorides owned great potential synthetic application, which could be readily converted to other chiral sulfonyl derivatives, such as sulfonamide and sulfonate through simple transformations with high yields.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgmentsWe are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21772147, 22071186, 22071187), Natural Science Foundation of Hubei Province (No. 2020CFA036) and Natural Science Foundation of Jiangsu Province (No. SKB2019041078). The Program of Introducing Talents of Discipline to Universities of China (111 Project) is also appreciated.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.063.
| [1] |
(a) S.G. Pyne, A.S. Davis, N.J. Gates, et al., Synlett (2004) 2670-2680; (b) A. Padwa, W. Pearson, in: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Wiley & Sons, New York, 2002; (c) J.P. Michael, Nat. Prod. Rep. 25 (2008) 139-165. |
| [2] |
(a) M. Kuhnert, A. Blum, H. Steuber, W.E. Diederich, J. Med. Chem. 58 (2015) 4845-4850; (b) M. Setoguchi, S. Iimura, Y. Sugimoto, et al., Bioorg. Med. Chem. 21 (2013) 42-61; (c) S.D. Roughley, A.M. Jordan, J. Med. Chem. 54 (2011) 3451-3479; (d) M.J.C. Scanio, X.B. Searle, B. Liu, et al., ACS Med. Chem. Lett. 10 (2019) 1543-1548; (e) Y. Zhao, A. Aguilar, D. Bernard, S. Wang, J. Med. Chem. 58 (2015) 1038-1052; (f) Y. Zhao, L. Liu, W. Sun, et al., J. Am. Chem. Soc. 135 (2013) 7223-7234; (g) K. Ding, Y. Lu, Z. Nikolovska-Coleska, et al., J. Am. Chem. Soc. 127 (2005) 10130-10131; (h) G.R. Cutting, Nat. Rev. Genet. 16 (2015) 45-56; (i) Q. Ding, Z. Zhang, J.J. Liu, et al., J. Med. Chem. 56 (2013) 5979-5983. |
| [3] |
(a) J. Dong, L. Krasnova, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 53 (2014) 9430-9448; (b) J. Chen, B. Huang, Z. Wang, X. Zhang, M. Yan, Org. Lett. 21 (2019) 9742-9746. |
| [4] |
(a) P.G. Baraldi, B. Cacciari, S. Moro, et al., J. Med. Chem. 44 (2001) 2735-2742; (b) N.N. Gushwa, S. Kang, J. Chen, J. Taunton, J. Am. Chem. Soc. 134 (2012) 20214-20217; (c) D.A. Shannon, C. Gu, C.J. McLaughlin, et al., ChemBioChem 13 (2012) 2327-2330; (d) A.J. Brouwer, A. Jonker, P. Werkhoven, et al., J. Med. Chem. 55 (2012) 10995-11003; (e) A. Baranczak, Y. Liu, S. Connelly, et al., J. Am. Chem. Soc. 137 (2015) 7404-7414; (f) E.C. Hett, H. Xu, K.F. Geoghegan, et al., ACS Chem. Biol. 10 (2015) 1094-1098; (g) A. Narayanan, L.H. Jones, Chem. Sci. 6 (2015) 2650-2659; (h) A.J. Brouwer, N.H. Álvarez, A. Ciaffoni, H. van de Langemheen, R.M. Liskamp, Bioorg. Med. Chem. 24 (2016) 3429-3435; (i) W. Chen, J. Dong, L. Plate, et al., J. Am. Chem. Soc. 138 (2016) 7353-7364; (j) Q. Zhao, X. Ouyang, X. Wan, et al., J. Am. Chem. Soc. 139 (2017) 680-685; (k) D.E. Mortenson, G.J. Brighty, L. Plate, et al., J. Am. Chem. Soc. 140 (2018) 200-210; (l) X. Chen, G.F. Zha, J.Q. Wang, X.H. Liu, J. Enzyme Inhib. Med. Chem. 33 (2018) 1266-1270; (m) R. Artschwager, D.J. Ward, S. Gannon, et al., J. Med. Chem. 61 (2018) 5395-5411; (n) G.F. Zha, S.M. Wang, K.P. Rakesh, et al., Eur. J. Med. Chem. 162 (2019) 364-377. |
| [5] |
W.E. Truce, F.D. Hoerger, J. Am. Chem. Soc. 76 (1954) 3230-3232. DOI:10.1021/ja01641a046 |
| [6] |
A. Ungureanu, A. Levens, L. Candish, D.W. Lupton, Angew. Chem. Int. Ed. 54 (2015) 11780-11784. DOI:10.1002/anie.201504633 |
| [7] |
(a) X. Chen, G.F. Zha, G.A.L. Bare, et al., Adv. Synth. Catal. 359 (2017) 3254-3260; (b) X. Chen, G.F. Zha, W.Y. Fang, K.P. Rakesh, H.L. Qin, Chem. Commun. 54 (2018) 9011-9014. |
| [8] |
(a) X.R. Li, H.J. Chen, W. Wang, et al., J. Organomet. Chem. 899 (2019) 120912-120915; (b) B. Moku, W.Y. Fang, J. Leng, et al., iScience 21 (2019) 695-705. |
| [9] |
(a) J. Adrio, J.C. Carretero, Chem. Commun. 50 (2014) 12434-12446; (b) T. Hashimoto, K. Maruoka, Chem. Rev. 115 (2015) 5366-5412; (c) X. Fang, C.J. Wang, Org. Biomol. Chem. 16 (2018) 2591-2601; (d) J. Adrio, J.C. Carretero, Chem. Commun. 55 (2019) 11979-11991; (e) L. Wei, X. Chang, C.J. Wang, Acc. Chem. Res. 53 (2020) 1084-1100; (f) B. Bdiria, B.J. Zhao, Z.M. Zhou, Tetrahedron: Asymmetry 28 (2017) 876-879; (g) T. Hashimoto, K. Maruoka, Chem. Rev. 115 (2015) 5366-5412; (h) C. Nejera, J.M. Sansano, Curr. Top. Med. Chem. 14 (2014) 1271-1282; (i) C. Nájera, J.M. Sansano, J. Organomet. Chem. 771 (2014) 78-92; (j) L. Wei, L. Xiao, Y. Hu, et al., Chin. J. Org. Chem. 39 (2019) 2119-2130. |
| [10] |
(a) F. Cheng, S.J. Kalita, Z.N. Zhao, et al., Angew. Chem. Int. Ed. 58 (2019) 16637-16643; (b) Y. Xiong, Z. Du, H. Chen, et al., J. Am. Chem. Soc. 141 (2019) 961-971; (c) B. Liu, W. Li, H.H. Wu, J. Zhang, Org. Chem. Front. 6 (2019) 694-698; (d) L. Liang, H.Y. Niu, D.C. Wang, et al., Chem. Commun. 55 (2019) 553-556; (e) X. Chang, X.S. Sun, C. Che, et al., Org. Lett. 21 (2019) 1191-1196; (f) Z. Gan, M. Zhi, R. Han, et al., Org. Lett. 21 (2019) 2782-2785; (g) G.S. Caleffi, O. Larranaga, M. Ferrandiz-Saperas, et al., J. Org. Chem. 84 (2019) 10593-10605; (h) Y.P. Zhang, Y. You, J.Q. Zhao, et al., Org. Chem. Front. 6 (2019) 1879-1884; (i) C. Shen, Y. Yang, L. Wei, et al., iScience 11 (2019) 146-159; (j) H. Deng, R. Jia, W.L. Yang, X. Yu, W.P. Deng, Chem. Commun. 55 (2019) 7346-7349; (k) R. Chowdhury, A.K. Dubey, S.K. Ghosh, J. Org. Chem. 84 (2019) 2404-2414; (l) Z. Wang, D.C. Wang, M.S. Xie, G.R. Qu, H.M. Guo, Org. Lett. 22 (2020) 164-167; (m) H. Cui, K. Li, Y. Wang, et al., Org. Biomol. Chem. 18 (2020) 3740-3746; (n) X. Cheng, D. Yan, X.Q. Dong, C.J. Wang, Asian J. Org. Chem. 9 (2020) 1567-1570; (o) K.Q. Zhang, Q.F. Deng, J. Luo, et al., ACS Catal. 11 (2021) 5100-5107; (p) X. Chang, Y. Yang, C. Shen, et al., J. Am. Chem. Soc. 143 (2021) 3519-3535; (q) W.R. Zhu, Q. Su, N. Lin, et al., Org. Chem. Front. 7 (2020) 3452-3458; (r) N. Chen, L. Zhu, L. Gan, et al., Eur. J. Org. Chem. (2018) 2939-2943; (s) Z. Dong, Y. Zhu, B. Li, et al., J. Org. Chem. 82 (2017) 3482-3490; (t) Y.M. Wang, H.H. Zhang, C. Li, T. Fan, F. Shi, Chem. Commun. 52 (2016) 1804-1807; (u) C. Zou, Y. Han, C. Zeng, et al., Chin. Chem. Lett. 31 (2020) 377-380. |
| [11] |
V.L. Mykhalchuk, V.S. Yarmolchuk, R.O. Doroschuk, A.A. Tolmachev, O.O. Grygorenko, Eur. J. Org. Chem. (2018) 2870-2876. DOI:10.1002/ejoc.201800521 |
| [12] |
A. López-Pérez, J. Adrio, J.C. Carretero, Angew. Chem. Int. Ed. 48 (2009) 340-343. DOI:10.1002/anie.200805063 |
| [13] |
S.I. Fukuzawa, H. Oki, Org. Lett. 10 (2008) 1747-1750. DOI:10.1021/ol8003996 |
| [14] |
G. Liang, M.C. Tong, C.J. Wang, Adv. Synth. Catal. 351 (2009) 3101-3106. DOI:10.1002/adsc.200900552 |
| [15] |
(a) P.K. Chinthakindi, K.B. Govender, A.S. Kumar, et al., Org. Lett. 19 (2017) 480-483; (b) S.A. Zhersh, O.P. Blahun, I.V. Sadkova, et al., Chem. Eur. J. 24 (2018) 8343-8349. |
| [16] |
C.J. Smedley, M.C. Giel, A. Molino, et al., Chem. Commun. 54 (2018) 6020-6023. DOI:10.1039/C8CC03400A |
 2021, Vol. 32
2021, Vol. 32