b College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China;
c Key Laboratory of Cellular Physiology, Ministry of Education, Shanxi Medical University, Taiyuan 030001, China
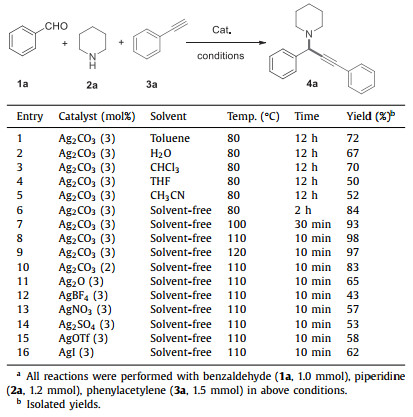
Propargylamines and chalcones are widely used as valuable compounds in organic synthesis, medicinal chemistry, and material science due to their importance as versatile synthetic blocks, biological activities and functional materials [1]. As a consequence, the preparation of propargylamines and chalcones has been intensively studied in the past decades [2]. In general, there are three main methods to prepare propargylamines (Scheme 1). The traditional method is nucleophilic addition of alkynyl–metal reagents to imines for the preparation of propargylamines (Scheme 1a). However, highly reactive reagents such as n-butyllithium or Grignard reagents used to prepare alkynyl–metal reagents are highly moisture sensitive and require harsh reaction conditions [3]. Transition-metal-catalyzed A3-coupling reaction of aldehydes, amines and alkynes is perhaps more attractive strategy [4]. Several transition metal catalysts such as AgI, AuBr, InCl3, CoCl2(PPh3)2, FeCl3, Mn(OAc)2, and metal nanometerials have been used in such three-component reactions (Scheme 1b) [5]. Nonetheless, some drawbacks such as high reaction temperature, using the toxic solvents, utilizing inert gas protection, long reaction time, laborious preparation of metal nanomaterial and moderate yields are rather common among these methods. Furthermore, most of the synthesized propargylamines are internal alkyne structures, and the synthesis of terminal propargylamines was rarely reported [6]. Alternatively, Li's group and other groups developed several methods based on copper-catalyzed oxidative coupling of terminal alkyne and amines followed by C-C bond formation to construct propargylamine (Scheme 1c) [7]. But the peroxide tert-BuOOH is indispensable during the catalytic process.
|
Download:
|
| Scheme 1. Synthesis of propargylamines and chalcones. | |
For the preparation of chalcones, the main methods are the Claisen-Schmidt condensation or hydration-condensation of aromatic alkynes with aldehydes (Scheme 1d) [8]. However, low selectivity and harsh reaction conditions limited their application. Therefore, further development of a simple, fast and practical approach to synthesize internal or terminal propargylamines and chalcones is very necessary in organic synthesis.
In recent years, silver salts as common catalysts are widely used in organic synthesis and industry due to the advantages of high catalytic activity and easy availability [9]. Furthermore, solvent-free synthetic reactions have attracted increasing attention from chemists [10]. In this paper, we reported Ag2CO3 as a commercial catalyst for the efficient preparation of internal or terminal propargylamines and chalcones via A3 coupling reaction of aldehydes, amines and alkynes under solvent-free condition (Scheme 1e). Compared to the reported methods [5], the present protocols provided several simple, fast, cost-effective and practical ways for the preparation of various propargylamines and chalcones.
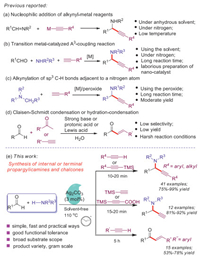
This study was commenced by optimizing the reaction conditions using benzaldehyde 1a, piperidine 2a and phenylacetylene 3a as model substrates (Table 1). A mixture of 1a (1 mmol), 2a (1.2 mmol), 3a (1.5 mmol) and Ag2CO3 (0.03 mmol) in toluene (3.0 mL) was stirred at 80 ℃ for 12 h. The reaction took place and gave 72% isolated a yield of 4a (entry 1). When we employed H2O, CHCl3, THF and CH3CN as the solvents, lower yields were observed (entries 2–5). Interestingly, the solvent-free reaction proceeded rapidly in good yields (entry 6). As the temperature continued to increase at 100 ℃, the yield of 4a increased to 93%. The highest yield was achieved during the temperature at 110 ℃ and the reaction time was only 10 min (entries 7–9). When the loading amount of Ag2CO3 was reduced, yield of 4a decreased to 83% (entry 10). We also examined other silver salts such as Ag2O, AgBF4, AgNO3, Ag2SO4, AgOTf and AgI, but moderate yields were observed (entries 11–16). Therefore, the optimal reaction conditions were as follows: aldehydes (1.0 mmol), amines (1.2 mmol), alkynes (1.5 mmol), and Ag2CO3 (0.03 mmol) at 110 ℃ under solvent-free condition.
|
|
Table 1 Optimization of reaction conditions.a |
With the optimal reaction conditions in hand, the substrate scope of the A3 coupling reaction was investigated with respect to aldehydes, amines and terminal alkynes (Scheme 2). First, several aldehydes bearing electron-rich (Me, OMe and OH) and electron-deficient (F, Cl) substituents on the aromatic ring were successfully converted into the corresponding propargylamines in up to 99% yield (4b-4h). Among them, slight decreases in reactivity were observed for 4c and 4h, probably because the relatively strong electron-donating effect and steric hindrance impeded the nucleophilic addition of phenylacetylene to an imine. The reactivity of the ortho position is lower than that of para and meta for chlorine-substituted benzaldehyde (4e-4g). When 5-bromothiophene-2-carbaldehyde, [1,1′-biphenyl]-4-carbaldehyde and N-(4-formylphenyl)acetamide were subjected to this process, the desired products 4i, 4j and 4k were obtained in 87%, 90% and 88% yield, respectively, showing good tolerance. Moreover, the cyclohexanecarbaldehyde was also effective to generate the target product with 91% yield (4l).

|
Download:
|
| Scheme 2. Reaction scope of aldehydes, secondary amines or terminal alkynes. Conditions: Catalyst Ag2CO3 (3 mol%), aldehydes (1, 1.0 mmol), secondary amines (2, 1.2 mmol), terminal alkynes (3, 1.5 mmol), solvent-free, 110 ℃, isolated yields. | |
Next, a variety of terminal alkynes and secondary amines were screened under optimal condition. As for electron-deficient and electron-rich phenylacetylenes with different substituents (F, Cl, OMe, Me, Et and n-C5H11), regardless of the location at the ortho-, meta-, or para-position, the products were obtained in good to excellent yields (4m-4t). 3,3-Diethoxyprop-1-yne with acetal group could also be tolerated to afford the desired product 4u in 85% yield. However, it is important to point out that trimethyl(prop-2-yn-1-yloxy)silane did not produce the corresponding product, instead desilylation of the TMS group (4v). Furthermore, the reaction using heterocyclic secondary amines such as pyrrolidine, azepane, morpholine, and thiomorpholine proceeded smoothly resulting in the desired products in high yields (4w-4z).
Guided by removing the silyl group of the product 4t, we investigated another A3-coupling reaction of benzaldehyde, piperidine and trimethyl(phenylethynyl)silane utilizing Ag2CO3-catalyzed activation of the C–Si bond (optimal conditions in Table S1, Supporting information). Gratifyingly, above Ag2CO3 catalytic system can promote the reaction to proceed smoothly producing the corresponding propargylamines. As illustrated in Scheme 3, both aromatic aldehydes with electron-withdrawing (F, NO2) and electron-donating (OMe) groups can react smoothly with piperidine and trimethyl(phenylethynyl)silane to obtain propargylamines (4a′–4c′). Meanwhile, alkynylsilanes possessing chlorine and alkyl groups at para- position underwent the reaction smoothly delivering the target products in excellent yields (4d′–4f′). Alkyl pivalaldehyde and hex-1-yn-1-yltrimethylsilane can be transformed to the corresponding products with 80% and 87% yield (4g′, 4h′). In addition, noncyclic secondary amines were also effective to generate the target products in good yields (4i′, 4j′). Thus, the above results prove that Ag2CO3 can be used as an effective catalyst to synthesize propargylamines easily and quickly, and showed great advantages over the reported catalytic systems [5].

|
Download:
|
| Scheme 3. Reaction scope of aldehydes, secondary amines or alkynylsilanes. Conditions: Catalyst Ag2CO3 (3 mol%), aldehydes (1, 1.0 mmol), secondary amines (2, 1.2 mmol), alkynylsilanes (5, 1.5 mmol), solvent-free, 110 ℃, isolated yields. | |
Although the preparation of the propargylamines with internal alkyne structure has a certain practicability, terminal propargylamines may provide a wider range of applications due to the unique reactivity of sp C-H bond. Therefore, we chose TMS–acetylene as the coupling reagent by direct sp C-Si cleavage with aldehydes and amines to synthesize terminal propargylamines catalyzed by Ag2CO3. As illustrated in Scheme 3, all the aldehydes and secondary amines substrates showed high reactivity delivering the terminal propargylamines in high yields (4k′–4p′). It was noteworthy that alkynylsilane containing TES could also be converted to the corresponding terminal propargylamine 4k′ (removing TES), which is in sharp contrast with that AgI-catalyzed A3-coupling reaction in the previous reported method (retaining TES) [5a]. Surprisingly, when using 3-(trimethylsilyl)propiolic acid as a substrate, the results showed the product 4k′ was also the terminal propargylamine, which was first disclosed by us. Likewise, substrates aldehydes and secondary amines were readily converted into valuable terminal alkynes in satisfied yields (4q′–4t′). Hence, both TMS–acetylene and 3-(trimethylsilyl)propiolic acid could be used as good coupling partners for the preparation of terminal propargylamines through A3-coupling reaction catalyzed by Ag2CO3 without using excessive F- reagent [11].
Accidentally, we extended the reaction time over 2 h for the reaction of benzaldehyde, piperidine and phenylacetylene catalyzed by Ag2CO3 at 110 ℃ under solvent-free condition. The other product was (E)-chalcone, instead of stopping at the A3-coupling step. So we developed a novel method based on Ag2CO3-catalyzed the reaction of aldehydes, piperidine and terminal alkynes to obtain valuable chalcones (optimal conditions in Table S3, Supporting information). As shown in Scheme 4, we obtained moderate-to-good yields of (E)-chalcones. Aromatic aldehydes and phenylacetylenes with electron-withdrawing groups (CN, F, Cl and Br) show excellent diastereoselectivity (6b–6f, 6h-6j). On the contrary, phenylacetylenes with electron-donating groups (Me, Et, n-Pr, t-Bu) show good diastereoselectivity except for CH3 at meta position (6k–6l). In addition, the π-extended 2-naphthyl aldehyde is also a suitable substrate for this transformation (6g). Unfortunately, aliphatic aldehydes and aliphatic alkynes were not active in this transformation.
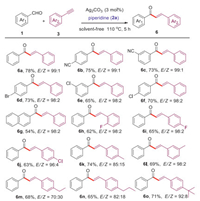
|
Download:
|
| Scheme 4. Reaction scope of aldehydes or terminal alkynes. Conditions: Catalyst Ag2CO3 (3 mol%), aldehydes (1, 1.0 mmol), piperidine (2a, 1.2 mmol), terminal alkynes (3, 1.5 mmol); solvent-free; 110 ℃, 5 h; Isolated yields; The ratio of E/Z was estimated by the integral area of aromatic ring hydrogen atom of the product in 1H NMR. | |
As a practical synthetic protocol, both the operational simplicity and scalability have great significance for the synthesis of propargylamines and chalcones in the laboratory, and even in the field of the pharmaceutical industry. Therefore, both large-scale preparation and bioactive molecule synthesis via A3-coupling reaction were further investigated. Pleasingly, 10 mmol of benzaldehyde (1a) was employed to react with piperidine (2a) and phenylacetylene (3a) under standard conditions and 92% yield of 4a was obtained (Scheme 5a), which is a positive aspect for industrial application. Importantly, the above catalysis system could be used for bioactive molecule synthesis such as ethisterone and N-ethyl-3-carbazolecarboxaldehyde to afford the corresponding products with yields of 88% and 92% (4u′, 4v′) (Scheme 5b), respectively. Furthermore, we screened the compound 4v′ for antiproliferative activities in two human cancer cell lines (colorectal carcinoma HCT116 cells and hepatoma HepG2 cells) using the CCK-8 assay. Consequently, compound 4v′ showed good inhibitory activity for these two human cancer cell lines at the relatively low µmol/L level, with IC50 values of 61.01 µmol/L and 72.58 µmol/L, respectively (Supporting information). A further bioactivity investigation is still underway in our laboratory. Moreover, the diastereoselectivity of Ag2CO3-catalyzed A3-coupling reaction was investigated by using benzaldehyde, (S)-N-benzyl-1-phenylethylamine and phenylacetylene as model substrates. It was worth noting that (S)-N-benzyl-1-phenylethylamine showed good diastereoselectivity (2.5:1). Meanwhile, when (S)-N-benzyl-1-phenylethylamine was reacted with formaldehyde and phenylacetylene, the desired products 4x′, which had no racemization, were afforded in 98% yield (Scheme 5c).
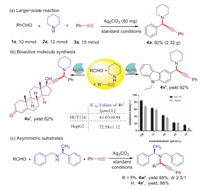
|
Download:
|
| Scheme 5. (a) Large-scale synthesis of 4a. (b) Bioactive molecule synthesis. (c) Investigation of substrate-controlled asymmetric Ag2CO3-catalyzed A3-coupling. | |
Besed on previously well-documented results [5a, 6a, 12], plausible mechanisms for the synthesis of different products are proposed in Scheme 6. The Ag2CO3 activated the C-H bond of terminal alkyne or C-Si bond of alkynylsilane to generate the silver acetylide intermediate, which reacted with the iminium ion I formed by aldehydes and piperidine to afford the propargylamines and releases the silver ion for further reaction (Path A). The alkynyl carbon bonded to 3-(trimethylsilyl)propiolate attacked the iminium ion I to form intermediate II, which decarboxylated to give the propargylamine with TMS group. At last, the Ag2CO3 activated C-Si bond, delivering the desired terminal propargylamine (Path B). Ag2CO3-catalyzed A3 coupling resulted in the formation of propargylamine, which was deprotonated by excessive base piperidine in the coordination of silver with the triple bond to form intermediate III. Subsequent protonation generated allenylamine intermediate IV, which was further hydrolyzed to give preference to the thermodynamically more stable E configuration product 6 (Path C).
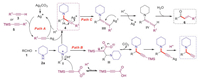
|
Download:
|
| Scheme 6. Plausible mechanisms. | |
In conclusion, we have developed several simple, efficient and practical methods for preparing internal or terminal propargylamines and chalcones via A3 coupling of aldehydes, amines and alkynes using easily available Ag2CO3 as a catalyst under solvent-free condition. The Ag2CO3 can effectively activate not only C-H bond in terminal alkyne, but C-Si bond in alkynylsilane to afford the corresponding products. Both TMS–acetylene and 3-(trimethylsilyl)propiolic acid could be used as good coupling partners delivering the terminal propargylamines. Besides, the above methods showed broad substrate group with good functional group tolerance and could be applied to synthesize 1-(1,3-diphenylprop-2-yn-1-yl)piperidine 4a in gram scale. Multifunctional compounds such as ethisterone and N-ethyl-3-carbazolecarboxaldehyde could also achieve the corresponding transformation and compound 4v′ showed good inhibitory activity against CHT116 cells and HepG2 cells. The stereoselectivity of A3-coupling reaction was also investigated. Given the operational simplicity, easily available commercial catalysts, short reaction time, high-efficiency and the diversity of products, these developed methods are expected to be ideal for organic intermediate synthesis and fine chemical production.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsWe are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21802093, 21536003), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0408), and the PhD Start-up Foundation of Shanxi Medical University (No. 03201501) for the financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.026.
| [1] |
(a) D.S. Ermolat'ev, J.B. Bariwal, H.P.L. Steenackers, et al., Angew. Chem. Int. Ed. 49 (2010) 9465–9468; (b) W. Chen, Y.C. Zhang, P.H. Li, et al., Org. Chem. Front. 5 (2018) 855–859; (c) Y. Yamamoto, H. Hayashi, T. Saigoku, et al., J. Am. Chem. Soc. 127 (2005) 10804–10805; (d) P. Kaur, B. Kumar, K.K. GurjarJ, et al., J. Org. Chem. 85 (2020) 2231–2241; (e) X.W. He, M.Q. Xie, R.X. Li, et al., Org. Lett. 22 (2020) 4306–4310; (f) J.F. Cui, K.K. Kung, H.M. Ko, et al., Adv. Synth. Catal. 356 (2014) 2965–2973; (g) Z. Wang, L. Yang, H.L. Liu, et al., Chin. J. Org. Chem. 10 (2018) 2639–2647; (h) Y.Y. Liu, Arkivoc i, (2014) 1–20. |
| [2] |
(a) K. Lauder, A. Toscani, N. Scalacci, et al., Chem. Rev. 117 (2017) 14091–14200; (b) S. Yan, S.G. Pan, T. Osako, et al., ACS Sustainable Chem. Eng. 7 (2019) 9097–9102; (c) F. Wang, H.D. Feng, H.Q. Li, et al., Chin. Chem. Lett. 31 (2020) 1558–1563; (d) J.Y. Zhang, X. Huang, Q.Y. Shen, et al., Chin. Chem. Lett. 29 (2018) 197–200; (e) M. Martinez-Amezaga, R.A. Giordano, D.N. Prada Gori, et al., Org. Biomol. Chem. 18 (2020) 2475–2486. |
| [3] |
(a) T. Takahashi, F.Y. Bao, G.H. Gao, et al., Org. Lett. 5 (2003) 3479–3481; (b) A. Tuulmets, V. Pällin, J. Tammiku-Taul, et al., J. Phys. Org. Chem. 15 (2002) 701–705; (c) T. Harada, T. Fujiwara, K. Iwazaki, et al., Org. Lett. 2 (2000) 1855–1857. |
| [4] |
(a) C.F. Zhao, D. Seidel, J. Am. Chem. Soc. 137 (2015) 4650–4653; (b) P. Li, S. Regati, H.C. Huang, et al., Chin. Chem. Lett. 26 (2015) 6–10; (c) F.S. Zhang, Q. Lai, X.D. Shi, et al., Chin. Chem. Lett. 30 (2019) 392–394. |
| [5] |
(a) C. Wei, Z. Li, C.J. Li, Org. Lett. 5 (2003) 4473–4475; (b) C. Wei, C.J. Li, J. Am. Chem. Soc. 125 (2003) 9584–9585; (c) Y.C. Zhang, P.H. Li, M. Wang, et al., J. Org. Chem. 74 (2009) 4364–4367; (d) W.W. Chen, H.P. Bi, C.J. Li, Synlett 3 (2010) 475–479; (e) P.H. Li, Y.C. Zhang, L. Wang, Chem. Eur. J. 15 (2009) 2045–2049; (f) S.N. Afraj, C.P. Chen, G.H. Lee, RSC Adv. 4 (2014) 26301–26308; (g) M. Gholinejad, F. Hamed, C. Nájera, Synlett 27 (2016) 1193–1201; (h) M. Mirabedini, E. Motamedi, M.Z. Kassaee, Chin. Chem. Lett. 26 (2015) 1085–1090; (i) R.N. Yi, Z.J. Wang, Z.W. Liang, et al., App. Organomet. Chem. 33 (2019) e4917; (j) D.P. Shi, Z.Y. Duan, Chin. J. Org. Chem. 40 (2020) 1316–1322. |
| [6] |
(a) X.P. Liu, X.X. Yin, Q.Q. Liu, et al., ChemistrySelect 2 (2017) 10215–10220; (b) Y.A. Zou, F.L. Zhu, Z.C. Duan, et al., Tetrahedron Lett. 55 (2014), 2033–2036. |
| [7] |
(a) Z. Li, C.J. Li, J. Am. Chem. Soc. 126 (2004) 11810–11811; (b) S.P. Teong, D. Yu, Y.N. Sum, et al., Green Chem. 18 (2016) 3499–3502; (c) C.M. Rao Volla, P. Vogel, Org. Lett. 11 (2009) 1701–1704. |
| [8] |
(a) Y.B. Zhou, Z.W. Li, X. Yang, et al., Synthesis (Mass) 48 (2016) 231–237; (b) C. Tamuly, I. Saikia, M. Hazarik, et al., RSC Adv. 5 (2015) 8604–8608; (c) R.H. Qiu, Y.M. Qiu, S.F. Yin, et al., Adv. Synth. Catal. 352 (2010) 153–162; (d) S. Wu, H.H. Yu, Q.Z. Hua, et al., Tetrahedron Lett. 58 (2017) 4763–4765; (e) C. Niu, A. Tuerxuntayi, G. Li, et al., Chin. Chem. Lett. 28 (2017) 1533–1538. |
| [9] |
(a) Y. Gu, L. Dai, J.G. Zhang, et al., J. Org. Chem. 86 (2021) 2173–2183; (b) X.H. Ouyang, R.J. Song, et al., Angew. Chem. Int. Ed. 55 (2016) 3187–3191; (c) A. Tlahuext-Aca, J.F. Hartwig, ACS Catal. 11 (2021) 1119–1127; (d) X.H. Meng, M. Yang, J.Y. Peng, et al., Adv. Synth. Catal. 363 (2021) 244–250; (e) R.N. Zhao, Z. Zhou, J.X. Liu, et al., Organic Lett. 22 (2020) 8144–8149; (f) H.Y. Peng, Y. Zhang, Y. Zhu, J. Org. Chem. 85 (2020) 13290–13297. |
| [10] |
(a) Z. Cao, Q. Zhu, Y.W. Lin, et al., Chin. Chem. Lett., 30 (2019) 2132–2138; (b) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241–3244; (c) Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22 (2020) 118–122; (d) Q.W. Gui, F. Teng, Z.C. Li, et al., Org. Chem. Front. 7 (2020) 4026–4030; (e) L. Yang, J.P. Wan, Green Chem. 22 (2020) 3074–3078; (f) Q. Yu, Y.T. Zhang, J.P. Wan, Green Chem. 21 (2019) 3436–3441. |
| [11] |
(a) F.S.P. Cardoso, K.A. Abboud, A. Aponick, J. Am. Chem. Soc. 135 (2013) 14548–14551; (b) N. Gommermann, C. Koradin, K. Polborn, et al., Angew. Chem. Int. Ed. 42 (2003) 5763–5766. |
| [12] |
(a) Y.W. Zhao, Q.L. Song, Org. Chem. Front. 3 (2016) 294–297; (b) D.M. Lustosa, S. Clemens, M. Rudolph, Adv. Synth. Catal. 361 (2019) 5050–5056; (c) N. Sakai, N. Uchida, T. Konakahara, Synlett (2008) 1515–1519; (d) R. Shang, L. Liu, Sci. China Chem. 54 (2011) 1670–1687. |
 2021, Vol. 32
2021, Vol. 32