Carbene transfer reactions are powerful synthetic tools in organic synthesis and open up a new window of reactivity patterns ranging from cross-coupling reactions [1-3]; rearrangement reactions [4-11], X-H insertion reactions [12-18], cycloadditions [19-24] to C-H activations [8, 25-29] (Scheme 1a). In the past few decades, although remarkable achievements have been made in carbene transfer reactions, the presence of expensive metal-based catalysts and toxic organic solvent limited their further development [7, 29-31]. In this context, the development of environmentally benign transformations remains highly desirable.

|
Download:
|
| Scheme 1. Carbene transfer reactions. | |
Only recently, the visible light photolysis of diazoalkanes has emerged as an important alternative to traditional metal-catalyzed carbene-transfer reactions (Scheme 1a) [32-37]. Furthermore, identifying sustainable and safe carbene precursors has also become one of the major tasks in the field of carbene transfer reactions. In this regard, hydrazones have become especially important carbene precursors through in situ generated diazo intermediates. Compared with diazo compounds, hydrazones are more stable, less explosive, and they can be easily prepared from aldehydes or ketones [31]. Consequently, we reasoned the possibility that carbene transfer reactions initiated by the visible light photolysis of hydrazones, which has recently been verified by the works of Koenigs et al. and ourselves, respectively (Scheme 1b) [34, 38-42].
On the other hand, cyclopropenes, as strained carbocyclic compounds, are widely used synthons in organic synthesis due to their high reactivity [43, 44]. A particularly practical reaction to access cyclopropylenes is the cyclopropenization reaction of alkynes, for which a variety of expensive, precious metal catalysts, based on AuII, RhII, IrI, and AgI have been reported [45-48]. Also, a metal-free cyclopropenization reaction using tosylhydrazones has been reported, despite the shortcomings of low yields and elevated temperature [34, 40, 49-51]. In the context of the development of environmentally benign methods for the synthesis of cyclopropylenes and our previous work, herein we developed a blue light-induced cyclopropenization reaction of N-tosylhydrazones with alkynes (Scheme 1c). Notably, this is the first report on light-induced metal-free cyclopropenizations of N-tosylhydrazones in water.
Furthermore, other carbene transfer reactions involving the visible light photolysis of N-tosylhydrazones, such as X-H insertions and cyclopropanations were proved to compatible with these water-mediated conditions.
Initially, the blue-light-mediated cyclopropenization of N-tosylhydrazone 1a (0.1 mmol) and phenylacetylene 2a (10 equiv.) was examined in dichloromethane (DCM) at 25 ℃ without the base. Unfortunately, no desired product was detected (Table 1, entry 1). Surprisingly, required methyl 1,2-diphenylcycloprop-2-ene-1-carboxylate (3a) was obtained in yields ranging from 30% to 55% when using KOH, t-BuOK, K2CO3, Et3N, and DBU as bases, and Et3N gave the best result (entries 2–6). After that, we further screened the solvent (entries 6–11), and the results showed that the reaction proceeded smoothly in water delivering the desired product in 66% isolated yield (entry 11). Besides, we investigated the equivalents of phenylacetylene and found that adding 10 equiv. of phenylacetylene significantly increased the yield of the target product (entry 13). Finally, through a detailed investigation of the amount of base and the reaction concentration, we have obtained the optimal reaction conditions.
|
|
Table 1 Optimization of the reaction conditions.a |
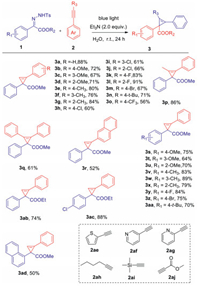
With the optimized conditions in hand (Table 1, entry 13), we next explored the functional group tolerance of both the N-tosylhydrazones 1 and alkynes 2 (Scheme 2). In general, substrates with different substituents were compatible with the optimized reaction conditions, and the corresponding cyclopropylenes were obtained with moderate to excellent yields (3a-3aa). Among them, the electronic effect of substituted functional groups had a strong influence on the yields of target compounds, which mainly manifested that the transformation efficiency of the electron-withdrawing substituents was generally lower than that of electron-donating substituents. Surprisingly, styrenes possessing fluorine at the benzene ring were exceptions and afforded the corresponding cyclopropenes in 83% and 91% yields (3k, 3l), respectively. It is worth mentioning that N-tosylhydrazone and alkyne substituted by naphthalene had poor compatibility with optimal conditions, and their corresponding cyclopropylenes were achieved in yields of 52% and 52% (3r, 3ad). Besides, we explored other substrates such as heterocyclic terminal alkynes, alkynes bearing electron-withdrawing groups, and non-aromatic terminal alkynes. Unfortunately, they were not suitable for this reaction condition, and no expected cyclopropenes were obtained (2ae-2aj).
|
Download:
|
| Scheme 2. Substrate scope of N-tosylhydrazones and alkynes. Reaction conditions: 1 (0.1 mmol), 2 (1.0 mmol) and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W, 470 nm) for 24 h. Isolated yield by chromatography on silica gel. | |
Based on the success of the light-mediated cyclopropenizations of N-tosylhydrazones in water, we further studied the applicability of this optimal condition to other carbene transfer reactions. First, we investigated the insertion reactions between N-tosylhydrazones and X-H bonds (Scheme 3). Primarily, we focused on testing N-tosylhydrazones and a variety of aromatic amines. The results showed that under the optimal conditions, both monosubstituted and disubstituted aromatic amines reacted smoothly with N-tosylhydrazones, and all the expected amino acid ester products were obtained in good to excellent yields (5a-5n). However, naphthalamine reacted with N-tosylhydrazons and afforded the desired product in 66% yield under these conditions (5o). Unfortunately, other aliphatic thiols, alcohols, phenols and amines did not provide the expected products. (4r-4v). Besides, we investigated the reaction of N-tosylhydrazons with monosubstituted aryl thiophenols, and the corresponding products were obtained with moderate yields (5p, 5q).

|
Download:
|
| Scheme 3. Substrate scope of N-tosylhydrazones and X-H bonds. | |
Reaction conditions: 1 (0.1 mmol), 4 (0.4 mmol), and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W, 470 nm) for 24 h. Isolated yield by chromatography on silica gel.
Besides, we tested the cyclopropane reactions between N-tosylhydrazones and aryl olefins in water (Scheme 4). The results show that the reaction of N-tosylhydrazones and aryl olefins can be successfully achieved under optimal conditions. Target products can be obtained more efficiently than we previously reported in DCM (7a-7n). Unfortunately, alkenes bearing following functional groups, such as methyl acrylate, acrolein, acrylonitrile, did not provide the expected products (6o-6q).

|
Download:
|
| Scheme 4. Substrate scope of N-tosylhydrazones and aryl olefins. Reaction conditions: 1 (0.1 mmol), 6 (1.0 mmol), and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W, 470 nm) for 24 h. Isolated yield by chromatography on silica gel. | |
To further study the efficiency and practicality of this transformation, a 10-fold scale-up of the reaction was conducted in 50 mL of H2O. The desired product 3a was isolated with 74% yield (Scheme 5).

|
Download:
|
| Scheme 5. 10-Fold scale-up reaction. | |
According to the existing literatures [30, 39, 52, 53], The mechanism proposed for this reaction comprises the following steps (Scheme 6): 1) decomposition of the hydrazone in the presence of the base to form the diazo compound, 2) and then formation of a carbene intermediate under blue light irradiation, 3) C-C bond formation by carbene migration insertion of the alkyne, alkene or other X-H bonds with loss of nitrogen to give the final product.

|
Download:
|
| Scheme 6. Proposed mechanism. | |
In summary, we reported on metal-free carbene transfer reactions of N-tosylhydrazones in water, which were induced by low energy blue light. Notably, this methodology allowed efficient cyclopropenizations, X-H insertion reactions (X = N, S), and cyclopropanations in the context of environmental harmony. We firmly believe that this water-mediated photolysis of N-tosylhydrazones would contribute to broadening the applications of free carbene in organic synthesis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 81373259; 81573286, 81773577; 81602954).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.031.
| [1] |
Y. Zhou, F. Ye, X. Wang, et al., J. Org. Chem. 80 (2015) 6109-6118. DOI:10.1021/acs.joc.5b00629 |
| [2] |
H. Zhao, K. Yang, H. Zheng, et al., Org. Lett. 17 (2015) 5744-5747. DOI:10.1021/acs.orglett.5b02783 |
| [3] |
S. Chen, J. Wang, Chem. Commun. (Camb) (2008) 4198-4200. DOI:10.1039/b806970k |
| [4] |
S.F. Zhu, Q.L. Zhou, Nat. Sci. Rev. 1 (2014) 580-603. DOI:10.1093/nsr/nwu019 |
| [5] |
Z. Zhang, Z. Sheng, W. Yu, et al., Nat. Chem. 9 (2017) 970-976. DOI:10.1038/nchem.2789 |
| [6] |
Z. Song, Y. Wu, T. Xin, et al., Chem. Commun. (Camb) 52 (2016) 6079-6082. DOI:10.1039/C6CC02641A |
| [7] |
Z. Sheng, Z.K. Zhang, C.H. Chu, et al., Tetrahedron 73 (2017) 4011-4022. |
| [8] |
R. Shang, L. Ilies, E. Nakamura, Chem. Rev. 117 (2017) 9086-9139. DOI:10.1021/acs.chemrev.6b00772 |
| [9] |
M. Liao, L. Peng, J. Wang, Org. Lett. 10 (2008) 693-696. DOI:10.1021/ol703058p |
| [10] |
D.M. Hodgson, F.Y.T.M. Pierard, P.A. Stupple, Chem. Soc. Rev. 30 (2001) 50-61. |
| [11] |
K.J. Hock, R.M. Koenigs, Angew. Chem. Int. Ed. 56 (2017) 13566-13568. DOI:10.1002/anie.201707092 |
| [12] |
S.F. Zhu, Q.L. Zhou, Acc Chem. Res. 45 (2012) 1365-1377. DOI:10.1021/ar300051u |
| [13] |
S.F. Zhu, B. Xu, G.P. Wang, et al., J. Am. Chem. Soc. 134 (2012) 436-442. DOI:10.1021/ja2084493 |
| [14] |
Y. Zhang, Y. Yao, L. He, et al., Adv. Syn. Catal. 359 (2017) 2754-2761. DOI:10.1002/adsc.201700572 |
| [15] |
B. Xu, S.F. Zhu, X.L. Xie, et al., Angew. Chem. Int. Ed. 50 (2011) 11483-11486. DOI:10.1002/anie.201105485 |
| [16] |
F. Tan, X. Liu, X. Hao, et al., ACS Catal 6 (2016) 6930-6934. DOI:10.1021/acscatal.6b02184 |
| [17] |
C. Qin, H.M. Davies, J. Am. Chem. Soc. 136 (2014) 9792-9796. DOI:10.1021/ja504797x |
| [18] |
Z. Hou, J. Wang, P. He, et al., Angew. Chem. Int. Ed. 49 (2010) 4763-4766. DOI:10.1002/anie.201001686 |
| [19] |
X. Xu, M.P. Doyle, Acc Chem. Res. 47 (2014) 1396-1405. DOI:10.1021/ar5000055 |
| [20] |
A. Padwa, Chem. Soc. Rev. 38 (2009) 3072-3081. DOI:10.1039/b816701j |
| [21] |
L.A. Lopez, J. Gonzalez, Org. Biomol. Chem. 17 (2019) 646-654. DOI:10.1039/c8ob02676a |
| [22] |
K. Dong, C. Pei, Q. Zeng, et al., Chem. Commun. (Camb) 55 (2019) 6393-6396. DOI:10.1039/c9cc02257k |
| [23] |
H.M.L. Davies, S.J. Hedley, Chem. Soc. Rev. 36 (2007) 1109-1119. DOI:10.1039/b607983k |
| [24] |
Q.Q. Cheng, Y. Deng, M. Lankelma, et al., Chem. Soc. Rev. 46 (2017) 5425-5443. DOI:10.1039/C7CS00324B |
| [25] |
Y. Yang, X. Wang, Y. Li, et al., Angew. Chem. Int. Ed. 54 (2015) 15400-15404. DOI:10.1002/anie.201508702 |
| [26] |
Y. Wu, Z. Chen, Y. Yang, et al., J. Am. Chem. Soc. 140 (2018) 42-45. DOI:10.1021/jacs.7b10349 |
| [27] |
J. Wang, M. Wang, K. Chen, et al., Org. Lett. 18 (2016) 1178-1181. DOI:10.1021/acs.orglett.6b00310 |
| [28] |
B. Li, B. Zhang, X. Zhang, et al., Chem. Commun. (Camb) 53 (2017) 1297-1300. DOI:10.1039/C6CC08377C |
| [29] |
H.M. Davies, J.R. Manning, Nature 451 (2008) 417-424. DOI:10.1038/nature06485 |
| [30] |
Y. Xia, J. Wang, Chem. Soc. Rev. 46 (2017) 2306-2362. DOI:10.1039/C6CS00737F |
| [31] |
M. Jia, S. Ma, Angew. Chem. Int. Ed. 55 (2016) 9134-9166. DOI:10.1002/anie.201508119 |
| [32] |
J. Yang, J. Wang, H. Huang, et al., Org. Lett. 21 (2019) 2654-2657. DOI:10.1021/acs.orglett.9b00647 |
| [33] |
T. Xiao, M. Mei, Y. He, et al., Chem. Commun. (Camb) 54 (2018) 8865-8868. DOI:10.1039/c8cc04609c |
| [34] |
R. Hommelsheim, Y. Guo, Z. Yang, et al., Angew. Chem. Int. Ed. 58 (2019) 1203-1207. DOI:10.1002/anie.201811991 |
| [35] |
F. He, F. Li, R.M. Koenigs, J. Org. Chem. 85 (2020) 1240-1246. DOI:10.1021/acs.joc.9b02605 |
| [36] |
Y. Guo, T.V. Nguyen, R.M. Koenigs, Org. Lett. 21 (2019) 8814-8818. DOI:10.1021/acs.orglett.9b03453 |
| [37] |
C. Empel, F.W. Patureau, R.M. Koenigs, J. Org. Chem. 84 (2019) 11316-11322. DOI:10.1021/acs.joc.9b01753 |
| [38] |
W. Xiong, C. Qi, H. He, et al., Angew. Chem. Int. Ed. 54 (2015) 3084-3087. DOI:10.1002/anie.201410605 |
| [39] |
M.C. Perez-Aguilar, C. Valdes, Angew. Chem. Int. Ed. 51 (2012) 5953-5957. DOI:10.1002/anie.201200683 |
| [40] |
Z. Liu, Q. Li, P. Liao, et al., Chemistry (Easton) 23 (2017) 4756-4760. DOI:10.1002/chem.201605335 |
| [41] |
J. Barluenga, C. Valdes, Angew. Chem. Int. Ed. 50 (2011) 7486-7500. DOI:10.1002/anie.201007961 |
| [42] |
J. Aziz, J.D. Brion, A. Hamze, et al., Adv. Syn. Catal. 355 (2013) 2417-2429. DOI:10.1002/adsc.201300466 |
| [43] |
Y. Xu, G. Lv, K. Yan, et al., Chem. Asian J. 15 (2020) 1945-1947. DOI:10.1002/asia.202000378 |
| [44] |
S. Jana, F. Li, C. Empel, et al., Chemistry (Easton) 26 (2020) 2586-2591. DOI:10.1002/chem.201904994 |
| [45] |
Z.B. Zhu, Y. Wei, M. Shi, Chem. Soc. Rev. 40 (2011) 5534-5563. DOI:10.1039/c1cs15074j |
| [46] |
I. Marek, S. Simaan, A. Masarwa, Angew. Chem. Int. Ed. 46 (2007) 7364-7376. DOI:10.1002/anie.200604774 |
| [47] |
M.J. Gonzalez, J. Gonzalez, L.A. Lopez, et al., Angew. Chem. Int. Ed. 54 (2015) 12139-12143. DOI:10.1002/anie.201505954 |
| [48] |
A. Archambeau, F. Miege, C. Meyer, et al., Acc Chem. Res. 48 (2015) 1021-1031. DOI:10.1021/acs.accounts.5b00016 |
| [49] |
B. Morandi, E.M. Carreira, Angew. Chem. Int. Ed. 49 (2010) 4294-4296. DOI:10.1002/anie.201000787 |
| [50] |
T. Goto, K. Takeda, N. Shimada, et al., Angew. Chem. Int. Ed. 50 (2011) 6803-6808. DOI:10.1002/anie.201101905 |
| [51] |
J.F. Briones, H.M. Davies, J. Am. Chem. Soc. 134 (2012) 11916-11919. DOI:10.1021/ja304506g |
| [52] |
W.Y. Yu, Y.T. Tsoi, Z. Zhou, et al., Org. Lett. 11 (2009) 469-472. DOI:10.1021/ol8026076 |
| [53] |
J. Barluenga, M. Tomas-Gamasa, F. Aznar, et al., Ang. Chem. Int. Ed. 49 (2010) 4993-4996. DOI:10.1002/anie.201001704 |
 2021, Vol. 32
2021, Vol. 32 


