b State Key Laboratory of Chemical Oncogenomics, Shenzhen Engineering Laboratory of Nano Drug Slow-Release, Peking University Shenzhen Graduate School, Shenzhen 518055, China;
c Key Laboratory of Coordination Chemistry and Functional Materials in Universities of Shandong, Dezhou College, Dezhou 253023, China
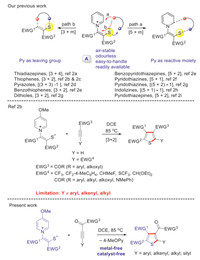
Recently, we embarked on a program to extensively explore the annulation reactions of one kind of novel pyridinium 1, 4-zwitterionic thiolate A (Scheme 1) [1], which could introduce a sulfur atom to organic molecular frameworks or use the sulfur atom to assist the bond-forming process. Thiolate A is air-stable, odourless, easy-to-handle, and readily available. In addition, its annulation reactions are often metal-free and catalyst-free, and only a base or heating is required for some special cases. Generally, it could mainly participate in two classes of reactions [i.e., (5 + m, path a) and (3 + m, path b), Scheme 1], which have been demonstrated in our previous reports [2]. Among them, the [3 + 2] reaction mode could be applied to the synthesis of a variety of tetra- and trisubstituted thiophenes [2b]. For this approach, the Y group is confined to suitable electron-withdrawing groups or hydrogen, as the alkyl or aryl group-substituted internal alkynes (e.g., methyl but-2-ynoate and 4-phenylbut-3-yn-2-one) gave no reaction at all. Thus, how to install other groups (e.g., amodifiedsubstrates would undergoryl, alkenyl, alkyl and silyl) in the corresponding position of Y is the problem always on our mind. Although there are already many synthetic approaches to access polysubstituted thiophenes reported in the literature [3], many of them involve metal catalysts, malodorous starting materials, and/or harsh reaction conditions. Thiophenes, one class of sulfur-containing five-membered heterocycles, widely present in pharmaceuticals, bioactive molecules [4], and functional materials [5], and they are often used as vital organic synthetic intermediates in organic chemistry and materials chemistry [6].
|
Download:
|
| Scheme 1. Reaction modes of pyridinium 1, 4-zwitterionic thiolates and this work. | |
Given our ongoing interest in sulfur chemistry [7], especially pyridinium 1, 4-zwitterionic thiolate A, we hope to use this versatile building block to synthesize more diverse thiophene derivatives and break through the limitation of substrate scope in our previous report [2b]. Inspired by the work of Hou [8], we envisaged that the reactivity of the original failed substrates (e.g., methyl but-2-ynoate and 4-phenylbut-3-yn-2-one) could be improved by introducing an additional carbonyl group between the alkynyl and EWG3 moieties, and the modified substrates would undergo a formal [3 + 2] annulation reaction, affording polysubstituted thiophenes with aryl, alkenyl, alkyl, or silyl moiety at the position of Y group. Herein, we will describe our preliminary explorations on this subject.
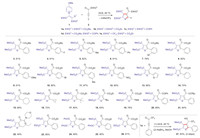
Based on our previous experience of the formal [3 + 2] annulation reaction, pyridinium 1, 4-zwitterionic thiolate 1a and methyl 2-oxo-4-phenylbut-3-ynoate (2a) were chosen as the model substrates to verify the design under the optimal reaction conditions in our previous report [2b]. Delightfully, when 1a and 2a were simply mixed, stirred, and heated at 85 ℃ in 1, 2-dichloroethane without any additives, the desired annulation reaction occurred smoothly, delivering tetrasubstituted thiophene 3 in 81% yield (Scheme 2), which demonstrated that the rational structural modification is effective for the previous failed substrate and an aryl group could be installed at the position of Y. Subsequently, other aryl and heteroaryl (e.g., thienyl) group-substituted alkynes were examined and all of them reacted well, delivering the annulation products in high yields (4–13). The substrates with substituents at the o, m and p-position of phenyl ring were all competent. Using this carbonyl insertion strategy, alkenyl and alkyl chains, even silyl moiety could be fixed on the thiophenes (14–19) and the steric hindrance of alkyl moiety had no obvious influence on the yield. The silyl group could be used to conduct further derivatization via the Hiyama coupling reaction. Except for the substrates with ester function as the EWG3, the ketone or amide-activated alkynes were also investigated. Substrates bearing ketone groups (20 and 21) performed well as those with ester moiety, while amide substrate (22) afforded a low yield of 40%. In contrast, N1, N4-dimethyl-N1, N4-diphenylbut-2-ynediamide and 1-(piperidin-1-yl)prop-2-yn-1-one did not work in our previous report [2b]. Finally, the scope of pyridinium 1, 4-zwitterionic thiolate was investigated (23–26). The annulation reactions of other thiolates (1b–1e) proceeded smoothly but diketone and ketoester substrates only gave low yields. It is worth noting that thiolate 1e could prepare CF3-containing thiophene (e.g., 26), and thiophenes 25 and 26 were decorated with four different substituents. In addition, it should be mentioned that 1, 1, 1-trifluoro-4-phenylbut-3-yn-2-one was also a viable alkyne substrate, and 4-(2, 2, 2-trifluoro-1-hydroxyethyl)thiophene derivative 27 was obtained in 83% yield via a two-step transformation.
|
Download:
|
| Scheme 2. Substrate scope of the formal [3 + 2] annulation reaction. Reaction conditions: 1 (1.5 equiv.), 2 (0.3 mmol), DCE (3 mL), 85 ℃, in air. | |
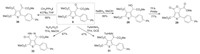
To further demonstrate the utility of the present synthetic protocol, a series of derivatization reactions were conducted (Scheme 3). The inserted carbonyl moiety could be reduced to a hydroxyl group, and then the resulting product 28 could undergo a lactonization reaction to afford 29 under the acidic conditions. The carbonyl moiety could also be readily transformed into the double bond (compound 30) or reactive intermediate hydrazone 31. In addition, the carbonyl and ester moieties could simultaneously be condensated with hydrazine hydrate to afford fused thiophene 32.
|
Download:
|
| Scheme 3. Further transformations of thiophenes. | |
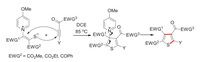
The present protocol follows the same mechanism proposed in the previous report (Scheme 4) [2b]. The annulation process is triggered by an S-Michael addition of pyridinium to activated alkyne followed by a C-Michael addition, and then a retro-Michael addition delivers the desired thiophene accompanied with 4-MeO-Py extrusion.
|
Download:
|
| Scheme 4. Proposed mechanism for [3 + 2] cascade cyclization reaction. | |
In summary, the synthesis of diverse tetrasubstituted thiophene derivatives was achieved via a formal [3 + 2] annulation of pyridinium 1, 4-zwitterionic thiolates and modified activated alkynes. Using the carbonyl insertion strategy could produce thiophenes with aryl, alkenyl, alkyl or silyl group at the position of Y group. The annulation reaction is metal-free and catalyst-free. The other explorations of pyridinium 1, 4-zwitterionic thiolates are in progress in our laboratory and will be reported in due course.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank National Natural Science Foundation of China (Nos. 21971092, 21901014, 21472072, 21871018, 21732001 and 21672017), Shenzhen Science and Technology Innovation Committee (No. JCYJ20200109141808025), and Characteristic Innovation Project of Guangdong Provincial Education Department (No. 2020KTSCX295) for the financial support.
| [1] |
L. Moafi, S. Ahadi, H.R. Khavasi, A. Bazgir, Synthesis (Mass) (2011) 1399-1402. |
| [2] |
(a) B. Cheng, Y. Li, T. Wang, et al., Chem. Commun. 55 (2019) 14606–14608; (b) B. Cheng, X. Duan, Y. Li, et al., Eur. J. Org. Chem. (2020) 1896–1906; (c) S. Zhai, X. Zhang, B. Cheng, et al., Chem. Commun. 56 (2020) 3085–3088; (d) B. Cheng, B. Bao, W. Xu, et al., Org. Biomol. Chem. 18 (2020) 2949–2955; (e) B. Cheng, Y. Li, T. Wang, et al., J. Org. Chem. 85 (2020) 6794–6802; (f) B. Cheng, X. Zhang, Y. Li, et al., Chem. Commun. 56 (2020) 8396–8399; (g) B. Cheng, Y. Li, X. Zhang, et al., Org. Lett. 22 (2020) 5817–5821; (h) B. Cheng, H. Li, S. Duan, et al., Org. Biomol. Chem. 18 (2020) 6253–6257; (i) B. Cheng, X. Zhang, H. Li, et al., Adv. Synth. Catal. 362 (2020) 4668–4672. |
| [3] |
(a) B. Godoi, R.F. Schumacher, G. Zeni, Chem. Rev. 111 (2011) 2937–2980; (b) K. Schaper, T.J.J. Müeller, Top. Curr. Chem. 376 (2018) 38; (c) F. Zamberlan, A. Fantinati, C. Trapella, Eur. J. Org. Chem. (2018) 3248–3264; (d) R. Mancuso, B. Gabriele, Molecules 19 (2014) 15687–15719; (e) J.A. Joule, Phosphorus Sulfur Silicon Relat. Elem. 188 (2013) 287–316; (f) S. Rajappa, A.R. Deshmunkh, Thiophenes and their benzo derivatives: reactivity, in: A.R. Katritzky, C.A. Ramsden, E.F.V. Scriven, R.J.K. Taylor (Eds. ), Comprehensive Heterocyclic Chemistry III, Elsevier Science, 2008, pp. 741–841; (g) S. Gronowitz, A.B. Hornfeldt, in: Thiophenes, Elsevier, Oxford, UK, 2004; (h) K.J. Hale, S. Manaviazar, Thiophenes, hydrothiophenes, benzothiophenes, and related compounds, in: M. Sainsbury (Ed. ), Second Supplements to the 2nd Edition of Rodd's Chemistry of Carbon Compounds, Elsevier, 2008. Vol. IV, Part A, 337-456; (i) L. Chen, H. Min, W. Zeng, et al., Org. Lett. 20 (2018) 7392–7395; (j) P. Fricero, L. Bialy, W. Czechtizky, M. Méndez, J.P.A. Harrity, Org. Lett. 20 (2018) 198–200; (k) M. Adib, S. Rajai-Daryasarei, R. Pashazadeh, et al., Eur. J. Org. Chem. (2018) 3001–3016; (l) J.K. Kim, H.J. Lim, K.C. Jeong, S.J. Park, Beilstein. J. Org. Chem. 14 (2018) 243–252; (m) D. Kurandina, V. Gevorgyan, Org. Lett. 18 (2016) 1804–1807; (n) S.N. Sahu, M.K. Gupta, S. Singh, et al., RSC Adv 5 (2015) 36979–36986; (o) A. Acharya, G. Parameshwarappa, S. Bonagiri, H. Ila, J. Org. Chem. 80 (2015) 414–427; (p) Z. Wang, Z. Qu, F. Xiao, H. Huang, G.J. Deng, Adv. Synth. Catal. 360 (2018) 796–800; (q) L.S. Ge, Z.L. Wang, X.L. An, X. Luo, W.P. Deng, Org. Biomol. Chem. 12 (2014) 8473–8479; (r) M. Adib, S. Rajai-Daryasarei, R. Pashazadeh, M. Jahani, M. Amanlou, Synlett 29 (2018) 1583–1588; (s) G. Bharathiraja, G. Sathishkannan, T. Punniyamurthy, J. Org. Chem. 81 (2016) 2670–2674. |
| [4] |
(a) K. Bozorov, L.F. Nie, J. Zhao, H.A. Aisa, Eur. J. Med. Chem. 140 (2017) 465–493; (b) M. Krátký, J. Vinsova, Curr. Top. Med. Chem. 16 (2016) 2921–2952; (c) D. Gramec, L.P. Mašič, M.S. Dolenc, Chem. Res. Toxicol. 27 (2014) 1344–1358; (d) K.K. Jha, S. Kumar, I. Tomer, R. Mishra, J. Pharm. Res. 5 (2012) 560–566. |
| [5] |
(a) C. Zhang, X. Zhu, Acc. Chem. Res. 50 (2017) 1342–1350; (b) G. Turkoglu, M.E. Cinar, T. Ozturk, Top. Curr. Chem. 375 (2017) 84; (c) S.C. Rasmussen, S.J. Evenson, C.B. McCausland, Chem. Commun. 51 (2015) 4528–4543; (d) A. Mishra, C.Q. Ma, P. Bäuerle, Chem. Rev. 109 (2009) 1141–1276. |
| [6] |
(a) B.H. Lipshutz, Chem. Rev. 86 (1986) 795–819; (b) G. Rassu, F. Zanardi, L. Battistini, G. Casiraghi, Chem. Soc. Rev. 29 (2000) 109–118; (c) C. Bianchini, A. Meli, Synlett 6 (1997) 643–649; (d) R. Kumar, R.K. Rej, S. Nanda, Tetrahedron Asymmetry 26 (2015) 751–759. |
| [7] |
(a) X. Jiang, in: Sulfur Chemistry, Springer, 2019; (b) M. Feng, B. Tang, S.H. Liang, X. Jiang, Curr. Top. Med. Chem. 16 (2016) 1200–1216; (c) N. Wang, P. Saidhareddy, X. Jiang, Nat. Prod. Rep. 37 (2020) 246–275. |
| [8] |
W.P. Ding, G.P. Zhang, Y.J. Jiang, et al., Org. Lett. 21 (2019) 6805-6810. DOI:10.1021/acs.orglett.9b02431 |
 2021, Vol. 32
2021, Vol. 32 

