Drawing from the natural designs, processes and assembly of molecular building blocks to generate high-performance soft materials is a promising approach for the construction of advanced drug delivery systems (ADDSs) with diverse functionalities [1–4]. Mimicking the transport and recognition processes inside organisms provides an excellent model for the design of ADDSs. For instance, the mimic of inherent enzymatic catalysis has generated a series of polymeric hydrogels for drug delivery, and mimics of the "bottom-up" way to build a complex structure has been implemented in a layer-by-layer (LBL) approach for the encapsulation of various compounds (e.g., drugs, genetic material) to notably enhance bioavailability [5–8]. The application of the biomimetic self-assembly not only renders the drug vehicles with bioinspired structure or functionality but also acts as a "self-delivery" packet without involving any external inserts [9,10].
In past three decades, the most efficient ADDSs reported are those based on amino acids, saccharides and lipids [11–17]. The discovery of the appropriate peptide sequence is the key for the generation of biomimetic systems. More recently, the PYRIN domain of apoptosis-associated speck-like protein (ASC) has been shown to bind the active form of vitamin B6 (pyridoxal phosphate, P5P) to fine tune the self-assembly of ASC, thus affecting its biological activity [18,19]. In addition to P5P, several other endogenous agents (e.g., ATP, AMP, and folinic acid) are able to bind to ASC [20]. In the early work on the crystal structure of ASC protein, the KKFKLKL sequence anchoring to the surface of the protein played indispensable roles in the interaction with the negatively charged domain for the oligomerization of ASC [21]. Such oligomerization is predominantly driven by the noncovalent interaction between the protonated ε-amine groups of KKFKLKL and the phosphate group on the endogenous agents [22–26]. This unique relationship provides a novel approach for the development of supramolecular hydrogels based on the interaction of peptide epitopes and endogenous small molecules. Inspired by these results, we rationally designed and synthesized a nonapeptide (NapFFKKFKLKL), which could co-assemble with Dexp to generate a supramolecular hydrogel. As shown in Fig. 1, it clearly observed that a transparent NapFFKKFKLKL/Dexp hydrogel formed instantly after the complexation of NapFFKKFKLKL and Dexp in aqueous solution.

|
Download:
|
| Fig. 1. Schematic formation of NapFFKKFKLKL/Dexp supramolecular hydrogel (2.5 mg/mL NapFFKKFKLKL and 10 mg/mL Dexp). | |
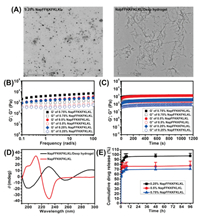
We used TEM to observe the morphology of the NapFFKKFKLKL solution and NapFFKKFKLKL/Dexp hydrogel. As shown in Fig. 2A, the NapFFKKFKLKL aqueous solution had spherical morphology, while the NapFFKKFKLKL/Dexp hydrogel exhibited a typical nanofibril structure with widths ranging from 20 nm to 50 nm and lengths that were several micrometers long. These nanofibers entangled with each other to support the 3D architecture of the hydrogels.
|
Download:
|
| Fig. 2. (A) TEM image of NapFFKKFKLKL solution (2.5 mg/mL) and NapFFKKFKLKL/Dexp hydrogel (2.5 mg/mL NapFFKKFKLKL and 10 mg/mL Dexp). (B) Frequency sweep of supramolecular hydrogel formed by different concentrations of NapFFKKFKLKL. (C) Time dependence of the dynamic storage moduli (G') and the loss moduli (G") of supramolecular hydrogel formed by different concentrations of NapFFKKFKLKL. (D) CD spectra of NapFFKKFKLKL solution (2.5 mg/mL) and NapFFKKFKLKL/Dexp hydrogel (2.5 mg/mL NapFFKKFKLKL and 10 mg/mL Dexp). (E) In vitro release profiles of Dexp from various NapFFKKFKLKL/Dexp hydrogels. | |
We thereafter measured the viscoelastic properties of hydrogels using a rheometer. The frequency-dependent storage modulus (G') and loss modulus (G") of hydrogels was presented in Fig. 2B, both G' and G" of all tested hydrogels were independent of frequency over the entire range tested (0.1–100 rad/s), suggesting the formation of a relatively strong elastic network in the hydrogel. Additionally, the mechanical strength was shown to be enhanced by increasing the NapFFKKFKLKL concentration, corresponding to an increase in the G' value. As shown in Fig. 2C, the domination of the G' over its G" in all the tested hydrogels over the entire study period indicated the formation of a stable hydrogel after the co-assembly of Dexp with NapFFKKFKLKL. Notably, the immediate increase of G' over G" in all tested hydrogels further confirmed that the hydrogelation occurred instantly.
To evaluate the possible molecular interaction of NapFFKKFKLKL and Dexp in the hydrogel, we obtained the emission spectra of the NapFFKKFKLKL aqueous solution and the NapFFKKFKLKL/Dexp hydrogel (Fig. S2 in Supporting information). It clearly observed that the emission spectrum of NapFFKKFKLKL in aqueous solution peaked at 335 nm in the range of 290–500 nm, corresponding to the 1Lb←1A transition [27,28]. For the NapFFKKFKLKL/Dexp hydrogel, the intensity of the emissions at 335 nm decreased significantly, indicating that the co-assembly of NapFFKKFKLKL with Dexp quenches most fluorescence.
We further adopted the CD spectrum to investigate the secondary structure of the NapFFKKFKLKL solution and the NapFFKKFKLKL/Dexp hydrogel (Fig. 2D). NapFFKKFKLKL solution displayed a typical α-helical conformation, which bears negative bands at 230 nm and a positive band at approximately 210 nm. After the co-assembly with Dexp, the NapFFKKFKLKL/Dexp hydrogel turned into a random coil conformation, which exhibits a negative band at approximately 198 nm and a positive band at 220 nm. This result strongly indicated that the introduction of Dexp to NapFFKKFKLKL solution significantly influenced the secondary conformation as well as molecular arrangement of NapFFKKFKLKL.
The in vitro release behavior of Dexp from various NapFFKKFKLKL/Dexp hydrogels was observed over a period of 96 h. As depicted in Fig. 2E, all hydrogel samples exhibited an initial burst release within the first 2 h of the study, and constant release occurred in the following 96 h. The volume of all the hydrogel samples was obviously decreased within the first 2 h of study, and the NapFFKKFKLKL concentration significantly affected the release profiles of Dexp from hydrogel. The initial burst release was lower for the hydrogel formed by 0.75% NapFFKKFKLKL compared with those hydrogels formed by 0.5% and 0.25% NapFFKKFKLKL, indicating that the release rates of Dexp from the hydrogel slowed down with an increase in the peptide concentration. In light of rheological measurements, it was found that the hydrogel formed by the higher NapFFKKFKLKL concentration displayed an enhanced mechanical strength. Owing to the enhanced mechanical strength of hydrogel at higher NapFFKKFKLKL concentrations, the ionic interaction between NapFFKKFKLKL and Dexp may have hindered Dexp diffusion, thus providing the slower drug release. Similarly, Liu and coworkers reported a self-assembling peptide hydrogel with a higher peptide concentration that had a longer half-release time of paclitaxel (PTX) from hydrogel [29]. Based on these results, the release rates of Dexp from supramolecular hydrogels can be finely tuned by changing the peptide concentration.
Before its further in vivo application, we assessed the in vitro cytotoxicity of NapFFKKFKLKL solution against L-929 cells and ARPE-19 cells. As depicted in Fig. S3 (Supporting information), the NapFFKKFKLKL peptide caused minimal cytotoxicity to L-929 cells and ARPE-19 cells with concentrations in a range of 0–200 μg/mL after 24 h incubation. However, the cell viability for ARPE-19 cells reduced significantly as the NapFFKKFKLKL concentration increased up to 400 μg/mL (P < 0.05). This result suggested that NapFFKKFKLKL hardly caused cytotoxicity under threshold concentration of 200 μg/mL towards both L-929 cells and ARPE-19 cells.
To determine the feasibility of the proposed supramolecular hydrogel for intraocular drug delivery, we thereafter assessed the intraocular biocompatibility of the supramolecular hydrogel following single intravitreal injection. All animal experiments complied with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources and were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University (Wenzhou, China). No clinical issues, such as infection, retinal hemorrhage, chemosis and conjunctival swelling, were observed in the eyes treated by PBS, NapFFKKFKLKL or the NapFFKKFKLKL/Dexp hydrogel during the one month of follow-up. The OCT measurements indicated that there was no significant difference in retinal thickness of treated eyes compared to retinal thickness before intravitreal injection (Figs. S4A and B in Supporting information). Furthermore, compared to the retinal thickness of eyes treated by PBS or NapFFKKFKLKL, there was also no significant difference in the retinal thickness of eyes treated with the NapFFKKFKLKL/Dexp hydrogel at each time point. Taking into consideration that the architecture of the retina was well preserved, it is reasonable to believe that there was no photoreceptor damage caused by the intravitreal injection of various formulations. We conducted the electroretinograms (ERGs) measurement to further evaluate the retinal functions of eyes treated by various formulations. ERGs can be used to monitor the loss of eyesight function related to different types of cells, such as cone cells, rod cells and bipolar cells [30]. As shown in Figs. S5A and B (Supporting information), eyes treated with PBS, NapFFKKFKLKL or the NapFFKKFKLKL/Dexp hydrogel did not result in a reduction of b-wave peak amplitudes during the one month of follow-up, indicating that the intravitreal injection of these formulations did not adversely impact eyesight function. Overall, the resulting NapFFKKFKLKL/Dexp hydrogel posed good intraocular biocompatibility, making it a promising carrier for treating posterior disorders (i.e., uveitis).
Using an EAU rat model, we further assessed in vivo therapeutic efficacy of various formulations on day 12 after immunization. In PBS- and NapFFKKFKLKL solution-treated rats, there were numerous inflammatory exudates in the anterior chamber accompanied by the occlusion of the pupil and severe iris congestion (Fig. 3A). Conversely, NapFFKKFKLKL/Dexp hydrogel treatment effectively attenuated the clinical signs of ocular inflammation, which provided more statistically significant clinical scores that those treated by PBS and NapFFKKFKLKL solution (Fig. 3B; P < 0.05).

|
Download:
|
| Fig. 3. (A) Clinical signs of EAU rats after treatment of PBS, NapFFKKFKLKL and NapFFKKFKLKL/Dexp hydrogel on 12th day post immunization; black arrow indicates the protein exudate and black star indicates the occlusion of pupil. (B) Clinical scores of EAU rats after treatment of PBS, NapFFKKFKLKL and NapFFKKFKLKL/Dexp hydrogel on 12th day post immunization. P < 0.05 as compared to PBS and NapFFKKFKLKL-treated group. (C) H&E score of EUA rats treated by PBS, NapFFKKFKLKL and NapFFKKFKLKL/Dexp hydrogel on 12th day post immunization. P < 0.05 as compared to PBS and NapFFKKFKLKL-treated group. (D) Histopathological observation of the retina from EUA rats treated by PBS, NapFFKKFKLKL, Dexp and NapFFKKFKLKL/Dexp hydrogel on 12th day post immunization. Black arrow indicates the retinal folds; black star indicates the inflammatory cell infiltration. | |
In line with the clinical observations, histological observations indicated that the morphology and architecture of the retina in eyes treated by PBS or NapFFKKFKLKL solution folded and damaged severely. Meanwhile, there were existence of numerous infiltration of inflammatory cells in vitreous cavity (Fig. 3D). In contrast, NapFFKKFKLKL/Dexp hydrogel treatment could maximally retain the normal morphology and architecture of retina, even though slight retinal fold were founded. Histological scores were 3 ± 0.5 and 2.8 ± 0.3 for the PBS- and NapFFKKFKLKL solution-treated groups (Fig. 3C), respectively. The histological scores of rats treated with NapFFKKFKLKL/Dexp hydrogel (1.5 ± 0.8) was significantly lower than those treated by PBS and NapFFKKFKLKL solution (P < 0.05). All these results evidenced that the resulting NapFFKKFKLKL/Dexp hydrogel might be a promising drug delivery system to treat various posterior disorders (i.e., uveitis).
In summary, we have fabricated a NapFFKKFKLKL/Dexp hydrogel based on the peptide epitope (KKFKLKL) of apoptosis-associated speck-like protein (ASC) and investigated its applicable in the field of ocular drug delivery. A single intravitreal injection of the NapFFKKFKLKL/Dexp hydrogel caused little damage to the retinal architecture and eyesight functions during the one month of follow-up, as indicated by OCT and ERGs measurements. Furthermore, the NapFFKKFKLKL/Dexp hydrogel had potent capacity to alleviate the intraocular inflammation and retain the morphology of retinal architecture, suggesting that the resulting NapFFKKFKLKL/Dexp hydrogel may be a promising drug carrier system to treat various posterior disorders (i.e., uveitis).
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis research was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LR18H300002) and the National Natural Science Foundation of China (Nos. 31671022, 81971732).
Appendix A. Supplementary dataSupplementarymaterial related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.037.
| [1] |
C. Alvarez-Lorenzo, A. Concheiro, Curr. Opin. Biotech. 24 (2013) 1167-1173. DOI:10.1016/j.copbio.2013.02.013 |
| [2] |
K. Ariga, K. Kawakami, M. Ebara, et al., New J. Chem. 38 (2014) 5149-5163. DOI:10.1039/C4NJ00864B |
| [3] |
A. Ribeiro, F. Veiga, D. Santos, et al., Biomacromolecules 12 (2011) 701-709. DOI:10.1021/bm101562v |
| [4] |
D. Fu, D. Liu, L. Zhang, L. Sun, Chin. Chem. Lett. 31 (2020) 3195-3199. DOI:10.1016/j.cclet.2020.07.011 |
| [5] |
Z. Feng, H. Wang, B. Xu, J. Am. Chem. Soc. 140 (2018) 16433-16437. DOI:10.1021/jacs.8b10542 |
| [6] |
Z. Feng, H. Wang, S. Wang, et al., J. Am. Chem. Soc. 140 (2018) 9566-9573. DOI:10.1021/jacs.8b04641 |
| [7] |
J.L. Brennan, A.G. Kanaras, P. Nativo, et al., Langmuir 26 (2010) 13590-13599. DOI:10.1021/la1018604 |
| [8] |
J. Hu, G. Zhang, S. Liu, Chem. Soc. Rev. 41 (2012) 5933-5949. DOI:10.1039/c2cs35103j |
| [9] |
S. Wen, K. Zhang, Y. Li, et al., Chin. Chem. Lett. 31 (2020) 3153-3157. DOI:10.1016/j.cclet.2020.03.077 |
| [10] |
L. Zhang, Y.M. Zhang, G. Liu, Y. Liu, Chin. Chem. Lett. 30 (2019) 120-122. DOI:10.1016/j.cclet.2018.04.028 |
| [11] |
K. Liu, R. Xing, Q. Zou, et al., Angew. Chem. Int. Ed. 55 (2016) 3036-3039. DOI:10.1002/anie.201509810 |
| [12] |
T. Ji, Y. Ding, Y. Zhao, et al., Adv. Mater. 27 (2015) 1865-1873. DOI:10.1002/adma.201404715 |
| [13] |
A. Altunbas, S.J. Lee, S.A. Rajasekaran, et al., Biomaterials 32 (2011) 5906-5914. DOI:10.1016/j.biomaterials.2011.04.069 |
| [14] |
A. Rösler, G.W.M. Vandermeulen, H.A. Klok, Adv. Drug. Deliver. Rev. 64 (2012) 270-279. DOI:10.1016/j.addr.2012.09.026 |
| [15] |
L. Zhang, J.M. Chan, F.X. Gu, et al., ACS Nano 2 (2008) 1696-1702. DOI:10.1021/nn800275r |
| [16] |
Y. Zhou, A. Fang, F. Wang, et al., Chin. Chem. Lett. 31 (2020) 494-500. DOI:10.1016/j.cclet.2019.04.048 |
| [17] |
W. Yu, M. Shevtsov, X. Chen, H. Gao, Chin. Chem. Lett. 31 (2020) 1366-1374. DOI:10.1016/j.cclet.2020.02.036 |
| [18] |
A. Lu, H. Wu, FEBS J. 282 (2015) 435-444. DOI:10.1111/febs.13133 |
| [19] |
A. Lu, V.G. Magupalli, J. Ruan, et al., Cell 156 (2014) 1193-1206. DOI:10.1016/j.cell.2014.02.008 |
| [20] |
H. Wang, Z. Feng, Y. Qin, et al., Angew. Chem. Int. Ed. 57 (2018) 4931-4935. DOI:10.1002/anie.201712834 |
| [21] |
J.Y. Bae, H.H. Park, J. Biol. Chem. 286 (2011) 39528-39536. DOI:10.1074/jbc.M111.278812 |
| [22] |
E. de Alba, J. Biol. Chem. 284 (2009) 32932-32941. DOI:10.1074/jbc.M109.024273 |
| [23] |
N.B. Bryan, A. Dorfleutner, S.J. Kramer, et al., J. Inflamm. 7 (2010) 23. DOI:10.1186/1476-9255-7-23 |
| [24] |
D.H. Abdelaziz, M.A. Gavrilin, A. Akhter, et al., J. Biol. Chem. 286 (2011) 3203-3208. DOI:10.1074/jbc.M110.197681 |
| [25] |
J.K. Dowling, C.E. Becker, N.M. Bourke, et al., J. Biol. Chem. 289 (2014) 6429-6437. DOI:10.1074/jbc.M113.539692 |
| [26] |
J.W. Yu, J. Wu, Z. Zhang, et al., Cell Death Differ. 13 (2006) 236-249. DOI:10.1038/sj.cdd.4401734 |
| [27] |
A.K. Dutta, K. Ray, T.K. Mandal, et al., Opt. Mater. 4 (1995) 609-616. DOI:10.1016/0925-3467(95)00016-X |
| [28] |
H. Wang, Z. Feng, A. Lu, et al., Angew. Chem. Int. Ed. 129 (2017) 7687-7698. DOI:10.1002/ange.201702783 |
| [29] |
J. Liu, L. Zhang, Z. Yang, X. Zhao, Int. J. Nanomed. 6 (2011) 2143-2153. DOI:10.2147/IJN.S24038 |
| [30] |
J. Luo, P. Baranov, S. Patel, et al., J. Biol. Chem. 289 (2014) 6362-6371. DOI:10.1074/jbc.M113.513713 |
 2021, Vol. 32
2021, Vol. 32 

