Chemiluminescence (CL) has been widely used for bioanalysis owing to its high sensitivity, low background, simplicity and rapidity. The common CL systems include luminol [1-3], peroxyoxalate [4, 5], lucigenin [6, 7], KMnO4 [8], etc. The CL of luminol and lucigenin is usually strong under alkaline conditions [9] but much weaker under physiological pH. KMnO4 system needs strong acid conditions to oxidize the CL regents and produce CL, and the CL reaction between CPPO and H2O2 was usually conducted in organic solvents [10, 11]. The demand of alkaline/acidic conditions or organic solvent always leads to the misfunction of biomolecules. For example, the harsh CL reaction conditions always resulted in the inactivation of enzymes that were widely used for amplified biosensing [12-14]. Hence, the development of CL system that can work under physiological conditions is still highly desired.
The CL systems of peroxyoxalate, because of their adjustable high quantum yields in the entire visible spectrum, have received extensive attention in recent years. In these systems, the energy from an excited intermediate can be transferred to a nearby fluorophore [4]. Such CL resonance energy transfer (CRET) process exited the fluorophore and produced CRET luminescence [15]. Bis(2-carbopentyloxy-3, 5, 6-trichlorophenyl) oxalate (CPPO), as a common peroxyoxalate CL system, has been usually combined with fluorochromes for biomarker sensing or even cancer diagnosis [10, 11]. However, the energy acceptor of CPPO-based CRET mainly focuses on small organic molecular dyes, such as rhodamine, coumarin, rubrene [4, 16, 17], fluorescein [18]. Notably, their application in biological research is limited due to the potential biotoxicity, poor biocompatibility of these dyes and weak luminescence in aqueous systems [4, 5].
Carbon dots (CDs) is an emerging type of fluorescent materials with many advantages, including high quantum efficiency, excellent water-solubility, environmental friendliness, low cost and preferable biocompatibility, etc. [19-21]. CDs have been widely used in medical imaging [22, 23], catalysis [24, 25], photodynamic therapy [26] and many other fields [27, 28]. The reported CDs-based CL systems mainly based on the reaction of CDs with various oxidants such as H2O2 [29], KMnO4 [30, 31], K3Fe(CN)6 [32]. Also the highly luminous CDs has been used as the acceptor of CPPO-based CRET in organic solvent [33], but not under physiological conditions.
In this work, we report a highly luminous CL system under physiological conditions based on CDs-CPPO micelles. The hydrophobic CDs and CPPO were simultaneously wrapped in micelles, which not only isolated the CPPO from water to prevent its hydrolysis but also shortened the distance between the donor and acceptor to improve the energy transfer efficiency of CRET. The CL quantum yields (QYs) of CDs was as high as 5.26 × 10−4 einsteins/mol under physiological conditions. Meanwhile, the constructed CL system is sensitive for the detection of oxidase substrates like glucose. Hence, the CDs-CPPO micelle CL system is promising for biological analysis and especially suitable for in-vivo biosensing.
Amphiphilic surfactant was used for forming the micelles that packed CPPO and hydrophobic CDs together (see CDs optical properties in Fig. S1 in Supporting information), as shown in Fig. 1A. The micelles isolate the CPPO from water to prevent its hydrolysis. In this CDs-CPPO CL process, CPPO first reacts spontaneously with H2O2 to generate high-energy intermediate, i.e., 1, 2-dioxetanedione by intermolecular electron transfer [34, 35], and then the high-energy intermediate transfer its energy to the CDs. As illustrated in Figs. 1B and C, the transmission electron microscopy (TEM) graph demonstrated that the CDs-CPPO micelles were much larger than CDs, confirming the successful formation of CDs-CPPO micelles. Such micelles made the close proximity between CDs and the CL intermediates from the oxidation of CPPO, thus enhancing the energy transfer efficiency as well as CDs QYs. Hence, the CL intensity of CDs-CPPO micelles was quite strong, and could last as long as 1000s (Fig. 1D). The CL emission of CDs-CPPO micelles can be even obviously observed by naked eyes (the inset picture in Fig. 1D).

|
Download:
|
| Fig. 1. (A) Construction of CDs-CPPO micelle CL system. (B) TEM graph of the CDs. (C) TEM graph of CDs-CPPO micelles. (D) The CL kinetics curve of CDs-CPPO micelle system (inset: the CL images of CPPO and CPPO-CDs). Experimental conditions: H2O2, 5 mmol/L; CPPO, 1 mmol/L; CDs, 2 mg/mL and imidazole, 2 mmol/L. | |
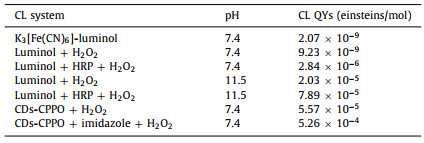
Most of the CPPO-based CL systems worked in organic systems. In order to explore the practicability of the CDs-CPPO CL system under physiological conditions, we tested the effect of different pH on the CL behavior. As shown in Fig. 2A, the CL intensity of the system was enhanced as the pH increased from 5.0 to 7.0, and then gradually decreased with the higher pH. The result demonstrated that the system could generate strong CL emission under physiological pH solution. The effects of CPPO, H2O2, CDs and imidazole concentrations on the CL intensity were also investigated (Fig. S2 in Supporting information). Under the optimal conditions, we tested the CL efficiency of CDs-CPPO system at physiological pH without organic solvent. The CL intensity of the CDs-CPPO micelles system is significantly higher comparing with those of K3[Fe(CN)6]-luminol, luminol-H2O2 and luminol-HRP-H2O2 systems (Fig. 2B). The CL QYs of these aqueous CL systems were calculated according to the equations in Fig. S3 (Supporting information) and the CL spectra in Fig. S4 (Supporting information), and the QYs were shown in Table 1. The CL QYs of CDs-CPPO micelles were as high as 5.57 × 10−5 einsteins/mol; when adding imidazole to the system, the CL intensity was significantly intensified and the CL QYs reached as high as 5.26 × 10−4 einsteins/mol, about 200-fold higher than that of the most commonly used luminol-HRP-H2O2 system under the same pH condition.
|
Download:
|
| Fig. 2. (A) The effect of pH on CL and (B) CL phenomena of different systems. Experimental conditions: H2O2, 5 mmol/L; CPPO, 1 mmol/L; CDs, 2 mg/mL and imidazole, 2 mmol/L. | |
|
|
Table 1 Comparison of CL quantum yields of CDs-CPPO micelle with other systems. |
As mentioned above, the CDs-CPPO CL system can work under physiological conditions, i.e., physiological solution pH and free of organic solvent. To illustrate its superiorities, the effect of pH and organic solvent on the enzyme activity were tested. Fig. 3A demonstrated the activity of the three enzymes, i.e., glucose oxidase (GOD), polyphenol oxidase (PPO) and cholesterol oxidase (COD) in different pH solutions. With pH value ranging from 1.0 to 10.0, the enzyme activity presents a typical bell-shaped curve. For the GOD, the enzyme activity was the best with the pH at 4.0–7.0, and the optimal pH for polyphenol and cholesterol oxidases are both 7.0. Fig. 3B showed the effects of different organic solvents on enzyme activity. It can be seen that enzyme activity is very weak in organic solvents (N, N-dimethylformamide (DMF), ethanol (EA), acetone (CP), glacial acetic acid (HAc), dimethylsulfoxide (DMSO), acetonitrile (ACN)), whereas the enzymes exhibited strong activity in aqueous solution at physiological pH. The effect of the proportion of organic solvent was also investigated. Fig. 3C showed that the enzyme activity decreases gradually as the ACN volume fraction (v/v, %) increases. Besides, 80% of ACN resulted in the loss of 75% enzymatic activity even within 5 min (Fig. 3D). The enzymatic activity can further decreased as the time increased, and eventually completely inactivated within about 60 min. These results demonstrated CDs-CPPO CL with high CL efficiency at physiological pH has great superiority for detection oxidase substrates.

|
Download:
|
| Fig. 3. (A) Enzyme activity of different pH solution. (B) The influence of different reagents on the enzyme activity. (C) The effect of different proportions of acetonitrile on the activity of different enzymes. (D) The effect of the mixing time of glucose oxidase in the solvent on the enzyme activity under 80% acetonitrile solution. Experimental conditions: H2O2, 5 mmol/L; CPPO, 1 mmol/L; CDs, 2 mg/mL and imidazole, 2 mmol/L. | |
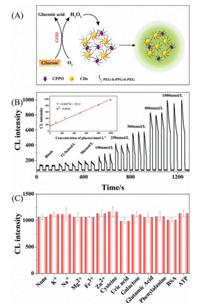
Under the optimal conditions, the CL system of CDs-CPPO micelle for quantitative detection of glucose was researched (Fig. 4A). Fig. 4B revealed the variance of the CL intensity with different concentration of glucose. The CL intensity of detecting glucose increased linearly in the range of 12.5~1000 nmol/L with a correlation coefficient of 0.9922 (Y = 0.8457X + 153.5, R2 = 0.9922). The limit of detection (LOD) for glucose was calculated to be 8.4 nmol/L (3σ), which is much lower than the reported optical methods for glucose assay (Table S1 in Supporting information). The superior sensitivity for glucose assay was mainly ascribed to the good enzymatic activity under physiological conditions as well as the high QYs of the CDs-CPPO micelle CL system.
|
Download:
|
| Fig. 4. (A) CDs-CPPO micelle CL system for detection glucose. (B) The CL response and linearity (insert picture) for detection of glucose by CDs-CPPO system. (C) Detection of glucose in the presence of potential interferents. Experimental conditions: H2O2, 5 mmol/L; CPPO, 1 mmol/L; CDs, 2 mg/mL and imidazole, 2 mmol/L. | |
In order to evaluate the anti-interference ability of the CL method, the effect of potential interferents from serum samples, including common inorganic ions and organic compounds (K+, Na+, Mg2+, Fe3+, Zn2+, cysteine, uric acid, galactose, glutamic acid, phenylalanine, bovine serum albumin (BSA) and adenosine 5′-triphosphate (ATP)) on the CL intensity of CDs-CPPO system was studied. Fig. 4C showed that the CL intensity for 500 nmol/L glucose was affected weakly by the investigated potential interferents (10 µmol/L, 20-fold higher than glucose). Hence, the CL system presents good anti-interference ability.
The applicability for real sample analysis was verified by determination of the glucose concentration in serum samples. The measured value of glucose in serum sample was 3.25 ± 0.051 mmol/L which was in good agreement with the referenced value (3.3 mmol/L). To further evaluate the accuracy, spike-and-recovery method was implemented. As shown in Table S2 (Supporting information), the recovery values for spiking 1.5 mmol/L, 3.2 mmol/L and 6 mmol/L of glucose were in the range of 99%~103% with a relative standard deviation (RSD) < 2.5%. These results indicated that the proposed method shows good reliability and reproducibility for real sample analysis.
In summary, this work proposed a highly luminous CDs-CPPO micelle CL system under physiological conditions. The micelles not only isolated the CPPO from water to prevent its hydrolysis, but also made the close proximity between CPPO and CDs, thus significantly enhancing the CDs quantum yield. The CL QYs of CDs-CPPO could be reach as high as 5.26 × 10−4 einsteins/mol. Hence, this CL system can be used for sensitive detection of glucose with an LOD as low as 8.4 nmol/L. Also, the sensing system exhibited high anti-interference ability for detection of glucose. This highly luminous CDs-CPPO CL system that can work under physiological conditions is promising for assay of biological samples and especially suitable for in-vivo imaging.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21475013) and the Sichuan Science and Technology Project (No. 2018JY0466).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.050.
| [1] |
Y. Huang, N. Yue, Y. Li, et al., Luminescence 36 (2021) 85-93. DOI:10.1002/bio.3916 |
| [2] |
H. Li, J. Wang, J. Du, Talanta 223 (2021) 121712. DOI:10.1016/j.talanta.2020.121712 |
| [3] |
H. Tan, Y. Zhao, X. Xu, et al., Microchim. Acta 187 (2019) 42. DOI:10.5430/ijh.v5n1p42 |
| [4] |
M.E. Quimbar, S.Q. Davis, S.T. Al-Farra, et al., Anal. Chem. 92 (2020) 14594-14600. DOI:10.1021/acs.analchem.0c02929 |
| [5] |
S.P. Souza, M. Khalid, F.A. Augusto, et al., J. Photoch. Photobio. A 321 (2016) 143-150. DOI:10.1016/j.jphotochem.2016.02.001 |
| [6] |
L.A. Montano, J.J.D. Ingle, Anal. Chem. 51 (1979) 919-926. DOI:10.1021/ac50043a032 |
| [7] |
C. Wang, Y. Lan, F. Yuan, et al., Microchim. Acta 187 (2019) 50. |
| [8] |
D. Yang, Y. He, F. Chen, Luminescence 32 (2017) 1077-1083. DOI:10.1002/bio.3294 |
| [9] |
L. Yue, Y.T. Liu, J. Phys. Chem. B 124 (2020) 7682-7693. DOI:10.1021/acs.jpcb.0c06301 |
| [10] |
J.Y. Koo, G.B. Schuster, J. Am. Chem. Soc. 100 (1978) 4496-4503. DOI:10.1021/ja00482a030 |
| [11] |
F.A. Augusto, G.A. de Souza, S.P. de Souza Junior, et al., Photochem. Photobiol. 89 (2013) 1299-1317. DOI:10.1111/php.12102 |
| [12] |
Q. Wang, H. Wei, Z. Zhang, et al., TrAC Trend. Anal. Chem. 105 (2018) 218-224. DOI:10.1016/j.trac.2018.05.012 |
| [13] |
Y. Wu, Q. Chen, S. Liu, et al., Chin. Chem. Lett. 30 (2019) 2186-2190. DOI:10.1016/j.cclet.2019.08.014 |
| [14] |
P. Zhu, Y. Zhang, S. Xu, et al., Chin. Chem. Lett. 30 (2019) 58-62. DOI:10.1016/j.cclet.2018.02.003 |
| [15] |
Y. Yan, X.Y. Wang, X. Hai, et al., TrAC Trend. Anal. Chem. 123 (2020) 115755. DOI:10.1016/j.trac.2019.115755 |
| [16] |
S. Cho, O. Hwang, I. Lee, et al., Adv. Funct. Mater. 22 (2012) 4038-4043. DOI:10.1002/adfm.201200773 |
| [17] |
Z. Li, B. Zhu, X. Duan, et al., Anal. Methods 11 (2019) 2763-2768. DOI:10.1039/c9ay00625g |
| [18] |
C.K. Lim, Y.D. Lee, J. Na, et al., Adv. Funct. Mater. 20 (2010) 2644-2648. DOI:10.1002/adfm.201000780 |
| [19] |
J. Feng, W.J. Wang, X. Hai, et al., J. Mater. Chem. B 4 (2016) 387-393. DOI:10.1039/C5TB01999K |
| [20] |
N. Wang, A.Q. Zheng, X. Liu, et al., ACS Appl. Mater. Interfaces 10 (2018) 7901-7909. DOI:10.1021/acsami.8b00947 |
| [21] |
P. Zuo, X. Lu, Z. Sun, et al., Microchim. Acta 183 (2015) 519-542. |
| [22] |
J. Feng, S. Chen, Y.L. Yu, et al., Talanta 217 (2020) 121014. DOI:10.1016/j.talanta.2020.121014 |
| [23] |
J. Zhong, X. Chen, M. Zhang, et al., Chin. Chem. Lett. 31 (2020) 769-773. DOI:10.1016/j.cclet.2020.01.007 |
| [24] |
Y. Li, Y. Yang, Y. Jiang, et al., Microchem. J. 157 (2020) 105113. DOI:10.1016/j.microc.2020.105113 |
| [25] |
Y. Li, S. Han, Microchem. J. 154 (2020) 104638-104644. DOI:10.1016/j.microc.2020.104638 |
| [26] |
L. Yue, H. Li, Q. Sun, et al., ACS Appl. Nano Mater. 3 (2020) 869-876. DOI:10.1021/acsanm.9b02394 |
| [27] |
N. Dhenadhayalan, K.C. Lin, T.A. Saleh, Small 16 (2020) 1905767. DOI:10.1002/smll.201905767 |
| [28] |
F. Cui, Y. Ye, J. Ping, et al., Biosens. Bioelectron. 156 (2020) 112085. DOI:10.1016/j.bios.2020.112085 |
| [29] |
S.N.A. Shah, X. Dou, M. Khan, et al., Talanta 196 (2019) 370-375. DOI:10.1016/j.talanta.2018.12.091 |
| [30] |
Z. Lin, W. Xue, H. Chen, et al., Chem. Commun. (Camb) 48 (2012) 1051-1053. DOI:10.1039/C1CC15290D |
| [31] |
S. Han, B. Liu, Z. Fan, et al., Luminescence 32 (2017) 1192-1196. DOI:10.1002/bio.3310 |
| [32] |
M. Amjadi, J.L. Manzoori, T. Hallaj, et al., Spectrochim. Acta. A 122 (2014) 715-720. DOI:10.1016/j.saa.2013.11.097 |
| [33] |
C.L. Shen, G.S. Zheng, M.Y. Wu, et al., Nanophotonics 9 (2020) 3597-3604. DOI:10.1515/nanoph-2020-0233 |
| [34] |
M. Chen, H. Zhou, X. Liu, et al., Small 16 (2020) 2002343. DOI:10.1002/smll.202002343 |
| [35] |
A. Singh, Y.H. Seo, C.K. Lim, et al., ACS Nano 9 (2015) 9906-9911. DOI:10.1021/acsnano.5b03377 |
 2021, Vol. 32
2021, Vol. 32