Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) are important small molecules, playing critical role in living organism. As the most abundant one, GSH participates in many cellular functions, including maintenance of intracellular redox balance, xenobiotic metabolism, gene regulation and intracellular signal transduction. Meanwhile, GSH can be oxidized into its oxidized state, glutathione disulfide (GSSG). The ratio of GSSH to GSH is a prominent indicator of some certain diseases, including but not limited to cancer, cardiopathy as well as many neurological disorders [1-4].
Glutathione levels in mitochondria are important medical signals. Mitochondrion is a pivotal organelle for cellular aerobic respiration, generating most of the energy for cell activity [5]. Meanwhile mitochondria are the primary site of oxygen consumption and the major source of reactive oxygen species (ROS), involving in the event of ROS-induced apoptosis [6-9]. In order to protect cells from the oxidative stress, mitochondria GSH as a crucial antioxidant can scavenge free-radical. Considering the key role of GSH in maintaining and indicating the cellular redox homeostasis, the detection of mitochondrial GSH is of practical significance [10-12].
Compared with the biological macromolecule, the small molecular fluorescent probe has the advantages of high sensitivity and little interference to cells, so it could be used as the fluorescent sensor for detecting small molecules, such as metal ions, reactive oxygen species, and mercaptans. At the same time, the fluorescence signal can also reflect the degree of oxidative stress of cells [13-16]. Some small molecular fluorescent probes have been reported for the detection of glutathione [17-20], however, there are few reports on glutathione fluorescent probes localized to mitochondria. Nitroazo [21] and 2, 4-dinitrobenzenesulfonyl (DNBS) [22] were usually introduced to the fluorophore as the GSH-selective reaction unit as well as the fluorescence quencher unit. Nevertheless, due to the approximate nucleophilic capacity of three different biothiols, these fluorescent probes exhibit poor selectivity towards glutathione, cysteine and homocysteine, and cannot eliminate the interference of Cys, and Hcy on GSH. Kim's group reported a heptamethine-based near infrared fluorescent probe for monitoring mitochondrial GSH with a high selectivity toward GSH over Cys/Hcy, and a low background autofluorescence [21]. Yin's and Yoon's groups developed a dual-channel fluorescent probe for detecting mitochondrial GSH with high selectivity [23]. The probe can monitor the changing of glutathione in mitochondria with high temporal and spatial resolution. It provides a new probe design strategy for real-time monitoring the level of GSH in mitochondria.
The detection mechanism of the reported glutathione probes mainly takes advantage of the nucleophilic sulfhydryl group on the GSH cysteine residue as the reaction group to react with the electrophilic detection site of the probe, giving changes in the fluorescence signal of the probe [24]. According to the groups that identify the nucleophilic sulfhydryl group, glutathione probes can be classified into such sorts as oxygen/sulfur ether type [21], sulfonate/sulfonamide type [25-27], disulfide bond type [28, 29], selenium-nitrogen type [30], halogen type [31, 32] and unsaturated bond type [33]. Based on the fact that cysteine and homocysteine also have the highly reactive nucleophilic sulfhydryl group, it is a very big challenge to design and develop a selective glutathione probe that can distinguish other biothiols such as cysteine and homocysteine [34]. Since the glutathione level in live cells is much higher than that of cysteine and homocysteine, some reported works ignore the influence of cysteine and homocysteine on glutathione [24]. Another fact is that GSH is a tripeptide, which has more reaction sites than cysteine and homocysteine, therefore, some other glutathione probes are designed by using multiple binding strategy to achieve the selectivity [2].
Native chemical ligation (NCL) technology is a polypeptide ligation technology, involving cascade reactions using a C-terminal thioester peptide and another peptide with N-terminal Cys-residue [35-37]. Herein, based on the mechanism of native chemical ligation (NCL) and fluorescence resonance energy transfer (FRET), we designed and synthesized a fluorescent probe QZ, a rhodamine B derivative, to detect mitochondrial GSH in living cells.
As for probe QZ, rhodamine B was selected as fluorophore and azo-based naphthalimide as the quencher [38]. The rationale was depicted in Scheme 1. The azo-based naphthalimide group of probe QZ would be removed through thioester exchange by sulfhydryl-containing amino acids, then the FRET effect disappeared. According to the action mechanism of NCL, the anti-thioesterification of aromatic thioesters will take place at a fast rate [39]. When the probe binds to Cys-and Hcy, their amino group tend to react with the Rhodamine fluorophore through nucleophilic addition, after the S, N-amide exchanging, forming a non-fluorescent five-membered or six-membered cyclic spironolactone derivative. As for GSH, the S, N-amide exchanging step could not take place, resulting in the rhodamine fluorophore will remain in a fluorescent open-loop state owning to the lack of amino group in the structure.
|
Download:
|
| Scheme 1. Plausible mechanism for the specific detection of GSH. | |
Probe QZ was synthesized according to the route shown in Scheme S1 (Supporting information). Since the reaction between protein amino-terminal Cys-residue and thioester was a spontaneous process, we selected aromatic thioester group as the recognition site to design and synthesis probe QZ. Compared with the aliphatic thioester, aromatic thioester group is a much electrophilic group, thus has a higher reactivity [39, 40]. QZ exhibited an obvious absorption peak at 568 nm and a characteristic fluorescence emission at 576 nm (Fig. 1A; Fig. S6 in Supporting information). The fluorescence quantum yield for QZ was calculated to be 0.041 in PBS with Rhodamine B as the standard reference in this work [41]. Upon addition of GSH, a red shift in the emission emerged from 576 nm to 587 nm with a three-fold increase in fluorescence intensity. When Cys-or Hcy was added, no significant change was observed, which is a preliminary testimony of our hypothesis. The detection mechanism was further investigated by mass spectrometry. As shown in Fig. S5 (Supporting information), the 732.2841 was found in the mass spectra, which means the signal of the hypothetical transthioesterification product was detected.

|
Download:
|
| Fig. 1. (A) Fluorescent spectra changes of probe QZ (1 µmol/L) in the presence of 100 equiv. of Cys, Hcy, GSH. (B) Time-dependent fluorescence spectral changes of QZ (1 µmol/L) with 100 equiv. of GSH. (C) Time curve of fluorescence intensity changes of QZ (1 µmol/L) in the presence of GSH (100 µmol/L). (D) Concentration-dependent fluorescence spectral changes QZ (1 µmol/L) upon addition of GSH (0–10 µmol/L). The linear relationship between the fluorescence intensity at 587 nm of QZ and the concentration of GSH, the liner regression equation is y = 3.16302 + 15.79428 × [GSH], R2 = 0.99906. The assay was conducted in PBS buffer (25 mmol/L, pH 7.4, 1% DMSO as co-solvent) at 37 ℃. λex = 540 nm, λem = 587 nm, slit: 5/5 nm. | |
The reaction kinetics between GSH and the probe was also examined. When QZ was treated with 100 equiv. of GSH in PBS buffer at 37 ℃, the fluorescence intensity of QZ increased gradually with time. After 10 min, the fluorescence enhancement became slow and finally reached the saturation after 30 min (Figs. 1B and C). Since the transthio-esterification reaction between glutathione and thioester in the probe is relatively fast, QZ can be used as a sensitive glutathione probe.
Then we investigated the fluorescence changes of QZ to different concentrations of GSH (0–10 µmol/L). Upon addition of GSH, the enhancement of fluorescent intensity was observed proportional to the GSH concentration with a limit of detection of 2.98 µmol/L (Fig. 1D). Considering that glutathione levels in mammalian cells are approximately in the millimolar concentration range, QZ have the potential to be developed as a sensitive glutathione probe.
In order to achieve a selective detection of GSH over Cys and Hcy, the influence of other substrate should be precluded, especially for those small molecular amino acids having similar structures. Thus a further selectivity and competitiveness experiments of QZ towards GSH was carried out over other 20 natural amino acids. Upon addition of GSH (100 µmol/L), the fluorescence intensity had a four-fold enhancement at 588 nm whereas other amino acid caused almost no changes under the same conditions (Fig. S9a in Supporting information). When GSH (10 mmol/L) was added to the solution of QZ in the presence of other analytes except Cys-and Hcy, the emission intensity also increased as demonstrated in Fig. S9b. Briefly, QZ is a sensitive GSH probe with high selectivity and has the ability to distinguish GSH in complex biological systems, even among other analogous biothiols.
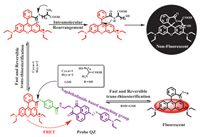
In cell imaging experiments, the ability of subcellular localization of QZ was investigated firstly. Commercial mitochondrial Mito TrackerⓇ Green and Lyso TrackerⓇ Green were used for co-localization. In the mitochondrial localization experiment, the Pearson sample correlation factors of QZ in red channel and Mito TrackerⓇ Green in the green channel were 0.83, showing a specific mitochondrial localization (Fig. 2). This locating capability is most likely due to the positive charge on the rhodamine dye. No localization was observed in lysosomes (Fig. S10 in Supporting information).
|
Download:
|
| Fig. 2. Confocal Fluorescence imaging of subcellular co-localization of QZ (10 µmol/L) with commercial mitochondrial Mito TrackerⓇ Green FM (100 nmol/L) in HeLa cells. (A) Fluorescence image in red channel (λex= 561 nm, λem= 570–620 nm) for QZ. (B) Fluorescence image in green channel (λex= 488 nm, λem= 500–550 nm) for Mito Tracker. (C) Merged images of (A) and (B). (D) Bright field image. (E) Intensity profiles of regions of interest (ROIs) across HeLa cells. (F) Intensity correlation plot of fluorescence signals in red channel and green channel, Pearson's correlation coefficient is 0.83. (A-D) Scale bar is 50 µm. | |
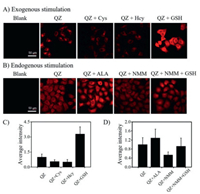
The selectivity of QZ towards GSH, Cys and Hcy was also investigated in HeLa cells. Cells in Confocal dish were incubated with GSH, Cys and Hcy respectively for 15 min and then washed three times before incubated with QZ for 30 min. It was observed that the fluorescence intensity of cell incubated with Cys and Hcy decreased, while the fluorescence intensity of cells pre-treated with GSH significantly increased, indicating that the probe specifically responded to GSH (Figs. 3A and C).
|
Download:
|
| Fig. 3. Confocal imaging of living HeLa cells with exogenous (A) and endogenous stimulation (B). Blank: HeLa cells; QZ: HeLa cells incubated with QZ (10 µmol/L) for 30 min; QZ+Cys: HeLa cells incubated with Cys (1 mmol/L) and QZ (10 µmol/L) for 30 min; QZ+Hcy: HeLa cells incubated with Hcy (1 mmol/L) and QZ (10 µmol/L) for 30 min; QZ+GSH: HeLa cells incubated with GSH (1 mmol/L) and QZ (10 µmol/L) for 30 min; QZ+ALA: HeLa cells pretreated with ALA (1 mmol/L) for 20 min, and then incubated with QZ (10 µmol/L) for 30 min; QZ+NMM: HeLa cells pretreated with NMM (1 mmol/L) for 20 min, and then incubated with QZ (10 µmol/L) for 30 min; QZ+NMM+GSH: HeLa cells pretreated with NMM (1 mmol/L) for 20 min, and then incubated with GSH (100 µmol/L) and QZ (10 µmol/L) for 30 min. (C) Relative average fluorescence intensity of HeLa cell in group (A). (D) Relative average fluorescence intensity of HeLa cell in group (B). Fluorescence images were captured in red channel (λex= 561 nm, λem= 570–620 nm). (A and B) Scale bar is 50 µm. | |
Subsequently, HeLa cells were pretreated with N-methylmaleimide (NMM), a commercial thiol-blocking reagent for 20 min before incubated with QZ for 30 min. The fluorescence intensity declined obviously. After adding GSH to the culture system, the fluorescence intensity recovered partially. By contrast, after pretreated with alpha lipoic acid, a thiol-enhancing reagent, the fluorescence intensity of cells enhanced (Figs. 3B and D) significantly. The results demonstrated that QZ maintained its selectivity in intracellular imaging experiments. MTT assay showed that QZ within 50 µmol/L had no obvious cytotoxicity. (Fig. S11 in Supporting information).
In summary, an "OFF-ON" type mitochondrial-target glutathione fluorescent probe QZ was designed and synthesized based on NCL and FRET mechanism, of which the high selectivity is ensured by the biological specificity of thiol sulfur aromatic ester bond recognition. QZ exhibits high sensitivity and rapid detection kinetics, which ensures its capacity to detect endogenous GSH in mitochondria organelles specifically. Cell imaging and MTT experiments showed excellent cell membrane permeability and low cytotoxicity, indicating that QZ has good application prospect in biological researches.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21878088, 21476077), and Key Project of the Shanghai Science and Technology Committee (No. 18DZ1112703) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.033.
| [1] |
K. Van Laer, C.J. Hamilton, J. Messens, Antioxid. Redox Signaling 18 (2013) 1642-1653. DOI:10.1089/ars.2012.4964 |
| [2] |
C.X. Yin, K.M. Xiong, F.J. Huo, J.C. Salamanca, R.M. Strongin, Angew. Chem. Int. Ed. 56 (2017) 13188-13198. DOI:10.1002/anie.201704084 |
| [3] |
L.E. Hernandez, J. Sobrino Plata, M.B. Montero Palmero, et al., J. Exp. Bot. 66 (2015) 2901-2911. DOI:10.1093/jxb/erv063 |
| [4] |
F.Q. Schafer, G.R. Buettner, Free Radicals Chem., Biol. Med. 30 (2001) 1191-1212. DOI:10.1016/S0891-5849(01)00480-4 |
| [5] |
R.S. Balaban, S. Nemoto, T. Finkel, Cell 120 (2005) 483-495. DOI:10.1016/j.cell.2005.02.001 |
| [6] |
H. Kamata, H. Hirata, Cell. Signalling 11 (1999) 1-14. DOI:10.1016/S0898-6568(98)00037-0 |
| [7] |
M.P. Murphy, Biochem. J. 417 (2009) 1-13. DOI:10.1042/BJ20081386 |
| [8] |
J.F. Turrens, J. Physiol. 552 (2003) 335-344. DOI:10.1113/jphysiol.2003.049478 |
| [9] |
P. Li, Y. Jia, N. Zhao, et al., Anal. Chem. 92 (2020) 12987-12995. DOI:10.1021/acs.analchem.0c01703 |
| [10] |
K.R. Atkuri, T.M. Cowan, T. Kwan, et al., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 3941-3945. DOI:10.1073/pnas.0813409106 |
| [11] |
M.T. Lin, M.F. Beal, Nature 443 (2006) 787-795. DOI:10.1038/nature05292 |
| [12] |
G. Calabrese, B. Morgan, J. Riemer, Antioxid. Redox Signaling 27 (2017) 1162-1177. DOI:10.1089/ars.2017.7121 |
| [13] |
Y. Wang, H. Duan, H. Shi, et al., Chin. Chem. Lett. 31 (2020) 2933-2936. DOI:10.1016/j.cclet.2020.05.028 |
| [14] |
H. Duan, Y. Ding, C. Huang, et al., Chin. Chem. Lett. 30 (2019) 55-57. DOI:10.1016/j.cclet.2018.03.016 |
| [15] |
L.M. Hyman, K.J. Franz, Coord. Chem. Rev. 256 (2012) 2333-2356. DOI:10.1016/j.ccr.2012.03.009 |
| [16] |
W. Xu, Z. Zeng, J.H. Jiang, Y.T. Chang, L. Yuan, Angew. Chem. Int. Ed. 55 (2016) 13658-13699. DOI:10.1002/anie.201510721 |
| [17] |
S. Lee, J. Li, X. Zhou, J. Yin, J. Yoon, Coord. Chem. Rev. 366 (2018) 29-68. DOI:10.1016/j.ccr.2018.03.021 |
| [18] |
Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798. DOI:10.1016/j.cclet.2019.08.013 |
| [19] |
Y. Yang, Y. Feng, H. Li, et al., Sens. Actuators B 333 (2021) 129189. DOI:10.1016/j.snb.2020.129189 |
| [20] |
Y.L. Zheng, H.C. Zhang, D.H. Tian, et al., Spectrochim. Acta A 238 (2020) 118429. DOI:10.1016/j.saa.2020.118429 |
| [21] |
S.Y. Lim, K.H. Hong, D.I. Kim, H. Kwon, H.J. Kim, J. Am. Chem. Soc. 136 (2014) 7018-7025. DOI:10.1021/ja500962u |
| [22] |
Y. Li, K.N. Wang, B. Liu, et al., Sens. Actuators B 255 (2018) 193-202. DOI:10.1016/j.snb.2017.08.041 |
| [23] |
Z. Xu, X. Huang, X. Han, et al., Chem 4 (2018) 1609-1628. DOI:10.1016/j.chempr.2018.04.003 |
| [24] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [25] |
G.J. Kim, D.H. Yoon, M.Y. Yun, et al., Sens. Actuators B 211 (2015) 245-249. DOI:10.1016/j.snb.2015.01.102 |
| [26] |
J.X. Lu, Y.C. Song, W. Shi, X.H. Li, H.M. Ma, Sens. Actuators B 161 (2012) 615-620. DOI:10.1016/j.snb.2011.11.009 |
| [27] |
J. Yin, Y. Kwon, D. Kim, et al., J. Am. Chem. Soc. 136 (2014) 5351-5358. DOI:10.1021/ja412628z |
| [28] |
Z. Yang, J.H. Lee, H.M. Jeon, et al., J. Am. Chem. Soc. 135 (2013) 11657-11662. DOI:10.1021/ja405372k |
| [29] |
X. Li, H. Wang, Y. Zhang, Q. Cao, Y. Chen, Chin. Chem. Lett. 32 (2020) 1541-1544. DOI:10.1016/j.cclet.2019.11.014 |
| [30] |
B. Tang, Y.L. Xing, P. Li, et al., J. Am. Chem. Soc. 129 (2007) 11666-11667. DOI:10.1021/ja072572q |
| [31] |
L. Chen, J.S. Park, D. Wu, C.H. Kim, J. Yoon, Sens. Actuators B 262 (2018) 306-312. DOI:10.1016/j.snb.2018.02.023 |
| [32] |
L.W. He, Q.Y. Xu, Y. Liu, et al., ACS Appl. Mater. Interfaces 7 (2015) 12809-12813. DOI:10.1021/acsami.5b01934 |
| [33] |
Z. Xue, L. Xiao, H. Chen, et al., Bioorg. Med. Chem. 26 (2018) 1823-1831. DOI:10.1016/j.bmc.2018.02.030 |
| [34] |
X. Ren, L. Liao, Z. Yang, et al., Chin. Chem. Lett. 32 (2020) 1061-1065. DOI:10.1016/j.cclet.2020.09.024 |
| [35] |
P.E. Dawson, S.B.H. Kent, Annu. Rev. Biochem. 69 (2000) 923-960. DOI:10.1146/annurev.biochem.69.1.923 |
| [36] |
J. Liu, Y.Q. Sun, H.X. Zhang, et al., RSC Adv. 4 (2014) 64542-64550. DOI:10.1039/C4RA10865E |
| [37] |
M.F. Tang, L.L. Wu, D. Wu, et al., RSC Adv. 6 (2016) 34996-35000. DOI:10.1039/C6RA00832A |
| [38] |
Q. Cai, T. Yu, W.P. Zhu, Y.F. Xu, X.H. Qian, Chem. Commun. 51 (2015) 14739-14741. DOI:10.1039/C5CC05518K |
| [39] |
J. Kalia, R.T. Raines, ChemBioChem 7 (2006) 1375-1383. DOI:10.1002/cbic.200600150 |
| [40] |
R. Miyajima, Y. Tsuda, T. Inokuma, et al., Pept. Sci. 106 (2016) 531-546. DOI:10.1002/bip.22757 |
| [41] |
K.G. Casey, E.L. Quitevis, J. Phys. Chem. 92 (1988) 6590-6594. DOI:10.1021/j100334a023 |
 2021, Vol. 32
2021, Vol. 32 
