b School of Pharmacy, Collaborative Innovation Center of Green Manufacturing Technology for Traditional Chinese Medicine in Shaanxi Province, Shaanxi Institute of International Trade & Commerce, Xi'an 712046, China;
c Department of Pharmacology and Toxicology and BIO5 Institute, University of Arizona, Tucson AZ 85721-0207, United States
A sensing or theranostic probe is generally designed for the detection of a single analyte [1-5]. Recently, multi-functional fluorescent imaging probes capable of sensing two or more targets have emerged as a powerful tool for the interrogation of biological systems [6, 7], which are often associated with cascade signaling events and activity-based sensing (ABS) tactic [3-5]. A straightforward approach for the design of such probes for multiply analytes employs different fluorescence responsive sensing units into a single molecule (Scheme S1a in Supporting information) [8-11]. However, probes designed in this way tend to have a large molecular weight and size, which could potentially cause undesired physicochemical properties, poor cell membrane permeability and/or cell toxicity. A compact probe adopting a single sensing moiety would be more desirable, but presents great challenges to design, which utilizes multiple reactive sites and divergent cascade reaction pathways for multiple analyte detection and differentiation. Recently, elegantly designed multiple reactive sites with divergent bond-formation cascade reactions were adopted for simultaneous sensing of cysteine, homocysteine, and glutathione (Scheme S1b in Supporting information) [12-14]. However, this bond-formation and analyte binding strategy is not suitable for analytes that may quench fluorophore's fluorescence, such as heavy metal ions. Herein, as a proof-of-concept study, we wish to demonstrate that multiple bond-cleavage sites with divergent analyte promoted bond-cleavage cascade reactions can be utilized to design a compact probe for detection and differentiation of multiple analytes (Scheme 1, Scheme S1c in Supporting information).

|
Download:
|
| Scheme 1. Probe design of COU-DPA-1 with three different bond-cleavage sites and divergent cascade reaction pathways. | |
Towards this end, a coumarin-thiazolidine masked D-penicillamine based fluorescent probe COU-DPA-1 was rationally designed for selective detection and differentiation of three heavy metal ions: Ag+, Hg2+, and Cu2+ (Scheme 1). The compact probe contains three cleavage sites: an initial C-S bond and two subsequent hydrolysis sites including a coumarin lactone and an imine. We envisioned that the heavy metal ion promoted C-S cleavage and potential imine complexation (Schemes S2a and b in Supporting information) [15-18], imine hydrolysis (Scheme S2c in Supporting information) [19], or lactone and imine hydrolysis (Scheme S2d in Supporting information) [20, 21], of the chiral probe may lead to divergent fluorescence (FL)/circular dichroism (CD) dual-modality responses (Scheme 1), in which enhanced CD signals come from exciton coupling between two neighboring fluorophores in formation of metal ion complexes [22]. Moreover, the cascade bond-cleavage reactions may release the drug D-penicillamine [23, 24] for potential treatments of Ag+ or Hg2+ poisoning [25, 26], or excess Cu2+ in disease conditions [27, 28].
The probe COU-DPA-1 was synthesized from 7-diethylaminocoumarin-3-aldehyde (1) and D-penicillamine (2) at refluxing condition in a mixture of methanol and water (Scheme S3 in Supporting information). The probe COU-DPA-1 contains inseparable mixture of trans- and cis-isomers in about 3:2 ratio. As C-S cleavage of the two isomers of the probe affords the same Schiff base ligand COU-DPA-1′, the probe was directly used without separation of its isomers. Four reference probes COU-DPA-2 ~ COU-DPA-5 were also synthesized (Schemes S4-S7 in Supporting information). All these reference probes were structurally characterized by 1H NMR, 13C NMR, and HRMS. More details can be found in Part Ⅱ in Supporting information.
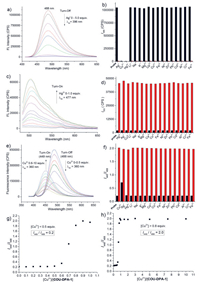
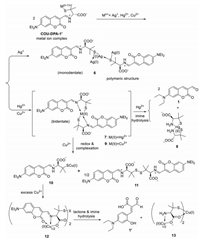
With the probe COU-DPA-1 in hand, its initial fluorescence responses upon 17 metal ions (Li+, Na+, K+, Mg2+, Ca2+, Co2+, Fe2+, Fe3+, Ni2+, Zn2+, Pb2+, Cd2+, Al3+, Cr3+, Ag+, Hg2+, Cu2+) were tested. Only three heavy metal ions (Ag+, Hg2+, Cu2+) gave immediate fluorescence turn-off responses (Fig. 1a, Fig. S2 in Supporting information). UV spectra showed red-shift of absorption from 399 nm to around 450 nm (Fig. S3 in Supporting information), while N-acetylated derivative COU-DPA-4 did not show any appreciable fluorescence and absorption spectra changes at the same condition (Fig. S10 in Supporting information), indicating C-S cleavage and formation of metal ion complexed COU-DPA-1′ is responsible for the initial fluorescence turn-off responses. For Ag+ at extended incubation, there were almost no fluorescence emission and no apparent UV–vis spectra changes (Figs. S4a and b in Supporting information). At incubation time of 30 s, Job's plot indicated that the fluorescence drop corresponded to the formation of 1:1 complex (Fig. S4d in Supporting information). From known polymeric structure of the 1:1 D-penicillamine Ag+ complex [29], we proposed that the Ag+ forms similar polymeric structures with COU-DPA-1′ (Scheme 2). For Hg2+, when the hydrolysis reaction product was selectively excited at 477 nm, it gave fluorescence turn-on response at 502 nm (Fig. 1c) with slight blue shift of absorption from 453 (Fig. S3b in Supporting information) to 446 nm (Fig. S5d in Supporting information). Evidences from comparison of normalized fluorescence spectra (Fig. S5e in Supporting information) and 1H NMR (Fig. S12 in Supporting information) and HRMS (Fig. S14 in Supporting information) studies of the reaction mixture confirmed that the product was the aldehyde 1. The hydrolysis reaction involved formation of 2:1 COU-DPA-1′ Hg2+ complex from the Job's plot (Fig. S5f in Supporting information), which was proposed to be a bidentate (N, S) coordination complex similar to a known D-penicillamine Hg2+ complex (Scheme 2) [30]. The probe's limits of detection (LOD) for Ag+ and Hg2+ were 1.9 and 10 nmol/L, respectively (Figs. S4c and S5c in Supporting information).
|
Download:
|
| Fig. 1. (a) Fluorescence spectra of the probe COU-DPA-1 (1 µmol/L, λex = 396 nm) upon addition of Ag+ ion (0–5.0 equiv., incubation time = 30 s). (b) Black column: I488 of the probe COU-DPA-1 (1 µmol/L, λex = 396 nm) or in response to 50 equiv. of other metal ions; red column: I488 of the probe in response to 5 equiv. Ag+ or 5 equiv. Ag+ in the presence of other metal ions (50 equiv. each) for 30 s. (c) Fluorescence spectra of probe COU-DPA-1 (1 µmol/L, λex = 477 nm) upon addition of increased concentrations of Hg2+ (0–1.0 equiv., incubation time = 15 min). (d) Black column: I502 of probe COU-DPA-1 (1 µmol/L, λex = 477 nm) or in response to 5 equiv. other metal ions; red column: I502 of probe in response to 0.5 equiv. Hg2+ or 0.5 equiv. Hg2+ in the presences of other metal ions (5 equiv. each) for 15 min. (e) Fluorescence spectra of probe COU-DPA-1 (1 µmol/L, λex = 360 nm) upon addition of Cu2+ ion (0–10 equiv., incubation time = 30 min). (f) Black column: I449/I488 of the probe COU-DPA-1 (1 µmol/L, λex = 360 nm) or the probe in response to 50 equiv. other metal ions; red column: I449/I488 of the probe (1 µmol/L, λex = 360 nm) in response to 5 equiv. Cu2+ or 5 equiv. Cu2+ in the presence of other metal ions (50 equiv. each, red columns) for 30 min. (g, h) Fluorescence intensity ratio (I449/I488) versus the equivalence of Cu2+ ([Cu2+]/[COU-DPA-1]). | |
|
Download:
|
| Scheme 2. Proposed metal ion binding promoted divergent cascade reaction of the Schiff base ligand COU-DPA-1′ in PBS buffer (pH 7.4). | |
The probe's fluorescence responses for Cu2+ at extended incubation showed non-typical ratiometric responses dependent on the equivalence of Cu2+ to the probe (Fig. 1e). At less than 0.5 equiv., the decrease of the probe's fluorescence at 488 nm was identified as typical turn-off response. Only when the probe's concentration was over 0.5 equiv., the product's fluorescence emission at 449 nm started to appear. Accordingly, the ratiometric ratio of I449/I488 increased from 0.2 to 2.0 (Figs. 1g and h). We termed this non-typical ratiometric fluorescence response as "self-threshold ratiometric fluorescence response", as there exists a threshold concentration of the analyte for the ratiometric response. We rationalized that the initial complex formed between the probe and less than 0.5 equiv. Cu2+ is non-fluorescent and resistance to hydrolysis as evidenced by only slight blue-shift of absorption peak from 452 nm to 445 nm at extended incubation (Fig. S6c in Supporting information). Excess Cu2+ over 0.5 equiv. leads to its binding to the second Cu2+ binding site and self-threshold ratiometric fluorescence response. From comparison with normalized fluorescence and absorption spectra of an aldehyde 1′ sample (Figs. S6g and h in Supporting information) from basic hydrolysis of the aldehyde 1 [20] and the aldehyde 1′ identified from HRMS of the reaction mixture (Fig. S15 in Supporting information), we proposed that the second Cu2+ binding site of COU-DPA-1′ functions as a trident (O, N, O) ligand and forms 2:1 complex with additional 0.5 equiv. Cu2+ to promote both lactone and imine hydrolysis (Scheme 2). Job's plot also confirmed that a total of 1 equiv. Cu2+ was required for formation of the aldehyde 1′ (Fig. S6f in Supporting information). The second Cu2+ binding site with 0.5 equiv. Cu2+ seems to involve the carboxylate anion, as the methyl ester probe COU-DPA-5 did not give the similar saddle-like absorption curve when incubation with excess Cu2+ (Fig. S11c in Supporting information). Further studies showed that Cu2+-promoted lactone hydrolysis is affected by substituents on the coumarin fluorophore. While 7-azetidinyl substituted probe COU-DPA-3 showed a similar self-threshold ratiometric fluorescence response for Cu2+ different from turn-on response for Hg2+ (Fig. S9 in Supporting information), the julolidine coumarin probe COU-DPA-2 showed fluorescence turn-on responses for both Cu2+ and Hg2+ due to formation of the same aldehyde 2 as product without lactone hydrolysis (Fig. S8 in Supporting information).
We then examined the probe's CD responses for the three heavy metal ions (Fig. 2). The probe (10 µmol/L) alone in PBS buffer showed very low CD signals. Upon addition of the three metal ions, intense bisignate CD signals (CD turn-on) due to metal ion complexation induced exciton coupling were observed. For Ag+, the initial CD signals did not decrease over 30 min (Fig. 2a), indicating formation rather stable silver ion complex with COU-DPA-1′. Moreover, the observed positive Cotton effect indicated that the neighbouring COU-DPA-1′ chromophore form clockwise orientation in Ag+ complex polymeric structures according to the Exciton Chirality Rule [22], different from the anti-clockwise orientation in complexation with Hg2+ and Cu2+ as indicated from the negative Cotton effect. Notably, CD and fluorescence signals compensate each other for improved sensing. For example, although the CD responses for Hg2+ and excess amount of Cu2+ can be differentiated by their zero-crossing wavelengths (Figs. 2b and c), they were better differentiated by distinct fluorescence responses (Figs. 1c and e). While fluorescence response cannot differentiate between Ag+ and less than 0.5 equiv. Cu2+, the two ions can be clearly differentiated by their distinct CD responses (Figs. 2a and d). Combined evidences from fluorescence emission (Fig. 1e), absorption (Fig. S6c) and CD (Fig. 2d) spectra, it was proposed that the reduction of Cu2+ to Cu+ by the thiol group [31] is possibly responsible for the observed reduction of CD signals (Scheme 2), during which a 1:1 complex was formed without neighbouring ligand and associated exciton coupling. Evidence from electron paramagnetic resonance (EPR) studies also suggested that a reduction reaction could happened (Fig. S13 in Supporting information).

|
Download:
|
| Fig. 2. Time-dependent CD spectra of the probe COU-DPA-1 (10 µmol/L) upon addition of 5 equiv. Ag+ (a), 0.5 equiv. Hg2+ (b), 5 equiv. Cu2+ (c), and 0.5 equiv. Cu2+ (d). The corresponding CD response of its enantiomer COU-LPA-1 in can be found in Fig. S16 (Supporting information). | |
Based on the above studies, the probe's divergent reaction pathway after C-S cleavage was shown in Scheme 2. Besides, its selectivity for the three heavy metal ions at both their specific fluorescence (Figs. 1b, d and e) and CD (Fig. S17 in Supporting information) detection conditions was high.
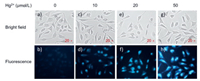
We further examined the potential use of the probe COU-DPA-1 in biological system. Unfortunately, the fluorescence "turn-off" response for Ag+ and the short excitation wavelength in UV region for Cu2+ was not ideal for cell imaging applications. The probe was tested for fluorescence imaging of Hg2+ and was capable of imaging increased Hg2+ levels inside HeLa cells from increased Hg2+ concentrations in the cell culture media (Fig. 3). Similarly, the methyl ester probe COU-DPA-5 can also detect increased Hg2+ and Cu2+ levels inside HeLa cells (Figs. S19 and S20 in Supporting information).
|
Download:
|
| Fig. 3. Fluorescence images of HeLa cells preincubated with COU-DPA-5 (20 µmol/L) and then incubated with increasing concentrations of Hg2+ (0, 10, 20, and 50 µmol/L) for 30 min. | |
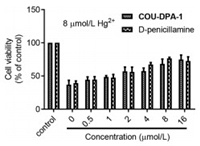
Finally, we examined the probe COU-DPA-1 for potential detoxification treatments of the heavy metal ions using the MCF-7 cells as the model cell line (see Supporting information part Ⅶ for more details). Both D-penicillamine and the probe COU-DPA-1 showed no significant toxicity up to 100 µmol/L (Fig. S21 in Supporting information). Among the three heavy metal ions, Hg2+ is the most toxic with IC50 = 7.52 ± 0.59 µmol/L (Fig. S21b). When MCF-7 cells were pretreated with 8 µmol/L toxic Hg2+ for 2 h, followed by addition of different concentrations (0~16 µmol/L) of the probe COU-DPA-1 and D-penicillamine to mimic acute heavy metal poisoning and detoxification treatments, increased cell viabilities were observed for both the probe and D-penicillamine treated cells, indicating that the probe showed protective effects against Hg2+-induced cytotoxicity (Fig. 4). Cell detoxification effects of the probe against Ag+ and Cu2+ were also observed (Fig. S23 in Supporting information).
|
Download:
|
| Fig. 4. Cell viability of MCF-7 cells pretreated with 8 µmol/L Hg2+ for 2 h and then incubated with increasing concentration (0 to 16 µmol/L) of the probe COU-DPA-1 and D-penicillamine for 24 h. | |
In conclusion, as a proof-of-concept study, we have successfully developed a compact dual-modality probe COU-DPA-1 with complimentary divergent fluorescence/circular dichroism responses for the three heavy metal ions via divergent cascade bond-cleavage reactions (metal ion promoted C-S cleavage and hydrolysis at two distinctive cleavage sites): FL "turn-off" and CD "turn-on" for Ag+ (no hydrolysis), FL "turn-on" and CD "turn-off" for Hg2+ (imine hydrolysis), and FL "self-threshold ratiometric" and CD "turn-off" for excess Cu2+ (lactone and imine hydrolysis), providing the first example of a fluorescence/CD dual-modality probe for differentiation of multiple species with complimentary responses. The bond-cleavage cascade reactions also lead to the formation of D-penicillamine heavy toxic metal complexes for detoxification treatments. The design principle by offering a way of creating compact probes via bond-cleavage cascade reactions and incorporating chirality and drug-release into the probe design may inspire future development of other chiral theranostic sensing systems for multiple analytes detection and disease treatments.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (Nos. 21577037 and 21738002), the State Key Laboratory of Bioreactor Engineering, Shanghai Natural Science Fund (No. 20ZR1414700) and Shanghai Sailing Program (No. 19YF1412500), Natural Science Basic Research Program of Shaanxi (No. 2019JQ-924), Key Breeding Program by Collaborative Innovation Center of Green Manufacturing Technology for Traditional Chinese Medicine in Shaanxi Province (No. 2019XT-1-03).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.047.
| [1] |
A.P. de Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, et al., Chem. Rev. 97 (1997) 1515-1566. DOI:10.1021/cr960386p |
| [2] |
Y. Yang, Q. Zhao, W. Feng, F. Li, Chem. Rev. 113 (2013) 192-270. DOI:10.1021/cr2004103 |
| [3] |
W. Sun, M. Li, J. Fan, X. Peng, Acc. Chem. Res. 52 (2019) 2818-2831. DOI:10.1021/acs.accounts.9b00340 |
| [4] |
K.J. Bruemmer, S.W.M. Crossley, C.J. Chang, Angew. Chem. Int. Ed. 59 (2020) 13734-13762. DOI:10.1002/anie.201909690 |
| [5] |
J. Ohata, K.J. Bruemmer, C.J. Chang, Acc. Chem. Res. 52 (2019) 2841-2848. DOI:10.1021/acs.accounts.9b00386 |
| [6] |
Y. Yue, F. Huo, F. Cheng, et al., Chem. Soc. Rev. 48 (2019) 4155-4177. DOI:10.1039/C8CS01006D |
| [7] |
J.L. Kolanowski, F. Liu, E.J. New, Chem. Soc. Rev. 47 (2018) 195-208. DOI:10.1039/C7CS00528H |
| [8] |
J. Han, X. Liu, H. Xiong, et al., Anal. Chem. 92 (2020) 5134-5142. DOI:10.1021/acs.analchem.9b05604 |
| [9] |
L. Yang, Y. Zhang, X. Ren, et al., Anal. Chem. 92 (2020) 4387-4394. DOI:10.1021/acs.analchem.9b05270 |
| [10] |
W. Zhang, F. Huo, F. Cheng, C. Yin, J. Am. Chem. Soc. 142 (2020) 6324-6331. DOI:10.1021/jacs.0c00992 |
| [11] |
L. Yuan, W. Lin, Y. Xie, B. Chen, S. Zhu, J. Am. Chem. Soc. 134 (2012) 1305-1315. DOI:10.1021/ja2100577 |
| [12] |
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577. DOI:10.1021/ja409578w |
| [13] |
C.X. Yin, K.M. Xiong, F.J. Huo, J.C. Salamanca, R.M. Strongin, Angew. Chem. Int. Ed. 56 (2017) 13188-13198. DOI:10.1002/anie.201704084 |
| [14] |
G. Yin, T. Niu, Y. Gan, et al., Angew. Chem. Int. Ed. 57 (2018) 4991-4994. DOI:10.1002/anie.201800485 |
| [15] |
Shaily A.Kumar, N. Ahmed, Ind. Eng. Chem. Res. 56 (2017) 6358-6368. DOI:10.1021/acs.iecr.7b00188 |
| [16] |
S. Areti, J.K. Khedkar, S. Bandaru, et al., Anal. Chim. Acta 873 (2015) 80-87. DOI:10.1016/j.aca.2015.02.065 |
| [17] |
M.A. Wani, P.K. Singh, R. Pandey, M.D. Pandey, J. Lumin. 171 (2016) 159-165. DOI:10.1016/j.jlumin.2015.11.017 |
| [18] |
K. Wang, W. Feng, Y. Wang, et al., Inorg. Chem. Commun. 71 (2016) 102-104. DOI:10.1016/j.inoche.2016.07.013 |
| [19] |
W. Lin, L. Yuan, X. Cao, Tetrahedron Lett. 49 (2008) 6585-6588. DOI:10.1016/j.tetlet.2008.09.029 |
| [20] |
N. Li, Y. Xiang, A. Tong, Chem. Commun. 46 (2010) 3363-3365. DOI:10.1039/c001408g |
| [21] |
K. Li, N. Li, X. Chen, A. Tong, Anal. Chim. Acta 712 (2012) 115-119. DOI:10.1016/j.aca.2011.10.066 |
| [22] |
D.A. Lightner, Tech. Instrum. Anal. Chem. 14 (1994) 131-174. |
| [23] |
W.M. Weigert, H. Offermanns, P. Scherberich, Angew. Chem. Int. Ed. 14 (1975) 330-336. DOI:10.1002/anie.197503301 |
| [24] |
M. Blanusa, V.M. Varnai, M. Piasek, K. Kostial, Curr. Med. Chem. 12 (2005) 2771-2794. DOI:10.2174/092986705774462987 |
| [25] |
H.T. Ratte, Environ. Toxicol. Chem. 18 (1999) 89-108. DOI:10.1002/etc.5620180112 |
| [26] |
T.W. Clarkson, L. Magos, G.J. Myers, N. Engl. J. Med. 349 (2003) 1731-1737. DOI:10.1056/NEJMra022471 |
| [27] |
U. Merle, M. Schaefer, P. Ferenci, W. Stremmel, Gut 56 (2007) 115-120. DOI:10.1136/gut.2005.087262 |
| [28] |
E. Gaggelli, H. Kozlowski, D. Valensin, G. Valensin, Chem. Rev. 106 (2006) 1995-2044. DOI:10.1021/cr040410w |
| [29] |
B.O. Leung, F. Jalilehvand, V. Mah, M. Parvez, Q. Wu, Inorg. Chem. 52 (2013) 4593-4602. DOI:10.1021/ic400192c |
| [30] |
B.O. Leung, F. Jalilehvand, V. Mah, Dalton Trans. (2007) 4666-4674. DOI:10.1039/B711436B |
| [31] |
Y. Sugiura, H. Tanaka, Chem. Pharm. Bull. 18 (1970) 368-373. DOI:10.1248/cpb.18.368 |
 2021, Vol. 32
2021, Vol. 32 

