Discotic polycyclic aromatic hydrocarbons (PAHs) have been of great interest in materials science and nanoscience [1]. Due to the structure tailorability, self-assembled ability and the appealing optical and electronical features of disc PAHs [2-8], they are ideal candidates for smart matters with diverse applications [9, 10]. For instance, up to now, different disc-PAHs have been designed and synthesized with the aim of using them for molecular recognition and fluorescent sensors [11, 12]. In this context, extending chromophoric group and doping heteroatoms are two practical strategies to improve the sensitivity and selectivity of the respective sensors, since the fluorescence intensity, energy level and intermolecular interactions could be fine-tuned.
1, 4, 5, 8, 9, 12-Hexaazatriphenylene (HAT, Fig. 1) represents the smallest two-dimensional N-containing PAH. The doped N atoms offer three chelating sites to the metal ions, so that the HAT derivatives have been widely used in fluorescent chemosensors and metal-containing supramolecular materials [13-15]. In most cases, HAT derivatives possess low fluorescence quantum yields partially due to the forbidden S0–S1 transition [1, 16]. Or, their absorption and emission colors are usually located in the violet and blue light region which are not sensitive to naked eyes [16, 17]. In this regard, more knowledge and examples about the improved sensitivity of the HAT based chemical sensors consisting of enhancing the fluorescence intensity and tuning the emission color are welcome.
|
Download:
|
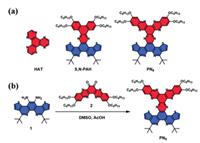
| Fig. 1. (a) Chemical structures of HAT, S, N-PAH and PN8. (b) Synthetic route of the target molecule PN8. | |
Recently, we reported a type of S, N-doped disc PAHs based on a π-extended, thiophene-fused phenanthroline unit (S, N-PAH, Fig. 1). The large conjugated mesogenic core with increased dipole moment derived from S, N heteroatoms not only facilitates the formation of highly ordered columnar superstructures, but also endows distinct bathochromic shifts of absorption and emission maxima compared to the smaller dibenzo[a, c]phenazine counterpart [18, 19]. Despite their excellent optical properties, their ion sensing feature was not observed, mainly due to the lack of efficient chelating sites. Based on our continuous interests in the synthesis and functions of large disc PAHs, in this work, we designed and explored a novel disc PAHs molecule with a unique metal-ion sensing character, by fusion the phenanthroline unit with a HAT moiety (PN8, Fig. 1). With two phenyl groups fused at the nitrogen-containing heterocycles, the core size of PN8 is larger than the reported S, N-PAH [19], with an expectation to the bathochromic shift of fluorescence colors, as well as the increase of overall dipole moment and anisotropic self-assembly ability. We also envisioned that the incorporated N heteroatoms in the aromatic core would provide more coordination sites, which may give rise to the recognition of metal ions for optical sensing applications. Consequently, PN8 exhibited a great tendency to self-assemble into long-range ordered aggregates. Moreover, both the self-assembled microfibers and the solutions were sensitively responsive to Cu2+ or Zn2+, accompanied by either quenched fluorescence or redshift emission colors. Meanwhile, the two metal ions could trigger dis-assembly of the microfibers. The efficient chelating ability of this newly emerged PAH molecule allowed its application as an adsorbent for the removal of Cu2+ and Zn2+ from wastewater.
The structure and synthetic route of the target molecule PN8 are presented in Fig. 1 and Scheme S1 (Supporting information). PN8 is an asymmetric π-extended HAT disc molecule surrounded by hexyloxy chains, which can be synthesized straightforwardly, by condensation of the thiophene-fused phenanthroline-11, 12-diamine 1 with quinoxalino[2, 3-a]-phenazine-6, 7-dione 2 in the presence of acetic acid in a yield of 26%. The target compound PN8 was unambiguously characterized by NMR spectroscopy (1H and 13C) and mass spectrometry (Figs. S9-S11 in Supporting information). The proton peaks of PN8 have been assigned explicitly in Fig. S9. And HR-ESI-MS spectrum of PN8 revealed a single species (m/z: 1111.5660 for C66H78N8O4S2H [M + H]+) in accordance with the calculated value.
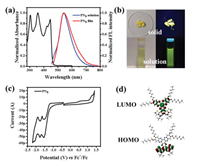
In dilute chloroform solution, PN8 showed intense UV-vis absorption ranging from 250 nm to 500 nm with four distinct bands centered at 303 nm (ε = 9.75 × 104 L mol−1 cm−1), 357 nm (ε = 10.25 × 104 L mol−1 cm−1), 417 nm (ε = 7.55 × 104 L mol−1 cm−1) and 445 nm (ε = 8.75 × 104 L mol−1 cm−1), assignable to the π-π* and n-π* transitions of the PAH core (Fig. 2a). PN8 displayed bright fluorescence emission in the green-yellow region at 500–750 nm with a maximum emission at approximately 540 nm. In solid state, PN8 exhibited bright yellow fluorescence with the absolute quantum yield rising from 2.32% in chloroform solution to 7.66%, suggesting the aggregation-enhanced emission (AEE) property [20] (Fig. 2b). Compared to many reported HAT derivatives, there are distinct bathochromic shifts of the absorption and emission colors, owing to the extended conjugation of the chromophore core [21].
|
Download:
|
| Fig. 2. (a) UV–vis absorption and fluorescence emission spectra of PN8 in chloroform (1 × 10−5 mol/L, λex = 445 nm) and in solid state. (b) The corresponding photographs under visible light (left) and 365 nm UV light (right). (c) Cyclic voltammograms of PN8 measured in dichloromethane with 0.1 mol/L TBAPF6 as electrolyte (scan rate = 100 mV/s). (d) DFT calculated molecular-orbital amplitude plots and energy levels for PN8 in the gas phase. | |
The electrochemical properties of PN8 were studied both experimentally and theoretically. First, the cyclic voltammograms of PN8 in dichloromethane are shown in Fig. 2c. Different from S, N-PAH that only exhibited one reversible reduction peak, three reduction waves at −1.55, −1.77 and −2.09 V for PN8 were observed, which were attributed to the consecutive reduction steps of three pyrazine moieties [22]. The onset of the first oxidation (Eox) and reduction onset (Ered) was 0.37 eV and −1.17 eV, respectively. The corresponding ionization potentials (IP) and electron affinities (EA) energy levels were thus estimated to be 5.03 and 3.53 eV, respectively. Compared to S, N-PAH, the reduced EA energy level for PN8 was ascribed to the electron-deficient nature of the fused HAT part. On the other hand, according to DFT calculation results, the calculated PN8 adopted a highly planar configuration, with the LUMO electrons delocalized over the HAT moiety, whereas the HOMO electrons unevenly distributed over the thiophene-fused 1, 10-phenanthroline part (Fig. 2d). It is noteworthy that the calculated dipole moment of PN8 is larger than that of S, N-PAH, possibly due to the joint effects from the π-expanded core and more doped nitrogen atoms (Table S1 in Supporting information).
As a large conjugated disc molecule with a strong local dipole, PN8 was readily to self-assemble into anisotropic superstructures. The self-assembled behavior of PN8 was first examined by concentration-dependent 1H NMR spectra. As shown in Fig. S1 (Supporting information), all the aromatic signals shifted upfield and became less-resolved with the concentration increasing. This indicated that PN8 formed stacked assemblies in which the aromatic protons were placed in the shielding regions produced by the neighboring aromatic rings. The peak assigned for –OCH2 protons also became broad and non-splitting at high concentration, implying that the alkyl chains were also involved in the assembly process [23]. Morphological study based on scanning electron microscopy (SEM) technique also confirmed the formation of microfibrils with high aspect ratios. For instance, in THF or mixed solutions, either three-dimensional networks composed of interdigitated long and rigid microfibers (in chloroform/acetone), or flexible high aspect ratio microwires (in chloroform/methylcyclohexane) were observed, with the widths in the range of 100–400 nm and the lengths up to tens of micrometers (Fig. S2 in Supporting information). Interestingly, these well-ordered 1D assemblies could experience reversibly morphological transitions upon alternate treatment with metal ions and EDTA solution. As shown in Fig. 3a, when Cu2+ or Zn2+ was added to the PN8 suspension, the initially formed fiber structures quickly crashed, resulting in ill-defined nanoaggregates. And the regular 1D assemblies could be re-generated when the resulted solution was treated with EDTA. Accompanied with morphological changes, appreciable color changes of the suspension and solutions could be easily detected by naked eyes (Fig. 3b). Such transition cycle can be repeated many times without the chemical decomposition of PN8, indicating the existence of supramolecular interactions between PN8 and metal ions.

|
Download:
|
| Fig. 3. (a) SEM images of assembled PN8 from THF (left), then treated with Zn2+ (middle), followed by treatment with EDTA (right) and (b) the corresponding photographs. | |
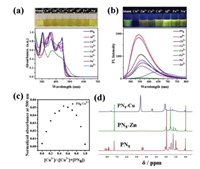
These results inspired us to further explore the ion-sensing properties of PN8. Its responsive behaviors in dilute solution were screened by adding different cations, like Al3+, Cd2+, Co2+, Cu2+, Fe3+, Na+ and Zn2+. PN8 was found selectively responsive to Cu2+ and Zn2+. In detail, the addition of Cu2+ and Zn2+ led to the red-shift of absorption onset, so the color of the solution became deep yellow (Fig 4a). More remarkably, according to FL spectra (Fig. 4b), Cu2+ caused almost complete fluorescence quenching, while for Zn2+, not only the decreased fluorescence intensity, but also a distinct red-shift emission color was detected for its mixed solution. The emission maximum varied from 547 nm to 630 nm after the treatment of Zn2+. Under the same conditions, an increase of emission intensity was observed after Al3+ or Fe3+ was added; while a slight decrease after treated with other ions. The enhanced emission might be due to the Lewis acidity of Al3+ and Fe3+, as a similar phenomenon appeared after TFA was added to the PN8 solution (Fig. S3 in Supporting information). In contrast to Cu2+ and Zn2+, such fluorescence change of the solution upon treated with the other tested metal ions was not readily discriminated by naked eyes, therefore, PN8 is promising to serve as a fluorescent optical probe for metal ions, such as Cu2+ and Zn2+.
|
Download:
|
| Fig. 4. (a) UV-vis absorption and (b) fluorescence spectra of PN8 in chloroform (1 × 10−5 mol/L, λex = 445 nm) with addition of different metal ions (4 equiv.), and the corresponding photographs under visible light and 365 nm UV light. (c) Job plot for the PN8–Cu2+ system. [PN8]+[Cu2+] = 0.1 mmol/L. (d) 1H NMR spectra of PN8 and PN8 with 4 equiv. metal ions in CDCl3. | |
Following, the detection limit was evaluated on the basis of fluorescence titration experiments [24]. The plot of fluorescence intensity of the PN8 (1 × 10−5 mol/L) vs. concentrations of Cu2+ in CHCl3 displayed a good linear relationship with R2 of 0.9878 during titration. The limit of detection (LOD) for Cu2+ was determined to be 8.71 × 10−7 mol/L for PN8 (Fig. S4 in Supporting information) based on LOD = 3σ/k, where σ is the standard deviation of blank measurements and k is the slope. Meanwhile, the fluorescence wavelength of the solutions showed a good linear relationship (R2 = 0.9954) with the concentration of Zn2+, with LOD for Zn2+ determined to be 8.57 × 10−8 mol/L. Both values are comparable to many reported ones [15, 25-27].
To shed more light on the ion-sensing mechanism, a set of experiments were carried out. First, no optical changes were detected after the acidification of PN8 solution, therefore, the responsive properties of PN8 towards Cu2+ and Zn2+ presumably derived from pH variation or protonation could be excluded. Also, the control compounds S, N-PAH and the thiophene-fused phenanthroline part alone TP were found not responsive to metal ions (Fig. S5 in Supporting information). Based on these facts and combined with the molecular structure, we inferred a binding mechanism mainly arisen from the fused HAT unit. Although attempts to obtain single crystals of PN8 with Cu2+ or Zn2+ were not successful, MALDI-TOF mass spectroscopic measurements on the mixtures of PN8 with the two metal ions could provide valuable information on the complex structures [13]. Typically, as for the mixture of PN8 and Cu2+, a strong peak at m/z of 1172.93 was observed, which was corresponding to the m/z of [1[PN8]+Cu2+-H+]+, thus suggesting the prevailing of the species in the form of 1:1 binding mode (Fig. S6 in Supporting information). Furthermore, the binding stoichiometry between PN8 and the ions was explored via UV–vis spectroscopic titration with a fixed concentration of 0.1 mmol/L [28]. A peak in the obtained Job plot at the molar fraction of 0.5 was found (Fig. 4c), again confirming the 1:1 (or n: n) binding stoichiometry. In addition, according to the 1H NMR spectra, the proton signals of the PN8 and Cu2+ mixture disappeared (for aromatic protons) or became less-resolved (for alkyl protons), which suggested a shielding effect very likely caused by supramolecular interactions between PN8 and Cu2+ (Fig. 4d). Similar results were found for Zn2+, except for the possible co-existence of two complexes ([1[PN8]+Zn2+-H+]+ and [2[PN8]+Zn2+-H+]+), since there were two peaks at m/z of 1173.22 and 2288.70 according to MALDI-TOF mass spectroscopy (Fig. S7 in Supporting information). Taken together, these characterizations jointly manifested the good selectivity and sensitivity of the ion-sensing/binding properties of PN8 towards Cu2+ and Zn2+, in colorimetric and fluorometric modes.
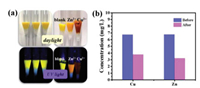
Since PN8 was not soluble in water and showed excellent and reversible metal ions binding ability in solution, its application as a solid-state adsorbent to remove Cu2+ and Zn2+ from aqueous solutions was examined. For demonstration, a small-size column with PN8 powder as the filler, methanol and water as the eluent was fabricated. As shown in Fig. 5a, the pristine powder was yellow and emitted bright yellow fluorescence. Then, after the aqueous solutions of Cu2+ or Zn2+ were poured into the column, the successful adsorption of Cu2+ and Zn2+ could be observed with the color of the column changed to brown and orange, respectively. And under UV light, the corresponding column exhibited either quenched emission or orange fluorescence. All these changes were consistent with those in the solution. At the same time, the metal ion concentration before and after filtration was measured by ICP-MS (Fig. 5b) [29], which confirmed that a small amount of PN8 (10 mg) could remove most of the Cu2+ and Zn2+ from the respective aqueous solution (1 × 10−4 mol/L, 4 mL). The removal efficiency was estimated to be 54.27% and 44.30% for Cu2+ and Zn2+, respectively, indicating good absorptivity of PN8 (Fig. S8 in Supporting information). Notably, benefiting greatly from its large PAH core, PN8 was highly hydrophobic and stable. So compared to other HAT-based materials, PN8 is more suitable to be utilized as an optical ion probe and adsorbent, particularly in solid state, with the merits of high (fluorescence) color contrast, good stability and recyclability [30-35].
|
Download:
|
| Fig. 5. (a) Photographs of glass pipettes filled with PN8 powders under daylight and UV light before and after adsorption of Cu2+ and Zn2+ (1 × 10−3 mol/L). (b) The ion concentrations before and after the aqueous solutions were filtrated. | |
In conclusion, we have successfully synthesized a new HAT based, large PAH disc molecule (PN8). PN8 exhibited fluorescent sensing characteristics, which was selective for Cu2+ and Zn2+ over many other ions. The enlarged PAH core (up to 11 fused aromatic rings) and the doped hetero atoms endowed PN8 good anisotropic self-assembly ability, metal-ion binding affinity and pronounced optical properties. Therefore, in self-assembled state, revisable morphological transition was found upon the alternate treatment of ions and EDTA. In solution state, the two ions triggered highly appreciable optical changes with high (fluorescence) color contrast and low detection limits, which were proved mainly based on the metal ion binding mode in different stoichiometric ratios. Importantly, due to its good stability and efficient ion binding capability, PN8 was demonstrated as an excellent adsorbent to remove Cu2+ and Zn2+ from wastewater. The current work thus will not only enrich the family and the functions of hetero-atom containing PAHs, but also be helpful for future applications of HAT-embedded disc molecules in advanced fluorescent sensing and imaging, water treatment and so on.
Declaration of competing interestThere are no conflicts to declare.
AcknowledgmentThis work was supported by the National Key Research and Development Program of China (Nos. 2017YFA0204503 and 2017YFA0207800).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.044.
| [1] |
J.L. Segu, J. Segura, R. Juárez, M. Ramos, C. Seoane, Chem. Soc. Rev. 44 (2015) 6850-6885. DOI:10.1039/C5CS00181A |
| [2] |
Z.Y. Xiao, X. Zhao, X.K. Jiang, Z.T. Li, Chem. Mater. 23 (2011) 1505-1511. DOI:10.1021/cm103182e |
| [3] |
M. Han, G.C. Wang, H.Q. Duan, Chin. Chem. Lett. 25 (2014) 51-54. DOI:10.1016/j.cclet.2013.09.017 |
| [4] |
G.R. Kiel, M.S. Ziegler, T.D. Tilley, Angew. Chem. Int. Ed. 56 (2017) 4839-4844. DOI:10.1002/anie.201700818 |
| [5] |
Y. Dorca, C. Naranjo, P. Delgado-Martínez, R. Gómez, L. Sánchez, Chem. Commun. 55 (2019) 6070-6073. DOI:10.1039/C9CC02000D |
| [6] |
M. Wang, Y. Li, H. Tong, et al., Org. Lett. 13 (2011) 4378-4381. DOI:10.1021/ol201717d |
| [7] |
Y. Li, M.G. Li, Y.J. Su, et al., Chin. Chem. Lett. 27 (2016) 475-480. DOI:10.1016/j.cclet.2015.12.024 |
| [8] |
J. Ma, K. Zhang, K.S. Schellhammer, et al., Chem. Sci. 10 (2019) 4025-4031. DOI:10.1039/C8SC05416A |
| [9] |
Z.G. Tao, X. Zhao, X.K. Jiang, Z.T. Li, Tetrahedron Lett. 53 (2012) 1840-1842. DOI:10.1016/j.tetlet.2012.01.137 |
| [10] |
W. Yuan, J. Cheng, X. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 9940-9945. DOI:10.1002/anie.201914900 |
| [11] |
X.Y. Yan, M.D. Lin, S.T. Zheng, et al., Tetrahedron Lett. 59 (2018) 592-604. DOI:10.1016/j.tetlet.2018.01.004 |
| [12] |
A. Yadav, D.K. Panda, S. Zhang, W. Zhou, S. Saha, ACS Appl. Mater. Interfaces 12 (2020) 40613-40619. DOI:10.1021/acsami.0c12388 |
| [13] |
X. Zhang, J. Fu, T.G. Zhan, et al., Tetrahedron Lett. 55 (2014) 6486-6489. DOI:10.1016/j.tetlet.2014.10.015 |
| [14] |
S. Ibáñez, M. Poyatos, E. Peris, Chem. Commun. 53 (2017) 3733-3736. DOI:10.1039/C7CC00525C |
| [15] |
T.S. Saeed, D. Maddipatla, B.B. Narakathu, et al., RSC Adv. 9 (2019) 39824-39833. DOI:10.1039/C9RA08825C |
| [16] |
R. Juárez, M.M. Oliva, M. Ramos, et al., Chem. Eur. J. 17 (2011) 10312-10322. DOI:10.1002/chem.201101198 |
| [17] |
Z.G. Tao, T.G. Zhan, T.Y. Zhou, X. Zhao, Z.T. Li, Chin. Chem. Lett. 24 (2013) 453-456. DOI:10.1016/j.cclet.2013.03.031 |
| [18] |
T. Wang, H. Wang, G. Li, et al., Macromolecules 49 (2016) 4088-4094. DOI:10.1021/acs.macromol.6b00236 |
| [19] |
W. Yuan, X. Ren, M. Li, et al., Angew. Chem. Int. Ed. 130 (2018) 6269-6273. DOI:10.1002/ange.201801488 |
| [20] |
J. Mei, N.L. Leung, R.T. Kwok, J.W. Lam, B.Z. Tang, Chem. Rev. 115 (2015) 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [21] |
J.K. Salunke, F.L. Wong, K. Feron, et al., J. Mater. Chem. C 4 (2016) 1009-1018. DOI:10.1039/C5TC03690A |
| [22] |
T. Ishi-i, R. Hirashima, N. Tsutsumi, et al., J. Org. Chem. 75 (2010) 6858-6868. DOI:10.1021/jo101212d |
| [23] |
M. Tanaka, T. Ikeda, J. Mack, N. Kobayashi, T. Haino, J. Org. Chem. 76 (2011) 5082-5091. DOI:10.1021/jo200766u |
| [24] |
Y. Han, W. Yuan, H. Wang, et al., J. Mater. Chem. C 6 (2018) 10456-10463. |
| [25] |
X.H. Zhang, Q. Zhao, X.M. Liu, et al., Talanta 108 (2013) 150-156. DOI:10.1016/j.talanta.2013.02.071 |
| [26] |
Z. Liu, C. Peng, Y. Wang, M. Pei, G. Zhang, Org. Biomol. Chem. 14 (2016) 4260-4266. DOI:10.1039/C6OB00476H |
| [27] |
L.C. da Silva, V.G. Machado, F.G. Menezes, Chem. Paper. 75 (2021) 1775-1793. DOI:10.1007/s11696-020-01484-9 |
| [28] |
T. Tian, T. Qian, T. Jiang, et al., Chem. Commun. 56 (2020) 3939-3942. DOI:10.1039/D0CC01350A |
| [29] |
D. Kou, W. Ma, S. Zhang, Adv. Funct. Mater. 31 (2021) 2007032. DOI:10.1002/adfm.202007032 |
| [30] |
R. Juárez, M. Ramos, J. Segura, et al., J. Org. Chem. 75 (2010) 7542-7549. DOI:10.1021/jo101311v |
| [31] |
X.H. Zhang, C.F. Zhao, Y. Li, et al., Talanta 119 (2014) 632-638. DOI:10.1016/j.talanta.2013.11.009 |
| [32] |
J.O. Moilanen, B.M. Day, T. Pugh, R.A. Layfield, Chem. Commun. 51 (2015) 11478-11481. DOI:10.1039/C5CC04004C |
| [33] |
J.O. Moilanen, N.F. Chilton, B.M. Day, T. Pugh, R.A. Layfield, Angew. Chem. Int. Ed. 55 (2016) 5521-5525. DOI:10.1002/anie.201600694 |
| [34] |
V.J. Bhanvadia, A.L. Patel, S.S. Zade, N. J. Chem. 42 (2018) 17700-17707. DOI:10.1039/C8NJ03793K |
| [35] |
A. Markovic, L. Gerhards, P. Sander, et al., ChemPhysChem 21 (2020) 2506-2514. DOI:10.1002/cphc.202000547 |
 2021, Vol. 32
2021, Vol. 32 

