b Department of Chemistry, Bengbu Medical College, Bengbu 233030, China
High-nuclearity metal complexes have flourished in an increasing number of fields and inspired researchers to assemble plentiful polymers or give an insight into the extensive properties, due to the aggregation of abundant metal ions resulting in the multiple pleasing structures and diversified properties [1-10]. As one kind of significant metal clusters, heterometallic transition-lanthanide (3d-4f) high-nuclearity clusters have acquired comprehensive attention on account of multiple structures, and smart applications, such as photocatalytic CO2 reduction, gas separation and magnetocaloric effect (MCE) [11-15].
More specially, numerous efforts have been encouraged by 3d-4f compounds as the promising molecule-based magnetic cooling materials which have the chance to replace rare and costly He-3 in the ultra-low-temperature scope [16]. And compared with pure 3d- or 4f-based compounds, 3d-4f mixed metal complexes with the virtues of different spin centers can provide more opportunities to build magnetic materials [16-23]. In order to achieve the remarkable MCE, high-nuclearity 3d-Gd compounds are chosen as the ideal magnetic materials, because a high-spin ground state, isotropic electron orbit and negligible magnetic anisotropy of Gd3+ ions are beneficial to the outstanding MCE and the large low-field magnetic entropy change [15, 24-29]. Moreover, it is generally believed that the correlation of structure-property is presented in nanoscale materials [21, 30]. For example, a certain number of explorations demonstrate that multidimensional metal polymers are powerful to the brilliant MCE with large magnetic density [3, 31]. Nevertheless, it is worth paying consideration to the explorations demonstrate that multidimensional metal polymers challenge of assembling 3d-4f complexes as distinguished magnetic coolants, resulting from the different coordination modes of transition and lanthanide ions and the indissolubility engendered by the over-aggregation of metal ions [32-34]. So far, although a host of high-nuclearity 3d-4f heterometallic metal clusters have been assembled and published [15, 35, 36], most of them presented isolated structure [27, 37]. In other words, constructing multidimensional 3d-4f mixed-metal high-nuclearity clusters manifesting favorable MCE is more challenging but pregnant.
Based on reasonable experimental design, if the isolated structures could be bridged by ligands to form the multidimensional structures? With the purpose of obtaining objective 3d-4f clusters coupled with aesthetically enjoyable structures and large MCE, anionic templates have been efficiently utilized and widely accepted in designing of fabricating metal clusters [12, 30, 35]. Additionally, the CO32- anions as the common template have been successfully used to induce 3d and 4f ions into pure metal clusters or mixed-metal compounds because of its versatile coordination modes and the relatively strong coordination bonding between CO32- and metal ions [29]. However, among publicly reported high-nuclearity clusters, most of CO32- anionic templates came from the adsorption of carbon dioxide in the atmosphere or the decomposition of ligands, which were all of high serendipity [30, 32]. Therefore, we look forward to building novel and tempting 3d-4f high-nuclearity clusters via quantitatively introducing CO32- anions from carbonate.
To put the above considerations into practice, two captivating chain-like coordination polymers based on Ln18Ni24(23.5) high-nuclearity 3d-4f clusters, 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n, are isolated through CO32- anionic templates and solvothermal treatment. In terms of the structures, compounds 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n are both linked by IDA2- ligands to constitute the scarce 3d-4f chain-shaped metal clusters up to now. Additionally, products in this work are both templated by CO32- anions that are quantitatively introduced by the adding of carbonate. More importantly, compound 1-(Gd18Ni24)n makes a new breakthrough in MCE, with -∆Sm = 35.30 J kg-1 K-1 (at 3.0 K with ∆H = 7.0 T) and -∆Sm = 20.95 J kg-1 K-1 (at 2.0 K with ∆H = 2.0 T).
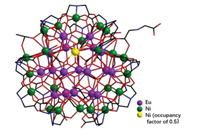
Single-crystal X-ray diffraction studies expose that compounds 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n both crystallize in the monoclinic space group P21/c. As shown in Fig. 1, in compound 2-(Eu18Ni23.5)n, [Eu18Ni23.5(IDA)22(IN)(CH3COO)2(NH2CH2COO)(μ3-OH)32(CO3)7(H2O)5Cl]·Cl6·(H2O)17, contains 18 Eu3+ ions, 24 Ni2+ ions (Ni24 with occupancy factor of 0.5), 7 CO32- ions, 22 IDA2- ligands, one glycine ligand (NH2CH2COO-) from the decomposition of H2IDA [18], one IN- ligand, two CH3COO-, 7 Cl- ions, 32 μ3-OH-, 5 coordination water molecules and 17 lattice water molecules. And compound 1-(Gd18Ni24)n, [Gd18Ni24(CO3)7(IDA)22(μ2-OH)3(μ3-OH)32(H2O)5Cl]·Cl8·(H2O)14, contains 18 Gd3+ ions, 24 Ni2+ ions, 7 CO32- ions, 22 IDA2- ligands, 9 Cl- ions, 32 μ3-OH-, 3 μ2-OH-, 5 coordination water molecules and 14 lattice water molecules (Figs. S6 and S7 in Supporting information).
|
Download:
|
| Fig. 1. Ball and stick pattern of compound 2-(Eu18Ni23.5)n. | |
In regard to structures of two complexes, here only compound 2-(Eu18Ni23.5)n as the example is discussed in detail due to the similarity of two compounds. {[Eu18Ni23.5(IDA)22(CO3)7(μ3-OH)32(H2O)5(IN)(CH3COO)2(NH2CH2COO)]Cl}n6n+ cation cluster (Fig. 2) of compound 2-(Eu18Ni23.5)n can be viewed as the core-shell configuration and can be divided into two parts: inter {Eu18Ni} core (Ni with occupancy factor of 0.5, Fig. 2b) and outer {Ni23} shell (Fig. 2c). Twenty-three Ni2+ ions connect with 21 IDA2- ligands and one CH3COO-, leading to a bowl-like {Ni23} shell. In addition, one IN- as the terminal ligand connects with one Ni2+ ion. As shown in Fig. 2b, inter {Eu18Ni} layer can be divided into {Eu11Ni} (top) and {Eu7} (bottom): in the top, 11 Eu3+ ions are linked through μ3-OH-; in the bottom, 7 Eu3+ ions are interconnected by μ3-OH-. Then {Eu11Ni} and {Eu7} are further connected by 7 CO32-, forming a unique {Eu18Ni} core (Fig. 2b). Interestingly, Ni24 exists in the core of {Eu11Ni}, as shown in Fig. 2b. The Ni24 ion is connected to adjacent six Eu3+ ions by oxygen atoms.

|
Download:
|
| Fig. 2. (a) The 2-(Eu18Ni23.5)n cluster. (b) The diagramming of inner {Eu18Ni} core building unit. (c) The outer {Ni23} shell. | |
The inter {Eu18Ni} core links with outer {Ni23} transition metal shell through μ3-OH-, CO32- (Fig. S4 in Supporting information), glycine (Fig. S5c in Supporting information) and IDA2- ligands (Fig. S3 in Supporting information), forming the core-shell {Eu18Ni24} cluster (Fig. 2a). The adjacent {Eu18Ni24} clusters interact with each other based on IDA2- ligands (Fig. 3b), forming the one-dimensional chain (Fig. 3).

|
Download:
|
| Fig. 3. (a) The 1D zigzag chain of (Eu18Ni23.5)n in 2; (b) The coordination mode of connector (IDA2-) between adjacent {Eu18Ni24} units (Color code: yellow, Ni24 with occupancy factor of 0.5). | |
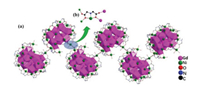
Although structure of 1-(Gd18Ni24)n is very similar to 2-(Eu18Ni23.5)n, there are still some distinctions between two products: (ⅰ) the occupancy factors of all Ni2+ ions in 1-(Gd18Ni24)n are one, but Ni24 in 2-(Eu18Ni23.5)n is 0.5; (ⅱ) μ2-OH- groups are only connected with metal ions in 1-(Gd18Ni24)n; (ⅲ) IN-, CH3COO- and NH2CH2COO- groups are only coordinated with metal ions in 2-(Eu18Ni23.5)n; (ⅳ) 9 Cl- and 14 free H2O groups are in 1-(Gd18Ni24)n, but 7 Cl- and 17 free H2O groups are in 2-(Eu18Ni23.5)n; (ⅴ) the chain-like structures of compounds 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n are both constructed by IDA2- ligand, but the coordination modes of IDA2- ligands are different (as depicted in Figs. 3 and 4)
|
Download:
|
| Fig. 4. (a) The 1D zigzag chain configuration of 1-(Gd18Ni24)n. (b) The coordination mode of connector (IDA2-) between adjacent {Gd18Ni24} units. | |
Meanwhile, CO32- anions act not only as the counter anions to balance the valence, but also as the anion templates to induce the synthesis of metal skeletons. As shown in Figs. S8 and S9 (Supporting information), CO32- anions are distributed in the middle of {Eu18Ni24} and {Gd18Ni24} building blocks as well display multidentate coordination patterns to link the neighbor subunits ({Eu11Ni} and {Eu7}, {Gd11Ni} and {Gd7}).
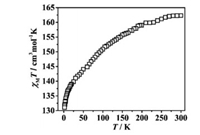
A great number of Gd3+ ions in compound 1-(Gd18Ni24)n encourage us to investigate the MCE. The plot of χMT-T for 1-(Gd18Ni24)n was studied with the scope of 1.8–300 K and under the appropriate magnetic field of 1000 Oe (Fig. 5). The value of χMT was 162.31 cm3 K/mol at room temperature, which was slightly inferior to the calculated result (165.75 cm3 K/mol; for 18 Gd3+ ions, S = 7/2, g = 2; and 24 Ni2+ ions S = 1, g = 2) [16]. Along with the cooling, χMT decreased in the scale of T = 1.8–300 K, and attained the minimum (131.06 cm3 K/mol). This behavior implied the existence of antiferromagnetic interactions in 1-(Gd18Ni24)n. The Curie–Weiss Law was studied to fit the χM-1 vs. T between 1.8 and 300 K, with two parameters C = 162.14 cm3 K/mol and θ = -4.10 K (Fig. S18 in Supporting information) [28]. The negative of θ indicated that weak antiferromagnetic interactions are presented in compound 1-(Gd18Ni24)n [30]. The research of M-H for 1-(Gd18Ni24)n was studied (T = 1.8–15 K, H = 0–7 T; Fig. S19 in Supporting information). As H increased, the M gradually rose, and attained the saturation value of 142.07 NµB at 7.0 T. The experimental value of M was smaller than the calculated result (174 NµB), which can be attributed to the antiferromagnetic exchanges of the metal ions [38, 39].
|
Download:
|
| Fig. 5. Temperature-dependent magnetic susceptibilities for compound 1-(Gd18Ni24)n. | |
The magnetization studies of 1-(Gd18Ni24)n were taken based on the scope of temperature (2.0–14.0 K) and the field (0.5–7.0 T), which were performed by the Maxwell relation for ∆Sm(T) = ∫[∂M (T, H)/∂T] [12]. The largest value of -∆Sm for 1-(Gd18Ni24)n was 35.3 J kg-1 K-1 at roughly 3.0 K with a field change of ∆H = 7.0 T (as exhibited in Fig. 6), which was inferior to the theoretical result (60.27 J kg-1 K-1). And the theoretical calculation formula is -∆Sm = nNiR ln (2SNi +1)/Mr+nGd R ln (2SGd +1)/Mr [35]. The discrepancy between theoretical value and experimental value may be ascribed to the antiferromagnetic behavior in 1-(Gd18Ni24)n [31]. Besides, the value of -∆Sm is relatively large in reported 3d-4f clusters, manifesting that 1-(Gd18Ni24)n is the potential magnetic cooling material (Table S4 in Supporting information). It is worth noting that the large values of -∆Sm at low fields come up to 12.25 J kg-1 K-1 (∆H = 1.0 T) and 20.95 J kg-1 K-1 (∆H = 2.0 T) at 2.0 K (Table S5 in Supporting information).

|
Download:
|
| Fig. 6. Values of -∆Sm for 1-(Gd18Ni24)n with varieties of temperatures (2.0–14.0 K) along with fields (0.5–7.0 T). | |
In summary, two attracting high-nuclearity 3d-4f mixed-metal clusters [1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n] were isolated in the existence of CO32- anionic templates and solvothermal treatment. Single-crystal X-ray diffraction studies suggested that products in this work are both bridged via IDA2- ligands to display the infrequent one-dimensional chain shapes. In addition, magnetic investigations indicated that 1-(Gd18Ni24)n is the potential magnetic coolant with -∆Sm = 35.3 J kg-1 K-1 at 3.0 K and ∆H = 7.0 T. Especially, the low-field magnetic entropy change of compound 1-(Gd18Ni24)n is remarkable (-∆Sm = 20.95 J kg-1 K-1 at 2.0 T and 2.0 K). This work offers a consultant for assembling novel metal clusters together with the large MCE.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Natural Science Foundation of China (No. 21571103) and Jiangsu (No. BK20191359); the Project of Natural Science Foundation of the Higher Education Institutions of Anhui Province, China (No. KJ2019A0350).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.042.
| [1] |
J. Zhou, Coord. Chem. Rev. 315 (2016) 112-134. DOI:10.1016/j.ccr.2016.01.009 |
| [2] |
Y.M. Han, N.F. Li, Y.Z. Yu, et al., RSC Adv. 10 (2020) 11365-11370. DOI:10.1039/D0RA01524E |
| [3] |
X. Liu, X. Yang, Y. Ma, et al., Chin. Chem. Lett. 32 (2021) 569-572. DOI:10.1016/j.cclet.2020.03.016 |
| [4] |
X.M. Luo, N.F. Li, Q.F. Lin, et al., Inorg. Chem. Front. 7 (2020) 2072-2079. DOI:10.1039/D0QI00226G |
| [5] |
B.Q. Ji, H.F. Su, M. Jagodic, et al., Inorg. Chem. 58 (2019) 3800-3806. DOI:10.1021/acs.inorgchem.8b03406 |
| [6] |
W. Muhammada, J. Nia, Z. Jaglici, et al., Chin. Chem. Lett. 31 (2020) 2503-2506. DOI:10.1016/j.cclet.2020.01.031 |
| [7] |
Y. Yu, X. Pan, C. Cui, et al., Inorg. Chem. 59 (2020) 5593-5599. DOI:10.1021/acs.inorgchem.0c00281 |
| [8] |
M.H. Yu, X. Jiang, S.D. Han, Q.L. Wang, X.H. Bu, Chin. Chem. Lett. 27 (2016) 317-320. DOI:10.1016/j.cclet.2016.01.001 |
| [9] |
Y.X. Sun, W.Y. Sun, Chin. Chem. Lett. 25 (2014) 823-828. DOI:10.1016/j.cclet.2014.04.032 |
| [10] |
J.H. Liu, L.D. Lin, G.Q. Wang, et al., Chem. Commun. 56 (2020) 10305-10308. DOI:10.1039/D0CC04070C |
| [11] |
J.B. Peng, Q.C. Zhang, X.J. Kong, et al., Int. Ed. 50 (2011) 10649-10652. DOI:10.1002/anie.201105147 |
| [12] |
W.P. Chen, P.Q. Liao, P.B. Jin, et al., J. Am. Chem. Soc. 142 (2020) 4663-4670. DOI:10.1021/jacs.9b11543 |
| [13] |
W.Z. Qiao, H. Xu, P. Cheng, B. Zhao, Cryst. Growth Des. 17 (2017) 3128-3133. DOI:10.1021/acs.cgd.7b00063 |
| [14] |
K. Sheng, N. Y.-.Liu, R.K. Gupta, M. Kurmoo, D. Sun, Sci. China Chem. 64 (2021) 419-425. DOI:10.1007/s11426-020-9886-5 |
| [15] |
W.P. Chen, P.Q. Liao, Y. Yu, et al., Angew. Chem. Int. Ed. 55 (2016) 9375-9379. DOI:10.1002/anie.201603907 |
| [16] |
W.P. Chen, J. Singleton, L. Qin, et al., Nat. Commun. 9 (2018) 1-6. DOI:10.1038/s41467-017-02088-w |
| [17] |
L. Chen, J.Y. Guo, X. Xu, et al., Chem. Commun. 49 (2013) 9728-9730. DOI:10.1039/c3cc43093f |
| [18] |
Q. Lin, J. Li, Y. Dong, et al., Dalton Trans. 46 (2017) 9745-9749. DOI:10.1039/C7DT01978E |
| [19] |
X. He, Y. Liu, Y. Lv, et al., Inorg. Chem. 55 (2016) 2048-2054. DOI:10.1021/acs.inorgchem.5b02372 |
| [20] |
H.R. Wen, J.J. Hu, K. Yang, et al., Inorg. Chem. 59 (2020) 2811-2824. DOI:10.1021/acs.inorgchem.9b03164 |
| [21] |
X.M. Luo, Z.B. Hu, Q.F. Lin, et al., J. Am. Chem. Soc. 140 (2018) 11219-11222. DOI:10.1021/jacs.8b07841 |
| [22] |
X. Yang, D. Schipper, R.A. Jones, et al., J. Am. Chem. Soc. 135 (2013) 8468-8471. DOI:10.1021/ja4031243 |
| [23] |
X.Y. Zheng, Y.H. Jiang, G.L. Zhuang, et al., J. Am. Chem. Soc. 139 (2017) 18178-18181. DOI:10.1021/jacs.7b11112 |
| [24] |
X.J. Kong, Y.P. Ren, W.X. Chen, et al., Angew. Chem. Int. Ed. 47 (2008) 2398-2401. DOI:10.1002/anie.200705731 |
| [25] |
Y.Z. Zheng, M. Evangelisti, R.E.P. Winpenny, Angew. Chem. Int. Ed. 50 (2011) 3692-3695. DOI:10.1002/anie.201008074 |
| [26] |
F.S. Guo, Y.C. Chen, J.L. Liu, et al., Chem. Commun. 48 (2012) 12219-12221. DOI:10.1039/c2cc37510a |
| [27] |
X.H. Miao, S.D. Han, S.J. Liu, X.H. Bu, Chin. Chem. Lett. 25 (2014) 829-834. DOI:10.1016/j.cclet.2014.05.025 |
| [28] |
J.D. Leng, J.L. Liu, M.L. Tong, Chem. Commun. 48 (2012) 5286-5288. DOI:10.1039/c2cc30521f |
| [29] |
J.B. Peng, Q.C. Zhang, X.J. Kong, et al., J. Am. Chem. Soc. 134 (2012) 3314-3317. DOI:10.1021/ja209752z |
| [30] |
N.F. Li, Q.F. Lin, X.M. Luo, J.P. Cao, Y. Xu, Inorg. Chem. 58 (2019) 10883-10889. DOI:10.1021/acs.inorgchem.9b01261 |
| [31] |
J.J. Yin, T.Q. Lu, C. Chen, et al., Cryst. Growth Des. 20 (2020) 4005-4012. DOI:10.1021/acs.cgd.0c00294 |
| [32] |
S. Fan, S.H. Xu, X.Y. Zheng, et al., CrystEngComm 20 (2018) 2120-2125. DOI:10.1039/C8CE00173A |
| [33] |
M. Li, Y. Lan, A.M. Ako, et al., Inorg. Chem. 49 (2010) 11587-11594. DOI:10.1021/ic101754g |
| [34] |
C. Cui, X. He, Q. Lin, X. Luo, Y. Xu, Inorg. Chem. Commun. 90 (2018) 101-104. DOI:10.1016/j.inoche.2018.02.002 |
| [35] |
Q.F. Lin, J. Li, X.M. Luo, et al., Inorg. Chem. 57 (2018) 4799-4802. DOI:10.1021/acs.inorgchem.8b00327 |
| [36] |
D.P. Liu, X.P. Lin, H. Zhang, et al., Angew. Chem. Int. Ed. 55 (2016) 4532-4536. DOI:10.1002/anie.201601199 |
| [37] |
X.Y. Zheng, J. Xie, X.J. Kong, L.S. Long, L.S. Zheng, Coord. Chem. Rev. 378 (2017) 222-236. |
| [38] |
Q. Lin, Y. Zhang, W. Cheng, Y. Liu, Y. Xu, Dalton Trans. 46 (2017) 643-646. DOI:10.1039/C6DT04118C |
| [39] |
H.J. Lun, M.H. Du, D.H. Wang, et al., Inorg. Chem. 59 (2020) 7900-7904. DOI:10.1021/acs.inorgchem.0c00613 |
 2021, Vol. 32
2021, Vol. 32 

