b CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics and National Center for Nanoscience and Technology, Chinese Academy of Sciences, Beijing 100049, China;
c College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China;
d GBA Research Innovation Institute for Nanotechnology, Guangzhou 510700, China
Ever since the mechanical exfoliation of graphene in 2004, there rises great research enthusiasm towards two-dimensional (2D) materials in various fields. Among all 2D materials, transition metal dichalcogenides (TMDCs) have gained tremendous interest due to their versatile properties. TMDCs refer to a huge group of layered materials with the formula of MX2, where M represents a transition metal element (e.g., Mo, W, Re, Ti, Hf, Nb, Ta), and X is a chalcogen (S, Se or Te) [1, 2]. The structural unit of bulk TMDC crystals is a sandwich-like X-M-X layer with X flanking in two external hexagonal planes separated by an internal plane of M [1]. Atoms of the same layer are covalently bonded, while adjacent layers are held by weak Van der Waals forces, thus allowing for the easy fabrication of few-layered TMDC nanosheets [3]. Resulting from the different stacking orders and metal atom coordination, TMDCs exist in two common structural phases, i.e., 1T or 2H phases, which correspond to the octahedral or trigonal prismatic coordination of M [2]. In addition to the abundant chemical composition and structural phase, TMDCs can also be modulated to exist in diverse forms with dimensions from 0D to 3D and layer numbers from multilayer to monolayer [4, 5]. The vast combination of atom composition, phase, dimension and thickness, along with various functionalization strategies collectively contribute to the huge diversity of TMDCs categories and properties, which allows for extensive biomedical applications. Generally, TMDC-based materials show intriguing electronical, optical, thermal, structural and mechanical properties. For example, by decreasing their thickness to a few-layer or monolayer, MoS2 nanosheets undergo a transition from indirect bandgap to the large direct bandgap, which further leads to strong photoluminescence [1]. Along with their merits such as large surface area, planar structures, transparency, flexibility, and ease to modulate and assemble, MoS2 nanosheets are highly desirable for electronical or optical biosensors as well as bioelectronics [4, 6-9]. Moreover, properties including high photothermal conversion, X-ray attenuation ability, good biocompatibility/biodegradability, high loading capacity, and ease of functionalization lay the foundation for TMDCs to act as cancer theranostic platforms, which integrate multifunction containing drug delivery, photothermal therapy (PTT), radiotherapy (RT), photoacoustic imaging (PAI), computed tomography (CT), etc. [10, 11]. Additionally, TMDCs also provide great opportunities for applications such as antibacterial, bone regeneration, radiation protection and treatment of Alzheimer's disease [11-15].
In order to identify and further promote the development of biomedical TMDCs, here we present a bibliometric analysis to explore their biomedical applications. Bibliometric analysis is especially useful when analyzing a mass of literature, which can draw the basic bibliometric landscapes of the development, most prolific and influential countries/institutes, and research focus/hotspots of a certain field [16]. In this work, we firstly provide a general bibliometric analysis towards the publications on biomedical TMDCs, which include the publication and citation trend, the most contributing countries/institutes, research area distribution based on Web of Science (WoS) category, and co-citation analysis of referenced sources. Following, a more detailed investigation of the research focuses/hotspots is conducted via combining the keyword co-occurrence analysis and deep exploration of the obtained dataset. Specifically, biosensing, bioelectronics, cancer theranostics, antibacterial and tissue engineering, are identified as the major applied biomedical contexts of TMDCs, in which the specific functions of TMDCs and their associated properties are stressed. Finally, prospects for designing novel TMDC-related biomaterials and promoting their clinical translation are proposed to shed light on the maximum exploration of TMDCs for biomedical applications.
2. Dataset and methodologyOur dataset was constructed via a two-step strategy, in which comprehensive search was performed using the advanced search of WoS core collection database (concrete retrieval strategy and results are presented in Table S1 in Supporting information), followed by a thorough screening process by checking the title and abstract parts of the retrieved literature manually. During the seriatim filtration, we excluded those off-topic publications which focus on other materials or TMDCs applied in non-biomedical fields such as the energy field. It is worth noting that meeting abstracts were excluded as well due to their incompleteness. Hence, 1387 records were obtained as our final dataset, which consists of 1193 articles and 194 reviews.
When it comes to bibliometric analysis towards the dataset, native functions of the WoS core collection database and bibliometric analytical software VOSviewer were harnessed. VOSviewer implements the visualization of similarities mapping technique to draw association landscapes of input publications by diverse parameters such as countries, institutes, journals, keywords, cited reference [17]. It can perform various types of analysis including co-authorship, co-occurrence of keywords, co-citation of cited journals/authors/references, etc. Herein, the WoS core collection database was used to generate root data of annual publication and citation statistics, and WoS category, which can shed light on the development of the TMDC-based biomedical field over the years and the major disciplinary involved in this field, respectively. WoS core collection database also produced the original files for VOSviewer, by which these files are further processed to visualize the bibliometric distributions by countries, referenced sources and author keyword co-occurrence.
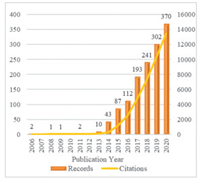
3. Annual publication and citation trendsTo investigate the development of TMDCs in the biomedical field, the annual publication and citation trends were detected and shown in Fig. 1. Generally, the last decade has witnessed the tremendous growth of TMDCs for biomedical applications. Despite TMDCs have a long and fruitful history that can be dating back to 1923 [2], employing TMDCs in the biomedical area did not appear until 2006, followed by a hover in the next 6 years. During this period, the studied TMDCs in the biomedical field were mostly in the forms of fullerene-like structures and nanotubes, as monolayer TMDCs could restack and scroll into these forms [18]. Followingly, there is a rapid expansion of TMDCs applied in the biomedical domain from 2013 to 2017, which may be attributed to the fact that stable 2D TMDC nanosheets can be fabricated in a facile and controllable manner, and their properties have been extensively explored. In more recent years, the development of TMDCs for biomedical applications has entered a steady increase stage. The highest annual publications and citations of the TMDC-based biomedical field are found in 2020, which indicates the increasing attraction of TMDCs to the biomedical scientific community.
|
Download:
|
| Fig. 1. The annual publication and citation growth of literature regarding TMDCs for biomedical applications. | |
To figure out the most productive and influential countries/regions as well as their cooperation network in the biomedical area based on TMDCs, we provide the general bibliometric statistics and co-authorship analysis of countries/regions. As shown in Table S2 (Supporting information), China holds a dominant lead in the rankings based on records and total citations (861 records, 23, 846 citations), followed by the USA (180 records, 6473 citations). In the average citation rank, Australia rises to 1st place (75.7 citations per record), followed by Singapore (72.1) and the UK (39.6). Since average citation equals total citation divides record, both higher total citation and lower record than the average level could result in a higher average citation value. Therefore, when identifying the most contributing countries, records, total citations and average citations should be combined to draw an impartial conclusion. In this sense, Singapore is identified in the contribution to the TMDC-based biomedical community from the perspectives of both quality and quantity.
As for the collaboration network among all 57 countries/regions, VOSviewer identified 5 clusters in different colors. The presence of a link between two countries indicates that they have a cooperation association, and the thicker the link is, the stronger collaboration they hold. Fig. S1 (Supporting information) reveals that China is at the center of the international cooperation network, linking with other 32 countries. Among them, the USA has the densest co-authorship association with China, followed by Singapore. These three countries are the major forces in the most contributing incorporation cluster (colored in blue) to the TMDC-based biomedical field. The second most contributing cooperation group lies in the green cluster, in which India and South Korea are the main drivers. As for the red cluster, it has the largest number of countries, which possess similar publication outcomes and are mostly European countries. The red and yellow clusters contain counties mostly from Europe, indicating their close collaboration with each other.
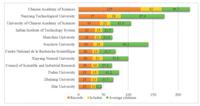
5. The most prolific and influential institutesTo identify the most contributing institutions in the TMDC-based biomedical field, we conduct an analysis of institutes with at least 25 publications from the perspective of productivity and scientific influence. As indicated in Fig. 2, the Chinese Academy of Sciences occupies the 1st position in record rank and h-index rank (125 records and h-index 42), followed by Nanyang Technological University (57, 28). H-index refers to the common maximum of both publications and citations of a certain institute/country/author. That is to say, a high h-index value demands for both high records and total citations possessed by the same entity. For example, if a researcher's h-index is 20, then it represents the scholar has at least 20 pieces of literature, each of which is cited at least 20 times [16]. As for the average citation rank, Soochow University stands out in this rank, indicating its average scientific value of the whole publications is high. To obtain a more thorough picture of institutes' contribution, records, h-index and average citations should be combined. It turns out that the Chinese Academy of Sciences, Nanyang Technological University and Soochow University are recognizable institutions in the TMDC-based biomedical area.
|
Download:
|
| Fig. 2. The most contributing institutes in the TMDC-based biomedical area. | |
Fig. 3 provides a list of the top 15 largest WoS categories of publications on TMDCs for biomedical applications. WoS categories are labels designated by the WoS database which suggest the general research areas/subjects of certain publications [17]. Therefore, detecting WoS categories of the retrieved dataset can give us a snapshot of their research subjects. As shown, Nanoscience Nanotechnology possesses the most publications (496), followed by Materials Science Multidisciplinary (487), which indicates the significant role of nanotechnology and the multidisciplinary nature of the biomedical research based on TMDCs. When it comes to their specific disciplines, chemistry, physics and biology are the three main pillars, in which categories like Chemistry Analytical, Electrochemistry, Physics Applied, Instruments Instrumentation, Biophysics and Optics deserve additional attention. This is consistent with the major applied biomedical contexts of TMDCs. For example, TMDCs are widely studied to develop various electrochemical and/or optical biosensors for the analysis of various biomarkers and bioelectronic devices for healthcare, which requires solid knowledge foundations of analytical chemistry, electrochemistry, optics, biophysics, applied physics, etc.

|
Download:
|
| Fig. 3. The top 15 WoS categories of literature on TMDCs for biomedical applications. | |
Co-citation analysis of referenced sources aims to identify the most influential journals that are cited by publications in the retrieved dataset. The co-citation relationship is recognized when two journals were cited together by the same literature [17]. Conducting such analysis can not only identify the core journals for researchers who are interested in the biomedical applications of TMDCs, but also provide insights into the research topics of literature, some of which can be simply reflected by the journal name [17]. As presented in Fig. S2 (Supporting information), the referenced sources are divided into 3 clusters marked in different colors. The circle size and brightness are suggestive of their citation times. For the blue cluster, Biosensors & Bioelectronics, Sensors and Actuators B-Chemical are the top 2 influential journals, which indicates the promising application of TMDCs as biosensors. The red cluster possesses journals in the material science and nanoscience area, which are represented by Advanced Materials, ACS Nano, ACS Applied Materials & Interfaces and Nanoscale, etc. This is simply due to that nanotechnology and material science are the foundation of the field of TMDCs for biomedical applications as we pointed out in Section 6. When it comes to the green cluster, there are many journals with respect to physics. This can be attributed to the fact that the biomedical applications of TMDCs are grounded on a deep understanding of their physical properties. In addition, journals on optics appear a great deal, such as Nature Photonics and Optics Express, which indicates that the optical properties of TMDCs are appealing to the biomedical scientific community.
8. Research hotspots in the TMDC-based biomedical filedAs indicated by the results of the WoS category and journal analysis, one can obtain a rough understanding of the research focuses in the TMDC-based biomedical field. To get more sense into that, an informative analysis towards the biomedical TMDCs is presented, which combines keyword co-occurrence analysis with the seriatim sorting and deep exploration of the dataset.
Keywords designated by authors imply the core content of literature. Therefore, performing keyword-related statistics towards a body of literature on a certain topic can offer useful insights into the hotspots of this research area [19]. To this end, a co-occurrence landscape of author keywords is given in Fig. 4. After performing the word clean-up and setting the minimum occurrence at 3, 220 out of 2265 author keywords are exhibited in 5 clusters with different colors. Node size reflects the occurrence frequency of terms. The larger a node is, the more frequently a term occurs. As indicated, the top 8 most frequently occurred author keywords are MoS2 nanosheets (occurred 346 times), biosensor (132), 2D materials (126), TMDCs (102), electrochemical biosensor (EC biosensor, 74), graphene (63), photothermal therapy (PTT, 62), and surface plasmon resonance (SPR, 60).

|
Download:
|
| Fig. 4. Co-occurrence analysis of author keywords with at least 3 occurrences. Node size indicates the occurrence frequency; Node color represents the cluster; Cluster resolution = 0.99. | |
By taking a closer look at this network visualization, common denominators among the items in each cluster become evident. Firstly, it is not difficult to find that the clusters colored in red and green share the same research topic, i.e., TMDCs applied in the biosensing field. As indicated, the most widely studied TMDCs for biosensing are MoS2 nanosheets, followed by WS2 nanosheets. Owing to the intriguing properties of TMDCs, these versatile nanomaterials can be used for different functions in different types of biosensors [6, 20], among which electrochemical biosensors, field-effect transistors (FET) biosensors, SPR biosensors, photoelectrochemical biosensors and fluorescent biosensors are extensively explored. For electrochemical biosensors, the main roles of TMDCs encompass: (1) act as the modifying materials of electrodes for signal amplification due to their desirable electronic properties including high carrier mobility and tunable bandgap [6, 21]. However, suffering from the relatively low electrical conductivity, TMDCs alone are not favorable as electrode materials. To this end, they have been cooperated with other promising materials such as graphene or gold nanoparticles to achieve better performance [22, 23]; (2) provide a large surface area for the biorecognition events that occurred between bioreceptors and analytes for enhanced sensitivity [24, 25]; (3) exert electrocatalytic functions by virtue of their enzymic mimetics activity (mostly the peroxidase-like activity) [26-29]. As for FET biosensors, semiconducting TMDCs are highly desirable as the sensing-channel materials for FET biosensors [30]. This can be attributed to their properties such as large band gap, high current on/off ratio, low leakage current, atomic-level thickness, pristine surface and large surface area [30-32].
Besides favoring the electrochemical biosensors, TMDCs also open great opportunities for various optical biosensors including fluorescent biosensors and SPR biosensors [4, 33-35]. In the cases of fluorescent biosensors, TMDCs can function as both fluorophores and fluorescent quenchers depending on their dimensions and thickness, which lead to distinct properties. On the one hand, monolayer TMDC quantum dots exhibit strong photoluminescence due to the enhanced quantum confinement and edge effects. Besides, quantum dots possess size-tunable emission, broad absorption spectra and robust photostability, which collectively make them outstanding fluorescent probes for biosensing and bioimaging [20, 36, 37]. On the other hand, 2D TMDC nanosheets have validated their effectiveness for being fluorescent quenchers. Their intrinsic quenching ability mainly results from the physical adsorption of fluorogenic probes onto the large basal plane of TMDC nanosheets. Among all quenching strategies, relying on the different affinity of TMDC nanosheets to single-stranded nucleic acid-based fluorescent probes versus the double-stranded counterparts is the most commonly adopted one [38-40]. Additionally, benefiting from their planar structure, nanoscale thickness, high refractive index (RI) and light absorption efficiency, TMDCs have received much interest in SPR biosensing. In this scenario, TMDCs are selected as dielectric coatings on the metal surface of SPR sensors, which greatly improve their sensitivity towards the minor alternations in RI when biorecognition events occur [34, 41, 42]. Collectively, these TMDC-related biosensors with various transducing mechanisms have been explored to detect a broad range of analytes including H2O2, glucose, cancer protein biomarkers such as carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA), microRNA, DNA, uric acid and pathogens including virus and bacteria, which play significant roles in disease diagnosis and life science research. Nowadays, as the SARS-CoV-2 virus rages on throughout the world, rapid and accurate diagnosis of the virus is of great significance to control the threatening global pandemic. TMDC-based biosensors are believed to play important roles in facilitating rapid and sensitive diagnosis of COVID-19 [43]. For instance, a near-infrared (NIR) SPR biosensor-based tellurene-MoS2–COOH heterostructure was created to rapidly and accurately detect the S glycoprotein of the SARS-CoV-2 virus [44]. Here, MoS2–COOH can not only enhance the detection sensitivity of SPR biosensors, but also capture angiotensin-converting enzyme Ⅱ, which serves as specific bioreceptors for S glycoprotein. What is more, TMDC-derived solid nanopore is a good alternative to protein nanopore and can be applied for next-generation sequencing and virus DNA/RNA detection, which also contributes to the combat with COVID-19 [45].
In addition to traditional biosensors, TMDCs can also serve as novel flexible mechanical sensors and gas sensors, which lay foundations for wearable and implantable bioelectronics [9]. Bioelectronics is a highly interdisciplinary subject involving biology, electronics, chemistry, physics, and information technology, which expects versatile properties towards the employed materials [46]. Due to their high mechanical flexibility, transparency, ease of modulation and integration, large surface area, superior electric and optical properties, TMDCs are highly desirable for bioelectronic devices, which facilitate not only personalized daily health monitoring but also early diagnosis, treatment feedback and recovery management of diseases, etc. [9, 47]. Specifically, TMDCs have gained great attention as flexible strain or pressure sensors, which detect physical signals such as heartbeat, intracranial pressure, cellular motion and limb motions, and thus play great roles in e-skin, biorobots, wearable devices and healthcare applications including respiration monitoring or rehabilitation guidance [48-52]. These mechanical sensors exhibit conformal flexibility, high transparency, excellent piezoresistivity and good repeatability, which result from the atomic thickness, chemical stability and piezoelectric properties of TMDCs [51, 53]. Besides detecting various physical signals, TMDC-based bioelectric devices can also perceive subtle chemical variations of physiology-related environments such as human breathing gas, sweat, saliva, urine [54-59]. Owing to their large surface area and almost complete exposure of atoms, TMDCs show great adsorption capability towards gas molecules. Along with the excellent electrochemical properties, they are highly appealing for gas sensing [55]. In the biomedical field, TMDCs have been widely studied to detect humidity, ammonia, acetone and other volatile organic compounds for human breath analysis, which open up opportunities for healthcare applications such as asthma management, early diagnosis of diabetes and lung cancer [56, 57, 60, 61].
Apart from the above flexible biosensors, TMDCs are also highly attractive as power supply and storage devices (e.g., nanogenerators and supercapacitors), which are of great importance for self-powered long-term human health monitoring systems [52, 62, 63]. For example, MoS2-based nanomaterials have been reported as ultrasensitive piezoelectric and triboelectric nanogenerators to harvest environmental or human mechanical energy [64-66]. Considering the versatility of TMDCs in biosensing, flexible devices, power supply and storage devices, they are highly envisioned to be suitable platforms for self-powered, automated, portable, user-friendly point of care devices when integrated with technologies such as microfluidics, paper strips and smartphone readouts [67]. However, there are still numerous challenges to overcome so as to fulfill this ambition. For example, the present biosensors suffer from complex sensing mechanisms, relatively poor uniformity in preparation, unsatisfactory performance in clinical samples, incapability to conduct multiplex sensing, etc. [67, 68]. Besides, the integration of diagnostic biosensors with wearable devices and/or smartphones requires deeper exploration. The achievement of future intelligent healthcare systems calls for cooperation from different fields including microelectronics, microfluidics, device manufacturing, computer programing, chemistry, physics and biology [67].
Benefiting from their ultrathin thickness, flexibility, biocompatibility and optoelectronic properties such as photo-absorption ability and photoresponsivity, TMDCs are great candidates for soft optoelectronic devices. For instance, photo-active semiconducting MoS2 nanosheets integrated with electrically conducting graphene have been reported as efficient and biocompatible photodetectors (Fig. S3 in Supporting information), which pave the path for the implantable retinal prosthesis to fight against retinal degenerative diseases [69, 70]. To conclude, TMDCs with favorable mechanical, flexible, electronical and optical properties provide great opportunities for traditional biosensors, wearable or implantable devices and intelligent healthcare systems.
The second-largest TMDC-based biomedical application lies in the blue cluster. It is obvious that TMDCs for cancer theranostics is its research focus, as validated by the frequently occurred keywords like PTT, nanomaterial, drug delivery, cancer, theranostics, synergistic therapy, RT, photodynamic therapy (PDT), chemodynamic therapy (CDT), bioimaging, PAI, CT, magnetic resonance imaging (MRI), etc. Theranostics refers to the combination of imaging techniques with therapeutic modalities, which holds great promise for precise and efficient cancer diagnosis and treatment [71]. Benefiting from their versatile properties including high photoabsorption ability, photothermal conversion efficiency and photostability, TMDCs are superior candidates for cancer theranostics, especially for PAI-guided PTT. However, to obtain a better cancer eradicating capability, the photothermal performance of TMDC-based platform should be further improved. Over the years, researchers have developed various strategies aimed at improving TMDCs' photothermal traits. For example, besides the most representative MoS2 nanosheets, TMDCs with novel chemical compositions such as WS2, WSe2, MoSe2, TiS2, ReS2, have been explored for PAI and/or PTT [72-76]. In addition, modulating the morphology of TMDCs is regarded as another useful strategy to promote their theranostic performance. Apart from regular nanosheets, TMDC-based nanodots, nanorods, flower-like nanoflakes, hollow spheres, radar-like nanoparticles, etc. have been reported to date. Distinct morphology can have great impacts on their surface-to-volume ratio and strain-related defects, thus enhancing their photothermal conversion efficiency [5, 77-79]. Furthermore, integration of semiconducting TMDCs with noble metals to form plasmonic nanoagent [80], construction of TMDC-based composite/hybrid [10, 81] and functionalization with coatings such as polydopamine [82] have also shown effectiveness in promoting PAI/PTT efficacy. Similarly, the good inherent optical and/or electronical properties such as great photo-harvest ability and strong photoluminescence make TMDCs suitable for PDT and fluorescence imaging (FI) applications [83-85]. Upon light irradiation, TMDCs with a narrow bandgap and good photoelectric conversion ability can be activated and react with the surrounding oxygen to form singlet oxygen, and thus serve as inherent photosensitizers [85, 86]. However, pristine TMDCs suffer from relatively poor ROS generation efficiency and inherent ROS scavenging capability due to their abundant defects, unpaired electrons and edge sites [15]. Therefore, pristine TMDCs are not perfectly suitable for PDT, and thus additional functionalization is required in order to improve their PDT performance. Besides their promising thermal, optical and electronical attributes, TMDC-based nanomaterials provide great opportunities for CT and/or RT. This application can be attributed to their containment of high atomic elements such as Re (75), W (74), Ta (73) and Mo (42), which endow them with superior X-ray attenuation capability, thereby enhancing their CT/RT performance [73, 87-90]. Given all this, versatile TMDCs are highly appealing as multifunctional platforms for combinational therapy, which can be achieved without relying on other additional materials. By far, PTT/RT has been identified as one of the reasonable paradigms of W or Re element containing TMDC-based nanocomposite [10, 91, 92]. More recently, potent anticancer effects on both primary and distant cancer cells have been achieved by further combining PTT/RT with immunotherapy [93, 94]. For instance, Dong et al. recently reported the combinational PTT/RT/checkpoint blockade immunotherapy (CBT) strategy based on WO2.9-WSe2-PEG NPs (WSP). In detail, WSP possess strong photothermal ability to kill radioresistant cancer cells and meanwhile improve the tumor oxygenation to further facilitate RT. Moreover, WSP with high atomic W elements exhibit high X-ray attenuation ability which can overcome PTT's penetration depth limitation and kill tumor cells in deep sites. Together with the heterojunction structure, it can effectively catalyze hydrogen peroxide in the tumor microenvironment under X-ray irradiation, thus achieving enhanced RT. Notably, both ROS generated by RT and hyperthermia derived from PTT can enhance tumor immunogenicity and hence benefit CBT. Meanwhile, CBT can offset the weakness of RT/PTT towards combating distant cancer cells. Finally, this strategy fulfilled the eradication of both local and metastatic tumors with lower radiation and NIR light dose and high anticancer efficiency (Fig. S4 in Supporting information).
Apart from making use of TMDCs' intrinsic properties, TMDC-related materials have expanded their applications to MRI, positron emission tomography (PET), PDT, chemotherapy and gene therapy by loading other contrast agents, photosensitizers, drugs or gene [95-98]. It has been verified that TMDCs are excellent nanocarriers for delivery applications owing to their large surface area and ease of functionalization. What is more, their good photothermal property allows for NIR- or heat-responsive release of cargos, thus achieving precise cancer treatment [97, 99]. By taking advantage of the favorable attributes of both the loaded cargos and TMDCs, TMDC-related materials can yield desirable synergistic theranostic performance. For instance, researchers have achieved MRI/PAI/CT-directed PTT/RT by doping Gd ions, integrating iron oxide nanoparticles or manganese dioxide with TMDCs [98, 100]. Besides, studies have reported that radioisotopes such as 64Cu and 89Zr can be stably labeled onto the surface of TMDCs via chelator-free approaches with high labeling yield, thus facilitating PET imaging [96, 101]. Furthermore, due to TMDCs' photothermal properties and chalcogen atoms, they have been widely explored as external stimuli (e.g., NIR) or internal stimuli (e.g., glutathione (GSH)) triggered drug/gene delivery systems for controllable chemotherapy or gene therapy [97, 102]. However, the additional functionalization of various contrast agents onto the surface of TMDCs would complicate their fabrication process, which, together with the leakage risks of the loaded theranostic agents especially via physical absorption, sets up barriers for their rapid and consistent industrial production and thus the clinical implementation [3]. In a nutshell, regardless of the above challenges, versatile TMDCs still hold great promise for multimodal imaging-guided synergistic cancer theranostic, in which the imaging techniques include PAI, CT, MRI, PET, FI, etc., and the therapeutic modalities encompass PTT, RT, PDT, chemotherapy, gene therapy, immunotherapy, etc. What is more, considering TMDCs' outstanding performance in the biosensing and theranostics applications, it is expected to fabricate diagnostic devices integrated with these two functions at the same platform, which greatly facilitate the intelligent diagnosis, treatment and monitor of diseases such as cancer [68].
As for the yellow-colored cluster, it mainly focuses on the antimicrobial (mostly antibacterial) applications of TMDCs. As displayed, the frequently occurred keywords include antibacterial, functionalization, photocatalysis, reactive oxidative species (ROS), photothermal, antimicrobial, peroxidase, nanozymes, antibiotics, etc. Due to the increasing amount of superbacteria and slow development of antibiotics, it is of urgent demand to develop novel bactericidal agents to combat bacterial infection, which has become one of the greatest threats to public health. TMDC-based biomaterials have exhibited great potential as alternative antibiotics. To date, researchers have recognized several bactericidal mechanisms of TMDCs, among which membrane stress and oxidative stress are widely acknowledged in the scientific community [12, 13, 103]. Membrane stress refers to the physical damage of bacterial wall/membrane caused by the sharp edges of TMDC nanosheets, which is also known as the nanoknife effect. This effect would result in the leakage of intracellular components and thus kill bacteria. The enhanced nanoknife effect can be achieved by rationally arranging the alignment of TMDC nanosheets. It has been reported that vertically aligned TMDCs onto other substrates possess higher antibacterial capability than their randomly aligned counterparts [104].
Oxidative stress is another well-recognized antibacterial mechanism of bactericidal TMDCs, which can be further divided into ROS-independent and ROS-dependent oxidative stress [13, 105]. In the former mode, TMDCs can directly oxidize cellular components or membranes without producing ROS, which has been verified to be the dominant mechanism of metallic 1T-TMDCs [105]. The oxidization capability is mostly derived from their facilitation of the charge transfer process between TMDCs nanosheets and bacteria structures [12, 105]. In this regard, improving the conductivity of TMDCs has been reported to elevate their oxidation antibacterial performance [12, 106]. As for the later mode, TMDCs can produce highly toxic ROS such as superoxide anions and peroxide to damage bacterial structures/components and thus kill them, which is reported to be the major antibacterial mechanism of semiconducting 2H-MoS2 nanosheets [103]. Besides relying on their inherent ROS generation ability, photo-enhanced ROS production of TMDCs has shown higher antibacterial efficiency. Semiconducting TMDCs with unique photoelectric properties can serve as photocatalysts to generate hole-electron pairs upon light irradiation, which can further react with the surrounding H2O and O2 to yield ROS and thus defeat bacteria [107, 108]. The photocatalytic capability is tightly related to the bandgap, thickness and size of TMDCs. Strategies such as reducing thickness, fabricating quantum dots, introducing sulfur vacancies and forming heterojunctions with other materials can enhance TMDCs' photocatalytic capability via facilitating the separation of electron-hole pairs [107-109]. Additionally, due to their favorable photoelectric conversion ability and large surface area, TMDCs can serve as instinct photosensitizers or nanocarriers loaded with other photosensitizers, thus achieving photodynamic antibacterial [110, 111]. Furthermore, TMDCs have been found to possess the intrinsic peroxidase-like ability, which can decompose H2O2 to generate •OH and thus exhibit the bactericidal effect [112, 113]. This nanozyme nature is derived from the catalytic ability of the active edges of TMDCs. Therefore, strategies with improving the exposure of active edges via introducing defects or doping nitrogen elements have shown enhanced nanozyme activity and bactericidal efficiency [113-115].
In addition, PTT based on the superior photothermal property of TMDCs has also been applied for antibacterial application [116]. The NIR-induced hyperthermia can damage bacteria via various thermal effects, such as breaking down cell membranes or denaturing proteins/enzymes [111]. What is more, PTT-induced heat can promote the oxidation of GSH, which can greatly improve the sensitivity of bacteria to ROS. In this regard, synergistic strategies such as PTT/photocatalytic therapy, PTT/nanozyme catalytic therapy and PTT/PDT have shown promising antibacterial efficiency [108, 111, 112]. For example, a latest study based on photo-responsive chitosan/Ag/MoS2 coating for rapid antibacterial has been reported. The good bactericidal capability can be attributed to the enhanced ROS generation due to the improved separation of photo-inspired electrons from MoS2 to Ag nanoparticles, positively charged chitosan surface, and photothermal performance of MoS2 (Fig. S5a and b in Supporting information) [108]. Benefiting from their large surface area, TMDCs can also function as delivery systems for various antibiotics, thus achieving PTT/chemotherapy for bacteria combat [117]. Collectively, versatile TMDC-based nanomaterials open up futuristic avenues for defeating antibiotic-resistant infections. Nevertheless, to maximize the antibacterial potential of TMDCs, several inevitable questions must be answered first, which include but are not limited to (1) the comprehensive understanding of nano-bacteria interaction and antibacterial mechanisms; (2) optimization of TMDCs' properties for "antibacterial-by-design" based on the full understanding of question (1); (3) exploration of the optimal combination for synergistic antibacterial, etc. [13].
When it comes to the purple-colored cluster, it indicates that TMDC-related nanocomposites can also be explored for regenerative medicine or tissue engineering. For bone regeneration, they not only serve as reinforcing agents with other polymers due to their excellent mechanical, flexible and chemical properties [118, 119], but also show positive biological effects on bone stem cells. Several studies have reported that MoS2-related nanofibrous scaffolds can promote the cell attachment, proliferation and differentiation of pre-osteoblasts and rat bone marrow mesenchymal stem cells [14, 120]. Their promotion on cell adhesion is mostly derived from the topographical property, which can increase the surface roughness, thus providing more anchor sites for cells to adhere to [121]. Additionally, whole-transcriptome sequencing of human stem cells indicated that MoS2-induced heat under NIR irradiation can upregulate the expression of cellular adhesion-related genes [122]. For the improvement in cell growth and differentiation, it can be attributed to the fact that the Mo element can upregulate the expression of osteogenic-related genes [123-125]. For instance, Wang et al. fabricated an integrated system by combining MoS2 nanosheets with 3D printed bioactive borosilicate glass scaffolds to yield MoS2-integrated borosilicate glass (BGM) scaffolds for combined bone cancer treatment and bone repairment [123]. MoS2 nanosheets serve as not only photothermal agents for cancer eradication but also inducers for osteogenic-related genes, as validated by in-vitro assessment of the osteogenic ability of BGM scaffolds (Fig. S5c and d in Supporting information). Similarly, for cardiac tissue engineering, besides taking advantage of their mechanical, topographical and electrical properties, MoS2 nanosheets can facilitate the attachment, maturity and differentiation of human-induced pluripotent stem cells due to their upregulated effects on cardiac functional genes [126]. Therefore, TMDCs-based materials hold great promise in tissue engineering.
9. Conclusion and prospectsThe last decade has witnessed the burgeoning development of TMDCs in the biomedical field. Benefiting from their versatile structural, mechanical, electronical, optical, thermal and chemical properties, TMDCs have received tremendous attention for applications such as conventional and flexible biosensors, cancer theranostics, antibacterial, and tissue engineering. Specifically, what makes TMDCs so appealing is that they are naturally suitable as multifunctional platforms. This derives from not only their versatile and tunable intrinsic properties, but also the giant TMDC family group, ease of functionalization, and easy integration with other superior materials. For instance, it has been demonstrated that TMDC-based materials hold great promise for multimodal imaging (e.g., PAI, CT, MRI, PET) guided synergistic therapy (e.g., PTT, PDT, CDT, RT, chemotherapy, gene therapy) for efficient and personalized cancer theranostics. So far, the biomedical research of TMDCs has been mostly concentrated on MoS2 and WS2 nanosheets, with inadequate research on other attractive TMDCs such as MoSe2, WSe2, TiS2, ReS2. Moreover, there are also plentiful TMDC members remaining unstudied or even un-synthesized. Hence, one of the research directions is to fabricate novel TMDCs with various properties such as phase, lateral size and thickness, and then explore their potential bio-applications [121]. Additionally, constructing versatile TMDC-based platforms with other materials in an ingenious manner is also highly appreciated. Furthermore, the possibility of utilizing TMDC nanosheets to form 3D matrixes, which obtain intriguing properties by increasing the available defects/vacancies/edges and harnessing the advantages of both micro and nanostructure, is largely overlooked, and thus remains another appealing research direction [11]. In addition, TMDCs also show potential application in several rarely explored biomedical fields such as gas therapy and antivirus [127]. Owing to their large surface area, containment of rich defects/vacancies and active edge sites, TMDCs in various forms including nanoparticles, monolayer sheets and quantum dots have been reported for catalyzing hydrogen evolution reaction to efficiently produce hydrogen [128-130]. The generated hydrogen via TMDCs catalysis can be used for hydrogen gas therapy to fight against various diseases resulted from oxidative stress such as cancer, inflammation, cardiovascular and nervous system diseases by depleting toxic hydroxyl radical but sparing other beneficial ROS [127, 131]. Hence, there remains much room to explore TMDC-based hydrogen gas therapy. What is more, TMDCs have been widely explored as virus biosensors, however, their antivirus applications are still poorly understood. A recent study compared the antibacterial and antiviral performance of graphene oxide and MoS2 nanosheets, which turned out MoS2 nanosheets possess lower antiviral capability [132]. Nevertheless, research on TMDCs for antiviral application is in its vacancy and thus represents a novel research direction.
Despite TMDCs have great potential in promoting human health, there are still hurdles to be tackled before their clinical implementation. First of all, similar to all the other medical supplies, the biosafety issue of TMDCs is of utmost importance to their successful clinical translation. By far, numerous in-vitro and in-vivo studies have primitively proven the favorable biocompatibility of various TMDCs to diverse living cells and mice/rats [50, 133-135]. However, several molecular dynamics simulations found that MoS2 nanosheets possess robust denaturation capability towards various proteins [136, 137]. Besides, although MoS2 and WS2 nanosheets elicited no cytotoxicity to macrophages, they stimulated macrophages to secret proinflammatory cytokines [138, 139]. Clearly, preclinical toxicological studies at both in-vitro and in-vivo levels are far from enough to draw a meaningful conclusion on TMDCs' biosafety. For ex-vivo studies, toxicity evaluations on various somatic cells from the potential exposed organs should be carried out instead of excessively relying on studies using tumor cell lines. Whereas for in-vivo tests, long-term investigations of TMDCs' biokinetics, bioeffects on the accumulated organs, immunogenicity, biodegradation, excretion, specific organ toxicity such as neurotoxicity, reproductive and development toxicity, and cellular or molecular mechanisms of toxicity are urgently required. Upon introducing into physiological environments, TMDCs may undergo two kinds of transformation. Firstly, TMDCs with different physicochemical properties can absorb serum proteins and form diverse protein coronas, which greatly affect their biofates and toxic effects [140]. Hence, further research on the role of protein coronas in bridging TMDCs' properties and the induced biological responses should be carefully explored. Secondly, different TMDCs obtain biodegradability to varying degrees. For example, MoS2, VS2, CoS2 possess rather quick degradation kinetics which relies on biofluid pH, H2O2, enzymes such as catalase and myeloperoxidase and their inherent properties, whereas WS2 and TiS2 nanosheets undergo extremely slow degradation [134, 135, 141, 142]. Since biodegradability is of great importance to the application scenario, toxicity mechanism and biosafety of TMDCs, it is urgent to design relevant bio-models either using cells or animals, rather than only relying on artificial fluids to test their biodegradability. This helps to identify the relationship between TMDCs' properties and the degradation kinetics, which ultimately facilitating the on-demand degradation of TMDCs for different biomedical applications. In addition, it is worth noting that when conducting these toxicological studies, the administrated dose of TMDCs should be equivalent or relative to the realistic application scenarios to avoid the false toxicity due to overdoes. Moreover, since contamination of TMDCs by endotoxins, exfoliated agents and stabilizing surfactants can also cause hazards to cells/animals, it is important to figure out how to bypass those impurities or contaminations before toxicity assessment [143, 144]. To conclude, the ultimate goal of toxicological research is to bridge the biological effects of TMDCs with multiple toxic-related factors including TMDCs' properties and dosage regimen, thus providing more desirable TMDC-based materials via "safety-by design" strategy.
Besides biosafety guarantee, the commercial implementation of TMDC-related medical products also requires the achievement of large-scale, cost-effective, repeatable and controllable synthesis of TMDCs [121, 145]. The prepared TMDCs should possess controllable lateral size, thickness, phase and surface chemistry since trivial iterations of these key properties can significantly impact the biomedical functions and biosafety of TMDCs [8]. Furthermore, the synthesis methods are expected to modulate the key properties of TMDCs in a separate manner, which is highly desirable for investigating the relationship of a certain property with their biological responses. Besides the intra-batch uniformity, high consistency between batches should also be reached to avoid device-to-device variation [8]. Current preparation strategies of TMDC include top-down approaches such as liquid-based exfoliation and bottom-up methods like chemical vapor deposition. These preparation strategies have their own merits and shortcomings. For example, chemical exfoliation suffers from low crystal quality, poor uniformity in thickness and size, and toxic residual solvents, yet it can provide large-scale production of few-layered TMDC nanosheets [3, 8]. In this regard, developing synthesis methods with high yield, control of TMDCs' properties, reproductivity and low cost is highly appreciated. For biomedical devices relying on the integration of TMDCs with other materials, assemble process should also be carefully designed to avoid introducing undesired changes in TMDCs' structures/properties.
To sum up, TMDCs with good biocompatibility and versatile properties have opened up considerable opportunities for various biomedical applications including biosensing, bioelectronics, cancer theranostics, antibacterial, tissue engineering, etc. Despite of big challenges such as thorough toxicity evaluation and industrial production, TMDCs still hold tremendous promise for biomedical purposes. Further endeavor and collaboration from chemists, material scientists, toxicologists, physicians and physicists are calling on to maximize the potential of TMDCs in the biomedical field.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB36000000); the National Basic Research Program of China (Nos. 2020YFA0710702 and 2016YFA2021600); the National Natural Science Foundation of China (Nos. 51822207, 51772292 and 11621505); Chinese Academy of Sciences Youth Innovation Promotion Association (No. 2013007) and CAS-Iranian Vice Presidency for Science and Technology Joint Research Project (No. 113111KYSB20190067).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.cclet.2021.04.023.
| [1] |
Q.H. Wang, K. Kalantar-Zadeh, A. Kis, et al., Nat. Nanotechnol. 7 (2012) 699-712. DOI:10.1038/nnano.2012.193 |
| [2] |
S. Manzeli, D. Ovchinnikov, D. Pasquier, et al., Nat. Rev. Mater. 2 (2017) 17033. DOI:10.1038/natrevmats.2017.33 |
| [3] |
Z. Li, S.L. Wong, Mater. Sci. Eng. C: Mater. Biol. Appl. 70 (2017) 1095-1106. DOI:10.1016/j.msec.2016.03.039 |
| [4] |
S. Barua, H.S. Dutta, S. Gogoi, et al., ACS Appl. Nano Mater. 1 (2018) 2-25. DOI:10.1021/acsanm.7b00157 |
| [5] |
T. Liu, Y. Chao, M. Gao, et al., Nano Res. 9 (2016) 3003-3017. DOI:10.1007/s12274-016-1183-x |
| [6] |
X.R. Gan, H.M. Zhao, X. Quan, Biosens. Bioelectron. 89 (2017) 56-71. DOI:10.1016/j.bios.2016.03.042 |
| [7] |
S. Singh, P.K. Singh, A. Umar, et al., Micromachines (Basel) 11 (2020) 779-806. DOI:10.3390/mi11080779 |
| [8] |
A. Bolotsky, D. Butler, C. Dong, et al., ACS Nano 13 (2019) 9781-9810. DOI:10.1021/acsnano.9b03632 |
| [9] |
C. Choi, Y. Lee, K.W. Cho, et al., Acc. Chem. Res. 52 (2019) 73-81. DOI:10.1021/acs.accounts.8b00491 |
| [10] |
S.G. Wang, X. Li, Y. Chen, et al., Adv. Mater. 27 (2015) 2775-2783. DOI:10.1002/adma.201500870 |
| [11] |
V. Agarwal, K. Chatterjee, Nanoscale 10 (2018) 16365-16397. DOI:10.1039/C8NR04284E |
| [12] |
X. Yang, J. Li, T. Liang, et al., Nanoscale 6 (2014) 10126-10133. DOI:10.1039/C4NR01965B |
| [13] |
L. Mei, S. Zhu, W. Yin, et al., Theranostics 10 (2020) 757-781. DOI:10.7150/thno.39701 |
| [14] |
G.P. Awasthi, V.K. Kaliannagounder, B. Maharjan, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 116 (2020) 111162. DOI:10.1016/j.msec.2020.111162 |
| [15] |
L. Wang, Y. Li, L. Zhao, et al., Nanoscale 12 (2020) 19516-19535. DOI:10.1039/D0NR05746K |
| [16] |
S. Zhu, L. Li, Z. Gu, et al., Small 16 (2020) 2000980. DOI:10.1002/smll.202000980 |
| [17] |
Y. Yang, G. Reniers, G. Chen, F. Goerlandt, Saf. Sci. 120 (2019) 14-24. DOI:10.1016/j.ssci.2019.06.022 |
| [18] |
R. Tenne, J. Mater. Res. 21 (2006) 2726-2743. DOI:10.1557/jmr.2006.0354 |
| [19] |
Y.M. Guo, Z.L. Huang, J. Guo, et al., Sustainability 11 (2019) 3606. DOI:10.3390/su11133606 |
| [20] |
Y.H. Wang, L.L. He, K.J. Huang, et al., Analyst 144 (2019) 2849-2866. DOI:10.1039/C9AN00081J |
| [21] |
Y.L. Hu, Y. Huang, C.L. Tan, et al., Mat. Chem. Front. 1 (2017) 24-36. DOI:10.1039/C6QM00195E |
| [22] |
K.J. Huang, L. Wang, J. Li, Y.M. Liu, Sens. Actuat. B: Chem. 178 (2013) 671-677. DOI:10.1016/j.snb.2013.01.028 |
| [23] |
O. Parlak, A. Incel, L. Uzun, et al., Biosens. Bioelectron. 89 (2017) 545-550. DOI:10.1016/j.bios.2016.03.024 |
| [24] |
K.J. Huang, Y.J. Liu, H.B. Wang, et al., Biosens. Bioelectron. 55 (2014) 195-202. DOI:10.1016/j.bios.2013.11.061 |
| [25] |
W. Zhang, Z. Dai, X. Liu, J. Yang, Biosens. Bioelectron. 105 (2018) 116-120. DOI:10.1016/j.bios.2018.01.038 |
| [26] |
X. Wang, C.C. Chu, L. Shen, et al., Sens. Actuat. B: Chem. 206 (2015) 30-36. DOI:10.1016/j.snb.2014.09.028 |
| [27] |
Y.G. Wang, Y.L. Wang, D. Wu, et al., Sens. Actuat. B: Chem. 255 (2018) 125-132. DOI:10.1016/j.snb.2017.07.129 |
| [28] |
X.Y. Li, X.Z. Du, Sens. Actuat. B: Chem. 239 (2017) 536-543. DOI:10.1016/j.snb.2016.08.048 |
| [29] |
J. Zheng, D.D. Song, H. Chen, et al., Chin. Chem. Lett. 31 (2019) 1109-1113. |
| [30] |
J. Liu, X. Chen, Q. Wang, et al., Nano Lett. 19 (2019) 1437-1444. DOI:10.1021/acs.nanolett.8b03818 |
| [31] |
D. Sarkar, W. Liu, X.J. Xie, et al., ACS Nano 8 (2014) 3992-4003. DOI:10.1021/nn5009148 |
| [32] |
D.W. Lee, J. Lee, I.Y. Sohn, et al., Nano Res. 8 (2015) 2340-2350. DOI:10.1007/s12274-015-0744-8 |
| [33] |
X. Xiang, J.B. Shi, F.H. Huang, et al., Biosens. Bioelectron. 74 (2015) 227-232. DOI:10.1016/j.bios.2015.06.045 |
| [34] |
S.W. Zeng, S.Y. Hu, J. Xia, et al., Sens. Actuat. B: Chem. 207 (2015) 801-810. DOI:10.1016/j.snb.2014.10.124 |
| [35] |
S. Kaushik, U.K. Tiwari, S.S. Pal, R.K. Sinha, Biosens. Bioelectron. 126 (2019) 501-509. DOI:10.1016/j.bios.2018.11.006 |
| [36] |
B.L. Li, J.P. Wang, H.L. Zou, et al., Adv. Funct. Mater. 26 (2016) 7034-7056. DOI:10.1002/adfm.201602136 |
| [37] |
W. Gu, Y.H. Yan, C.L. Zhang, et al., ACS Appl. Mater. Interfaces 8 (2016) 11272-11279. DOI:10.1021/acsami.6b01166 |
| [38] |
Q. Xi, D.M. Zhou, Y.Y. Kan, et al., Anal. Chem. 86 (2014) 1361-1365. DOI:10.1021/ac403944c |
| [39] |
J. Shi, J. Lyu, F. Tian, M. Yang, Biosens. Bioelectron. 93 (2017) 182-188. DOI:10.1016/j.bios.2016.09.012 |
| [40] |
C. Zhu, Z. Zeng, H. Li, et al., J. Am. Chem. Soc. 135 (2013) 5998-6001. DOI:10.1021/ja4019572 |
| [41] |
Y. Chen, S. Hu, H. Wang, et al., Adv. Opt. Mater. 7 (2019) 1900479. |
| [42] |
L.M. Wu, Y. Jia, L.Y. Jiang, et al., J. Lightwave Technol. 35 (2017) 82-87. DOI:10.1109/JLT.2016.2624982 |
| [43] |
M. Asif, M. Ajmal, G. Ashraf, et al., Curr. Opin. Electrochem. 23 (2020) 174-184. DOI:10.1016/j.coelec.2020.08.011 |
| [44] |
X. Peng, Y.X. Zhou, K.X. Nie, et al., New J. Phys. 22 (2020) 11. |
| [45] |
Z. Qin, R. Peng, I. Baravik, X. Liu, Matter 3 (2020) 628-651. DOI:10.1016/j.matt.2020.06.015 |
| [46] |
B. Wang, Y. Sun, H. Ding, et al., Adv. Funct. Mater. 30 (2020) 2003732. DOI:10.1002/adfm.202003732 |
| [47] |
P. Kang, M.C. Wang, S. Nam, Microelectron. Eng. 161 (2016) 18-35. DOI:10.1016/j.mee.2016.04.003 |
| [48] |
Y.J. Park, B.K. Sharma, S.M. Shinde, et al., ACS Nano 13 (2019) 3023-3030. DOI:10.1021/acsnano.8b07995 |
| [49] |
J. Jang, H. Kim, S. Ji, et al., Nano Lett. 20 (2020) 66-74. DOI:10.1021/acs.nanolett.9b02978 |
| [50] |
X. Chen, Y.J. Park, M. Kang, et al., Nat. Commun. 9 (2018) 1690-1701. DOI:10.1038/s41467-018-03956-9 |
| [51] |
M. Park, Y.J. Park, X. Chen, et al., Adv. Mater. 28 (2016) 2556-2563. DOI:10.1002/adma.201505124 |
| [52] |
L. Gao, Small 13 (2017) 1603994. DOI:10.1002/smll.201603994 |
| [53] |
S.J. Kim, S. Mondal, B.K. Min, C.G. Choi, ACS Appl. Mater. Interfaces 10 (2018) 36377-36384. DOI:10.1021/acsami.8b11233 |
| [54] |
S. Veeralingam, P. Sahatiya, A. Kadu, et al., ACS Appl. Electron. Mater. 1 (2019) 558-568. DOI:10.1021/acsaelm.9b00022 |
| [55] |
H.Y. Guo, C.Y. Lan, Z.F. Zhou, et al., Nanoscale 9 (2017) 6246-6253. DOI:10.1039/C7NR01016H |
| [56] |
J.S. Kim, H.W. Yoo, H.O. Choi, H.T. Jung, Nano Lett. 14 (2014) 5941-5947. DOI:10.1021/nl502906a |
| [57] |
H. Yan, M. Zhong, Z. Lv, P. Wan, Small 13 (2017) 1701697. DOI:10.1002/smll.201701697 |
| [58] |
S. Veeralingam, P. Sahatiya, S. Badhulika, Sens. Actuator. B: Chem. 297 (2019) 126725. DOI:10.1016/j.snb.2019.126725 |
| [59] |
D. Kinnamon, R. Ghanta, K.C. Lin, et al., Sci. Rep. 7 (2017) 13312. DOI:10.1038/s41598-017-13684-7 |
| [60] |
C. Gao, X. Liu, H. Yang, et al., Vacuum 182 (2020) 109729. DOI:10.1016/j.vacuum.2020.109729 |
| [61] |
Y. Pang, Z. Yang, Y. Yang, T.L. Ren, Small 16 (2020) 1901124. DOI:10.1002/smll.201901124 |
| [62] |
S. Gong, W. Cheng, Adv. Energy Mater. 7 (2017) 1700648. DOI:10.1002/aenm.201700648 |
| [63] |
J.V. Vaghasiya, C.C. Mayorga-Martinez, J. Vyskočil, et al., Adv. Funct. Mater. 30 (2020) 2003673. DOI:10.1002/adfm.202003673 |
| [64] |
D. Zhang, Z. Yang, P. Li, et al., Nano Energy 65 (2019) 103974. DOI:10.1016/j.nanoen.2019.103974 |
| [65] |
K. Maity, B. Mahanty, T.K. Sinha, et al., Energy Technol. 5 (2017) 234-243. DOI:10.1002/ente.201600419 |
| [66] |
L. Lan, T. Yin, C. Jiang, et al., Nano Energy 62 (2019) 319-328. DOI:10.1016/j.nanoen.2019.05.041 |
| [67] |
M. Mohammadniaei, H.V. Nguyen, M.V. Tieu, M.H. Lee, Micromachines (Basel) 10 (2019) 662. DOI:10.3390/mi10100662 |
| [68] |
S. Meng, Y. Zhang, H. Wang, et al., Biomaterials 269 (2020) 120471. |
| [69] |
R.F. Hossain, I.G. Deaguero, T. Boland, A.B. Kaul, npj 2D Mater. Appl. 1 (2017) 28-37. DOI:10.1038/s41699-017-0034-2 |
| [70] |
C. Choi, M.K. Choi, S.Y. Liu, et al., Nat. Commun. 8 (2017) 1664. DOI:10.1038/s41467-017-01824-6 |
| [71] |
Y.J. Chen, Y.K. Wu, B.B. Sun, et al., Small 13 (2017) 1603446. DOI:10.1002/smll.201603446 |
| [72] |
X.X. Qian, S.D. Shen, T. Liu, et al., Nanoscale 7 (2015) 6380-6387. DOI:10.1039/C5NR00893J |
| [73] |
S.G. Wang, J.L. Zhao, H.L. Yang, et al., Acta Biomater. 58 (2017) 442-454. DOI:10.1016/j.actbio.2017.06.014 |
| [74] |
Z.H. Miao, L.X. Lv, K. Li, et al., Small 14 (2018) 1703789. DOI:10.1002/smll.201703789 |
| [75] |
O. Adetunji Moses, M.I. Khan, Q. Fang, et al., Nanotechnology 30 (2019) 65102. DOI:10.1088/1361-6528/aaf151 |
| [76] |
Z.Y. Lei, W.C. Zhu, S.J. Xu, et al., ACS Appl. Mater. Interfaces 8 (2016) 20900-20908. DOI:10.1021/acsami.6b07326 |
| [77] |
W. Feng, L. Chen, M. Qin, et al., Sci. Rep. 5 (2015) 17422. DOI:10.1038/srep17422 |
| [78] |
L.F. Tan, S.P. Wang, K. Xu, et al., Small 12 (2016) 2046-2055. DOI:10.1002/smll.201600191 |
| [79] |
Z. Huang, Y. Qi, D. Yu, J. Zhan, RSC Adv. 6 (2016) 31031-31036. DOI:10.1039/C6RA03226E |
| [80] |
M.R. Younis, C. Wang, R. An, et al., ACS Nano 13 (2019) 2544-2557. |
| [81] |
H. Lee, H. Kim, T.P. Nguyen, et al., ACS Appl. Mater. Interfaces 8 (2016) 29213-29219. DOI:10.1021/acsami.6b10763 |
| [82] |
C. Wang, J. Bai, Y. Liu, et al., ACS Biomater. Sci. Eng. 2 (2016) 2011-2017. DOI:10.1021/acsbiomaterials.6b00416 |
| [83] |
H.F. Dong, S.S. Tang, Y.S. Hao, et al., ACS Appl. Mater. Interfaces 8 (2016) 3107-3114. DOI:10.1021/acsami.5b10459 |
| [84] |
C. Sweet, A. Pramanik, S. Jones, P.C. Ray, ACS Omega 2 (2017) 1826-1835. DOI:10.1021/acsomega.7b00229 |
| [85] |
D.K. Ji, Y. Zhang, Y. Zang, et al., Adv. Mater. 28 (2016) 9356-9364. DOI:10.1002/adma.201602748 |
| [86] |
Y. Wang, F. Zhang, H. Lin, F. Qu, ACS Appl. Mater. Interfaces 11 (2019) 43964-43975. DOI:10.1021/acsami.9b17237 |
| [87] |
S.D. Shen, Y. Chao, Z.L. Dong, et al., Adv. Funct. Mater. 27 (2017) 1700250. DOI:10.1002/adfm.201700250 |
| [88] |
Y. Liu, X. Ji, J. Liu, et al., Adv. Funct. Mater. 27 (2017) 1703261. DOI:10.1002/adfm.201703261 |
| [89] |
J.P. Wang, X.X. Tan, X.J. Pang, et al., ACS Appl. Mater. Interfaces 8 (2016) 24331-24338. DOI:10.1021/acsami.6b08391 |
| [90] |
X.Z. Cui, Z.G. Zhou, Y. Yang, et al., Chin. Chem. Lett. 26 (2015) 749-754. DOI:10.1016/j.cclet.2015.03.034 |
| [91] |
Y. Yong, X. Cheng, T. Bao, et al., ACS Nano 9 (2015) 12451-12463. DOI:10.1021/acsnano.5b05825 |
| [92] |
W. Yin, L. Yan, J. Yu, et al., ACS Nano 8 (2014) 6922-6933. DOI:10.1021/nn501647j |
| [93] |
X. Dong, R. Cheng, S. Zhu, et al., ACS Nano 14 (2020) 5400-5416. DOI:10.1021/acsnano.9b08962 |
| [94] |
D. Zhang, P. Cui, Z. Dai, et al., Nanoscale 11 (2019) 19912-19922. DOI:10.1039/C9NR05684J |
| [95] |
X. Jing, Z. Zhi, D. Wang, et al., Bioconjugate Chem. 29 (2018) 559-570. DOI:10.1021/acs.bioconjchem.8b00053 |
| [96] |
T. Liu, S.X. Shi, C. Liang, et al., ACS Nano 9 (2015) 950-960. DOI:10.1021/nn506757x |
| [97] |
J. Kim, H. Kim, W.J. Kim, Small 12 (2016) 1184-1192. DOI:10.1002/smll.201501655 |
| [98] |
G.B. Yang, R. Zhang, C. Liang, et al., Small 14 (2018) 1702664. DOI:10.1002/smll.201702664 |
| [99] |
L.Q. Peng, X. Mei, J. He, et al., Adv. Mater. 30 (2018) 1707389. DOI:10.1002/adma.201707389 |
| [100] |
L. Cheng, C. Yuan, S.D. Shen, et al., ACS Nano 9 (2015) 11090-11101. DOI:10.1021/acsnano.5b04606 |
| [101] |
L. Cheng, A. Kamkaew, S. Shen, et al., Small 12 (2016) 5750-5758. DOI:10.1002/smll.201601696 |
| [102] |
X.H. Dong, W.Y. Yin, X. Zhang, et al., ACS Appl. Mater. Interfaces 10 (2018) 4271-4284. DOI:10.1021/acsami.7b17506 |
| [103] |
S. Karunakaran, S. Pandit, B. Basu, M. De, J. Am. Chem. Soc. 140 (2018) 12634-12644. DOI:10.1021/jacs.8b08994 |
| [104] |
F. Alimohammadi, M. Sharifian Gh, N.H. Attanayake, et al., Langmuir 34 (2018) 7192-7200. DOI:10.1021/acs.langmuir.8b00262 |
| [105] |
T.I. Kim, J. Kim, I.J. Park, et al., 2D Mater 6 (2019) 25025. DOI:10.1088/2053-1583/ab070e |
| [106] |
T.I. Kim, B. Kwon, J. Yoon, et al., ACS Appl. Mater. Interfaces 9 (2017) 7908-7917. DOI:10.1021/acsami.6b12464 |
| [107] |
X. Tian, Y. Sun, S. Fan, et al., ACS Appl. Mater. Interfaces 11 (2019) 4858-4866. DOI:10.1021/acsami.8b19958 |
| [108] |
M. Zhu, X. Liu, L. Tan, et al., J. Hazard. Mater. 383 (2020) 121122. DOI:10.1016/j.jhazmat.2019.121122 |
| [109] |
X. Hou, T. Shi, C. Wei, et al., Biomaterials 243 (2020) 119937-119948. DOI:10.1016/j.biomaterials.2020.119937 |
| [110] |
Z.Z. Feng, X.M. Liu, L. Tan, et al., Small 14 (2018) 1704347. DOI:10.1002/smll.201704347 |
| [111] |
M. Li, L. Li, K. Su, et al., Adv. Sci. 6 (2019) 1900599. DOI:10.1002/advs.201900599 |
| [112] |
W.Y. Yin, J. Yu, F.T. Lv, et al., ACS Nano 10 (2016) 11000-11011. DOI:10.1021/acsnano.6b05810 |
| [113] |
F. Cao, L. Zhang, H. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 16236-16242. DOI:10.1002/anie.201908289 |
| [114] |
T. Wang, X. Zhang, L. Mei, et al., Nanoscale 12 (2020) 8415-8424. DOI:10.1039/D0NR00192A |
| [115] |
L. Wang, F. Gao, A. Wang, et al., Adv. Mater. 32 (2020) 2005423. DOI:10.1002/adma.202005423 |
| [116] |
W. Zhang, S. Shi, Y. Wang, et al., Nanoscale 8 (2016) 11642-11648. DOI:10.1039/C6NR01243D |
| [117] |
C. Zhang, D.F. Hu, J.W. Xu, et al., ACS Nano 12 (2018) 12347-12356. DOI:10.1021/acsnano.8b06321 |
| [118] |
G. Lalwani, A.M. Henslee, B. Farshid, et al., Acta Biomater. 9 (2013) 8365-8373. DOI:10.1016/j.actbio.2013.05.018 |
| [119] |
M. Naffakh, A.M. Diez-Pascual, J. Mat. Chem. B 2 (2014) 4509-4520. DOI:10.1039/c4tb00557k |
| [120] |
X. Zhang, J. Nie, X. Yang, et al., Appl. Mater. Today 10 (2018) 164-172. DOI:10.1016/j.apmt.2017.12.001 |
| [121] |
E.P. Nguyen, C. de Carvalho Castro Silva, A. Merkoci, Nanoscale 12 (2020) 19043-19067. DOI:10.1039/D0NR05287F |
| [122] |
J.K. Carrow, K.A. Singh, M.K. Jaiswal, et al., Proc. Natl. Acad. Sci. U. S. A. 117 (2020) 13329-13338. DOI:10.1073/pnas.1914345117 |
| [123] |
H. Wang, X. Zeng, L. Pang, et al., Chem. Eng. J. 396 (2020) 125081. DOI:10.1016/j.cej.2020.125081 |
| [124] |
U. Yadav, H. Mishra, V. Singh, et al., ACS Biomater. Sci. Eng. 5 (2019) 4511-4521. DOI:10.1021/acsbiomaterials.9b00227 |
| [125] |
S. Wu, J. Wang, L. Jin, et al., ACS Appl. Nano Mater. 1 (2017) 337-343. |
| [126] |
H. Nazari, A. Heirani-Tabasi, M. Hajiabbas, et al., Polym. Adv. Technol. 31 (2019) 248-259. |
| [127] |
L. Chen, S. Zhou, L. Su, J. Song, ACS Nano 13 (2019) 10887-10917. DOI:10.1021/acsnano.9b04954 |
| [128] |
B. Hinnemann, P. Moses, J. Bonde, et al., J. Am. Chem. Soc. 127 (2005) 5308-5309. DOI:10.1021/ja0504690 |
| [129] |
H.L. Duan, C. Wang, G.N. Li, et al., Angew. Chem. Int. Ed. 60 (2021) 7251-7258. DOI:10.1002/anie.202014968 |
| [130] |
S.J. Xu, D. Li, P.Y. Wu, Adv. Funct. Mater. 25 (2015) 1127-1136. DOI:10.1002/adfm.201403863 |
| [131] |
N. Matei, R. Camara, J.H. Zhang, Med. Gas Res. 8 (2018) 98-102. DOI:10.4103/2045-9912.239959 |
| [132] |
M. Singh, C. Zannella, V. Folliero, et al., Front. Bioeng. Biotechnol. 8 (2020) 569967. DOI:10.3389/fbioe.2020.569967 |
| [133] |
R. Kurapati, L. Muzi, A.P.R. de Garibay, et al., Adv. Funct. Mater. 27 (2017) 1605176. DOI:10.1002/adfm.201605176 |
| [134] |
J.L. Hao, G.S. Song, T. Liu, et al., Adv. Sci. 4 (2017) 1600160. DOI:10.1002/advs.201600160 |
| [135] |
L. Mei, X. Zhang, W. Yin, et al., Nanoscale 11 (2019) 4767-4780. DOI:10.1039/C8NR10319D |
| [136] |
Z. Gu, L.D. Plant, X.Y. Meng, et al., ACS Nano 12 (2018) 705-717. DOI:10.1021/acsnano.7b07871 |
| [137] |
Z. Gu, Z. Yang, S.G. Kang, et al., Sci. Rep. 6 (2016) 28252. DOI:10.1038/srep28252 |
| [138] |
Z. Gu, S.H. Chen, Z. Ding, et al., Nanoscale 11 (2019) 22293-22304. DOI:10.1039/C9NR04358F |
| [139] |
P. Yuan, X. Hu, Q. Zhou, Nanotoxicology 14 (2020) 1137-1155. DOI:10.1080/17435390.2020.1817598 |
| [140] |
D. Baimanov, J. Wu, R. Chu, et al., ACS Nano 14 (2020) 5429-5442. |
| [141] |
Z.Y. Wang, A. von dem Bussche, Y. Qiu, et al., Environ. Sci. Technol. 50 (2016) 7208-7217. DOI:10.1021/acs.est.6b01881 |
| [142] |
X. Wang, X. Zhong, Z. Zha, et al., Appl. Mater. Today 18 (2020) 100464. DOI:10.1016/j.apmt.2019.100464 |
| [143] |
W. Chen, W. Qi, W. Lu, et al., Small 14 (2018) 1702600. DOI:10.1002/smll.201702600 |
| [144] |
C. Moore, D. Movia, R.J. Smith, et al., 2D Mater. 4 (2017) 25065. DOI:10.1088/2053-1583/aa673f |
| [145] |
Y. Chen, C. Tan, H. Zhang, L. Wang, Chem. Soc. Rev. 44 (2015) 2681-2701. DOI:10.1039/C4CS00300D |
 2021, Vol. 32
2021, Vol. 32 

