b Department of Chemical Physics, University of Science and Technology of China, Hefei 230026, China;
c Vacuum Interconnected Nanotech Workstation, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
With the rapid development of society and growth of population, traditional energy structure with fossil energy as primary energy and electricity as secondary energy is facing severe challenges in the new era [1-3]. Meanwhile, various harmful and greenhouse gases are emitted during the conversion process, causing serious environmental pollution issues and almost irreversible damage to the global ecology. Therefore, the search of clean and green renewable energy to replace original energy architecture is imperative. Common renewable energy generally belongs to intermittent energy, such as solar energy, wind, tidal energy. During the power generation process, it is impossible to furnish a continuous output of stable current, thus posing higher requirements to the storage of electricity. In addition, with the booming development of multifarious mobile portable devices and hybrid electric vehicles, there is also an urgent appetite to improve the endurance of energy storage devices. At present, a wide variety of batteries dominate the main position in the field of energy storage, such as traditional lead-acid batteries, nickel metal hydride batteries and lithium-ion batteries [4]. This type of devices generally requires constant voltage and current charging, displaying low power density, so that cannot be charged and discharged at high current densities [5]. Although traditional capacitors exhibit high power density, their energy density is relatively too low and still cannot meet the needs of large-scale practical applications [6]. In this regard, Supercapacitors (SCs) as a new type of energy storage device between traditional capacitors and batteries, have shown significant power density (quick charge and discharge behavior within a few seconds), excellent reversibility and long cycle life (> 106 cycles), attracting widespread attention from academia and industry recently [7, 8]. According to the storage mechanism, SCs can be classified into electrical double-layer capacitors (EDLCs) and pseudocapacitors which store energy through physically rapid ion absorption and desorption at the interface between electrodes and electrolytes or through a chemically reversible faradaic redox reaction, respectively [9, 10]. Neither of these mechanisms depends on the diffusion of ions within the bulk of materials, like batteries. However, the limitation for SCs is their insufficient energy density, resulting in a certain gap with the actual application requirements.
It is well known that the energy density of SCs is calculated by the formula of 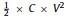
In this review, recent advances of the applications of non-carbon 2D materials in the aqueous electrolyte-based SCs are summarized in detail (Fig. 1). Firstly, we bring some brief introductory background and main development challenges on SCs, facilitating a review and understanding of the current research state in this field. Thereafter, the specific discussion based on various 2D materials of TMOs, TMHs, TMCs, MXenes and MOFs, etc. are taken up. Different synthetic strategies and novel electrode engineering designs to precisely control the microstructural morphology, optimize the conductivity and stability, tune the surface chemical/electronic properties are thoroughly presented. Besides, the properties of their corresponding derivatives and the rational integration with other electrochemical active materials to construct hybrid electrodes are also emphasized. The impact of unique crystal structures and intrinsic chemical properties inherent to these non-carbon 2D materials on the final electrode performances is then highlighted. On the basis of this review, we will outline some prospects and future tasks to boost the development of state-of-the-art 2D materials for new type energy storage device.

|
Download:
|
| Fig. 1. Schematic diagram for the current research field of 2D non-carbon material-based supercapacitor electrodes. | |
SCs are a kind of energy storage device that can provide fast charge and discharge processes. The rise of nanomaterials shortens the ion diffusion distance and increases the external surface area, enabling the greatly improved utilization efficiency of some pseudocapacitive materials in SCs. Unfortunately, many battery-type electrodes that exhibit faradaic behavior are assumed to be pseudocapacitive materials deservedly [33]. Besides, an increasing number of electrode materials show electrochemical characteristics that are neither purely capacitive nor purely faradaic. It is indispensable to distinguish the concepts of pseudocapacitive SC-type electrodes and faradaic battery-type electrodes clearly. This section mainly introduces the definition of EDLCs and pseudocapacitors, and sorts out some misunderstandings in actual work.
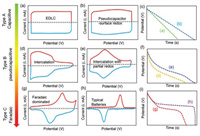
The energy storage in EDLCs results from the charge separation that occurs at the interface between the electrodes and the electrolytes without any electron transfer and faradaic redox reactions. According to the physical electrostatic process, the formation and relaxation time of the double electric layer is about 10−8 s, less than the redox reaction time of the pseudocapacitance (10−2~10−4 s) [34]. EDLCs with a nano-level plate spacing possess an effective specific surface area several orders of magnitude higher than that of traditional dielectric capacitors [35]. Carbon-based materials, from commercial activated carbon [36], carbon aerogels [37], templated carbon [38] to carbon nanomaterials (such as graphene and carbon nanotubes) [39, 40], due to high specific surface area, excellent electrochemical stability and open porosity towards electrolyte ions, are widely used in EDLC electrodes. In sum, the characteristic of EDLC is fast charge/discharge but limited charge storage. Generally speaking, good cycling stability and power density of EDLCs can be characterized by a capacitor-like response with a rectangular cyclic voltammogram (CV) (Fig. 2a) and a linear voltage response with a triangular-shaped profile during the galvanostatic charging/discharging process (GCD) (Fig. 2c).The rectangular CV curve is derived from the basic property of the double-layer storage mechanism: in the absence of diffusion constraints, there is an instantaneous charge separation (i.e., polarization) in the capacitor under an external electric field, so dV/dt remains constant.
|
Download:
|
| Fig. 2. Schematic CV curves and corresponding GCD profiles of capacitive, pseudocapacitive and battery-like materials. Reproduced with permission [41]. Copyright 2018, American Chemical Society. | |
The energy storage mechanism of pseudocapacitors is related to the change of valence state caused by electron transfer, which originates from the rapid reversible thermodynamic process of surface redox reactions. Based on Conway's research, different faradaic processes result in different pseudocapacitive characteristics, (i) underpotential deposition: the electrodeposition behavior of ions on a 2D metal-electrolyte interface at the positive potential of the reversible redox potential; (ii) surface redox pseudocapacitance: reversible fast redox reactions on or near the surface of active materials, such as RuO2, MnO2 and some conductive polymers; (iii) intercalation pseudocapacitance: reversible redox reactions resulting from ions intercalation into the redox active materials without crystal phase transition, such as Nb2O5 and MXenes. Among them, redox and intercalation pseudocapacitive materials are the most commonly used in the electrode design. As shown in Fig. 2b, the typical CV curve of surface redox pseudocapacitance generally exhibits a rectangle-like shape within the potential window, possibly due to a series of closely arranged, symmetrical bimodal superpositions. Pseudocapacitance is featured with relatively large charge storage but slow charging. On the other hand, batteries display prominent and separated redox peaks corresponding to metal center charge storage (Figs. 2g and h). Meanwhile, constant current discharge also presents a profoundly nonlinear E versus t plot (Fig. 2i), showing a nearly constant potential plateaus associated with the faradaic reduction or oxidation of the metal centers. The battery-like electrode materials exhibit the merits of large charge storage but suffer from sluggish reactions. Intercalation pseudocapacitors mainly depend on the non-diffusion-controlled intercalation/de-intercalation processes of cations (such as Li+, Na+, K+, H+) within the 2D nanochannels of active materials, showing the CV and GCD behaviors intermediate between EDLCs and batteries (Figs. 2d–f). If the electrode material belongs to the EDLC/pseudocapacitor category, capacitance (C in Farads) can be used to evaluate the performance of devices. If there is a plateau during charge/discharge process or an obvious redox peak in the CV curve, the capacitance value will be affected by the selection of the potential window and is not applicable. Instead, the concept of capacity (Q/3.6, mAh) should be adopted [41, 42].
To further analyze the kinetic difference of electrode materials, CV investigation can be applied to reveal the voltametric response at various scan rates based on a power law [42-44]. The specific formula is as follows:

|
(1) |
where i is the measured current at a fixed potential, v is the scan rate, a and b are both adjustable parameters. It has been clearly demonstrated that the voltametric response of battery behavior can be summarized as [33]:

|
(2) |
where n is the number of electrons involved in the electrode reaction, F is the faradaic constant, A is the surface area of the electrode, C* is the surface concentration of the electrode material, D is the diffusion coefficient, α is the transfer coefficient, R is the molar gas constant, T is the temperature and the function χ(bt) is the normalized current. Thereinto, for a battery-type electrode, the peak current (ip) response will vary linearly with the square root of the scan rate (ip = av0.5, b = 0.5). For a capacitor-type or pseudocapacitor-type electrode, the current (i) will be proportional to the scan rate (b = 1) according to the equation below [45]:

|
(3) |
where Cd is the capacitance and A is the surface area of the electrode material. Recently, it has also been discovered that not all capacity in some electrode materials comes from intercalation pseudocapacitances, but their intercalation pseudocapacitances account for a large part of the total capacity. In other words, the value of b is very close to but slightly less than 1. Therefore, it is particularly important to separate the overall current into the contribution from double-layer capacitances and faradaic pseudocapacitances on one side, and faradaic battery-type processes on the other side. The current response of each material at a fixed potential to the scan rate is different, depending on whether the redox reaction is a surface-controlled (k1v) or diffusion-controlled (k2v1/2) process. This relationship can be expressed by the following formula [44-46]:
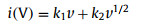
|
(4) |
By calculating the values of k1 and k2 at each potential, the contribution of the (pseudo)capacitive process and diffusion-controlled cation intercalation process can be given respectively. In addition, it should also be pointed out that although the b value of intercalation pseudocapacitive behavior is close to capacitive or traditional pseudocapacitive behavior, the CV curve of such electrodes is still similar to battery-type electrodes.
3. Non-carbon 2D materials for the aqueous electrolyte-based SCsCurrently, the formation of some novel non-caron 2D materials can provide ideal material platforms to fabricate superior SC electrodes. In this section, different physicochemical aspects of 2D materials including synthetic methods, special morphology and electrochemical performances are presented to demonstrate their promising application as electrode materials in the aqueous electrolyte-based SCs.
3.1. 2D transition metal oxides (TMOs) electrodes 3.1.1. 2D TMOs and their composite materialsVarious 2D binary TMOs, such as MnO2 [47, 48], Fe2O3 [49], NiO [50] and Co3O4 [51, 52], have attracted tremendous attention as SC electrode materials due to low cost, non-toxicity, enlarged surface area, excellent environmental compatibility and rich redox activity. Among them, MnO2 with a high theoretical capacitance of 1370 F/g and outstanding structural flexibility, showing wide applications in electrocatalysis, heterogeneous catalysis and energy storage [53]. To overcome its intrinsic drawback of low conductivity and strong aggregation tendency, conductive materials are employed to improve the electron collection efficiency and optimize the electrode interface.
Wu et al. [54] grafted MnO2 nanosheets vertically on the carbon nanotube (CNT) to form core-shell architectures through a secondary (seeded) growth and then scaffolded them in 2D reduced graphene oxide with the aid of electrostatic self-assembly via a simple vacuum filtration method. The SEM image clearly confirmed the vertically aligned MnO2 nanosheets on the CNT surface densely, providing open and porous architectures. The hierarchically sandwich-like structure afforded effective electrolyte ion transport channels, guaranteeing the sustainable redox reaction of MnO2. These ternary flexible hybrid films exhibit a high specific capacitance of 298 F/g in 1 mol/L Na2SO4 electrolyte at a current density 0.5 A/g, exceptional rate capability and excellent cycling stability of 90.3% retention and approximately 100% Coulombic efficiency over 5000 cycles. In another report, Gao et al. [55] directly electrodeposited 2D vertically aligned MnO2 nanoplates on nickel foam as the positive electrode without further processing and prepared porous graphene hydrogel as the negative electrode, constructing an ASC with a wide voltage window of 2.0 V. The hierarchical nickel foam rendered higher mass loading per unit area, which was conducive to the transport of electrolyte ions and the optimization of electrochemical properties for active materials. Moreover, the electrodeposition method endowed a robust contact between MnO2 and conductive support, allowing the elimination of any binders or additives. Therefore, the fabricated ASC exhibited a maximum energy density of 23.2 Wh/kg at a power density of 1.0 kW/kg and remarkable cycling performance over 5000 cycles. Li et al. [56] successfully prepared a new 2D free standing and flexible MnO2/graphene film electrode through a spin-coating followed by in-situ hydrothermal process. The graphene film without binders not only supported the vertical alignment of 2D MnO2 nanosheets, but also served as a base current collector. This integrated electrode exhibited remarkable electrochemical performance in 1 mol/L Na2SO4 aqueous solution electrolyte, such as a largest specific capacitance of 280 F/g at a current density of 1 A/g and no prominent capacitance decay over 10, 000 cycles. Composite materials without binders can also be obtained in large quantities through a one-pot electrical exfoliation process. In-situ self-assembly of 2D MnO2/graphene relied on the effective interactions between materials simultaneously exfoliated from the cathode and anode [57]. The flexible symmetric SCs fabricated by such MnO2/graphene film presented excellent specific capacitance of 265 F/g at 2.5 A/g and a largest energy density of 20.7 Wh/kg, which remained unchanged under folded or rolled conditions. H+/K+ is prone to insert and remove in 2D α-MoO3, presenting multiple oxidation states and redox reactions. MoO3/PANI coaxial heterostructure nanobelts were prepared by a simple and green in-situ polymerization approach at room temperature [58]. The as-synthesized MoO3/PANI electrode delivered a specific capacitance of 632 F/g at 1 A/g in an aqueous 1 mol/L H2SO4 electrolyte. Fe2O3 as a common negative electrode material could be combined with graphene according to the electrostatic self-assembly [59]. The obtained 2D layered hybrid electrode delivered a high specific capacitance of 714 F/g at 1 A/g in 2 mol/L KOH within the negative potential range from −1.2 V to −0.2 V (vs. Hg/HgO).
3.1.2. 2D cation modified TMO materialsAtomic-level structure engineering of TMOs, including cation doping and oxygen vacancy modification can give rise to influence the physicochemical properties of materials substantially, and even will adjust the charge storage mechanism from surface redox pseudocapacitive behavior to bulk ion intercalation [60, 61].
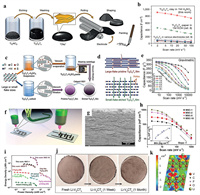
Jabeen et al. [62] applied an electrochemical oxidation to prepare high Na+ content Birnessite Na0.5MnO2 nanosheet array in situ on carbon cloth. The high Na+ content endowed the electrode with a large specific capacitance of 366 F/g and extended the positive potential window to 0–1.3 V (vs. Ag/AgCl), further providing the opportunity to enable the working voltage of aqueous ASC beyond 2.0 V. On this basis, Zhu et al. [63] designed a peculiar β-MnO2/Birnessite core-shell structure, where the outer parallel open sheet provided the possibility for entrance of electrolyte ions. The synergy of balancing cations (Na+) and reduced lattice interlayer spacing were able to activate the Mn(IV) from the bulk to exploit more pseudocapacitance. The ASC based on this material delivered an enlarged voltage of 2.2 V in 1 mol/L Na2SO4 aqueous electrolyte and a maximum energy density of 40.4 Wh/kg. Hu et al. [64] put forward a versatile and rapid mass production methodology of 2D ion-intercalated metal oxides via the molten salts method (MSM). Fig. 3a illustrated the experimental process of MSM. The precursors were added to the nitrates that had been heated to the molten state, reacted for only 1 min, then cooled and filtered to remove the soluble salts to obtain the final products. The critical feature of this method is the direct use of unhydrated bare ions in molten salts to rapidly facilitate the formation of 2D metal oxides. The representative SEM and TEM image of Na2W2O13 confirmed the presence of stiff 2D structure (Figs. 3b and c). Fig. 3d showed the selected area electron diffraction (SAED) and high-resolution TEM (HRTEM) images demonstrating single crystalline nature of Na2W2O13. As shown in Fig. 3e, Na+ ions were intercalated into the interlayer of 2D metal oxides, which can be further verified by the results of EDS, XRD and XPS, respectively. The growth mechanism of Na2W2O13 can be divided into two steps: (i) the nucleation of [MO6]octahedron seeds; (ii) the formation of 2D plane with intercalated cations to balance the crystal charge. The optimized Na2W4O13 electrode exhibited good wetting behavior in favor of the contact with electrolyte and displayed the maximum volumetric capacitance of 310 F/cm3 at a scan rate of 5 mV/s in 0.5 mol/L Na2SO4. The flexible solid-state SC was assembled by a H3PO4/polyvinyl alcohol (PVA) electrolyte and two symmetric A4 paper@CNT@Na2W4O13 electrodes (Fig. 3f). CV curves remained rectangular shape without obvious distortion even at 100 mV/s, indicating high ion migration rate and good rate performance (Fig. 3g). As illustrated in Fig. 3h, the largest energy density of 3.83 mWh/cm3 (1.33 Wh/kg) at a power density of 1.2 W/cm3 (598.4 W/kg) can be achieved in the Ragone plot, higher than that of WO3@MoO3-ASCs and VOx/VN-ASCs. Four cells connected in series with a working voltage of 3.2 V could lit a commercial red light-emitting-diode (LED), reflecting the application in flexible and wearable electronic devices (Fig. 3i).
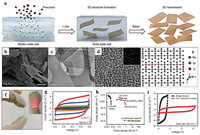
|
Download:
|
| Fig. 3. (a) Schematic of the MSM synthetic approach. Characterization of Na2W4O13: (b) SEM image, (c) low-resolution TEM image, (d) HRTEM image, (e) atomic structure. (f) Image of the assembled solid-state SC at the bending state. (g) CV curves at different scan rates of the symmetric solid-state SC. (h) Ragone plot. (i) CV curves at a scan rate of 200 mV/s of the single SC and four SCs connected in series. Reproduced with permission [64]. Copyright 2017, Nature. | |
On the other hand, the substitution of various cations (Co, Fe, Mg, Mn, Ni and Zn) on In2O3 nanosheet ground has been realized by a simple hydrothermal method [65]. Therein, compared with pure In2O3, the Co-In2O3 electrode with the optimal performance increased by 4.8 times in specific capacity and 1.3 times in rate capability. Furthermore, different cation substitutions affected the capacitance contribution of all electrodes. Mg and Zn substitution enhanced the capacitive contribution, while Co, Fe, Mn and Ni substitution increased the diffusion contribution. First principles calculations based on the density functional theory (DFT) confirmed the relatively lower formation energies and metallic character of cations substitution system to optimize the electrochemical performances. Among all seven ASCs, the device based on Co-In2O3 positive electrode delivered a largest energy density of 32 Wh/kg.
3.1.3. 2D ternary TMOs and their composite materialsBesides, 2D ternary TMOs with a formula of AB2O4 owing to rich electrochemical activity, abundant redox reactions, high electron conductivity and excellent cycling stability have been widely recognized as the competitive candidates for the selection of SC electrode materials [66, 67]. Zhang et al. [68] innovatively synthesized NiCo2O4 nanosheet arrays in situ on Ni foam through an optimized hydrothermal reaction in terms of crystal growth dynamics and a subsequent pyrolysis process (Fig. 4a). SEM and TEM images both confirmed the existence of cross-linked structure of ultrathin pyrolyzed NiCo2O4 nanosheets (Figs. 4b and c). More exposed active sites and plentiful diffusion channels were provided by the virtue of reasonable structure modulation and ordered crystal orientations, which also ultimately reduced the inner resistance and buffered the stress resulted from the phase transition during the charging/discharging process. Impressively, the NiCo2O4 nanosheet electrode exhibited admirable electrochemical evaluation, including drastically increased specific capacitance of 2017.8 F/g at 1 A/g in 6 mol/L KOH, distinguished rate performance of 93.2% and remarkable capacitance retention of 90.9% after 5000 cycles (Fig. 4d). It can also be observed from SEM that the array-like NiCo2O4 nanosheet structure was maintained well after cycling (Fig. 4e). For further practical application, the NiCo2O4/NF electrode matched with activated carbon (AC) electrode was fabricated into a flexible solid-state ASC using a PVA-CMC-KOH electrolyte (Fig. 4f). There was no apparent deformation of the collected CV curves under repeated bending angles from 0° to 120°, indicating the outstanding mechanical flexibility for this ASC device (Fig. 4g). The specific capacitance of the ASC was calculated to be 186.7 F/g at 1 A/g from GCD curves (Fig. 4h), and the rate capacity was 69.6% at 30 A/g.

|
Download:
|
| Fig. 4. (a) Schematic illustration of the NiCo2O4 preparation process. (b) SEM and (c) TEM images of NiCo2O4 nanosheets. (d) Specific capacitance retention of the NiCo2O4 electrode after 5000 cycles. (e) SEM image of the NiCo2O4 electrode after cycling. (f) A schematic diagram of solid-state NiCo2O4/NF//AC ASC. (g) Electrochemical stability measurement of ASC under repeated bending angles. (h) GCD curves of the ASC device. Reproduced with permission [68]. Copyright 2020, Wiley-VCH. (i) SEM and (j) TEM images of ZnCo2O4 nanowires. (k) SEM and (l) TEM images of ZnCo2O4 ultra-thin curved sheets. Reproduced with permission [70]. Copyright 2019, Elsevier. | |
A freestanding and dual hierarchical MnCo2O4/Ni electrode has been established for high-performance SCs through a simple hydrothermal method without any binder followed by mild electrodeposition [69]. The highly hierarchical porosity which was constructed by macroporous Ni foam, vertical MnCo2O4 nanoflakes and mesoporous MnCo2O4 nanosheets, offered integrated diffusion channels from bottom to top and allowed the access of OH− into the inner active sites. This electrode exhibited an outstanding specific capability of 283 mAh/g (2265 F/g) at 2 mA/cm2 (1.67 A/g) in 2 mol/L KOH and high cycling duration of 15% capacitance decay after 2000 cycles.
In another work, ZnCo2O4 ultra-thin curved sheets directly anchored on Ni foam was prepared via a facile hydrothermal method with the involvement of sodium dodecyl sulfate (SDS) chains [70]. Interestingly, ZnCo2O4 nanowires can only be obtained without the addition of SDS (Figs. 4i and j). The authors explained that a large number of hydrophilic adsorption sites can be supplied by SDS for Zn2+ and Co2+. The nucleus can grow with the SDS chains and formed a parallel chain arrays owing to the electrostatic repulsion, which was finally transformed into the ultra-thin curved sheets (Figs. 4k and l). Afterwards, the ZnCo2O4 sheet-type electrode exhibited a high specific capacity of 832 C/g at the current density of 5 A/g in 2 mol/L KOH aqueous electrolyte and superior cycling stability of only 14.5% capacitance attenuation after 5000 cycles at a super-high current density of up to 50 A/g. The fabricated ASC with a working voltage of 1.7 V can output a maximum energy density of 20.31 Wh/kg at a power density of 855 W/kg.
3.2. 2D transition metal hydroxides (TMHs) electrodes 3.2.1. 2D TMH materialsTMHs, similar to TMOs, have been extensively investigated as advanced electrode materials in SCs. However, the bulk electrode morphology of TMHs often suffers from the sluggish reaction kinetics, resulting in the insufficient utilization of active sites [71]. Accordingly, spreading the bulk TMHs into their 2D counterparts can achieve fast ion/electron transport, flexible strain adaptation during cycling process and reduced ion diffusion retardation.
In this regard, a lot of work was carried out around Ni(OH)2 and Co(OH)2. For instance, Zhu et al. [72] reported the mass synthesis of high-quality ultrathin 2D α-Ni(OH)2 nanosheets by a microwave-assisted liquid phase growth method. TEM and SEM images further confirmed the existence of a well-defined sheet-like 2D structure with micro-sized flat regions and less than 2 nm of thickness. Ni K-edge XANES spectra manifested the observed spectral peaks of nanosheets slightly shifted to the higher energy direction relative to bulk counterparts (Fig. 5a), reflecting the interlayer scattering from few layers α-Ni(OH)2 nanosheets with 1.52 nm thickness. Ni K-EXAFS oscillation curve of nanosheets showed a small reduction in amplitude (Fig. 5b), suggesting the special local atomic arrangement different from bulk Ni(OH)2. There were also three noticeable differences of the Fourier transform curves (Fig. 5c), implying the structural contraction in the surface of nanosheets to balance the surplus surface energy and improve the stability of materials. The α-Ni(OH)2 electrode with a high surface atomic ratio displayed a largest specific capacitance of 4172.5 F/g at 1 A/g and excellent capacitance retention of 98.5% over 2000 cycles at a high current density of 16 A/g in 6 mol/L KOH. Hu et al. [73] also prepared ultrathin 2D Ni(OH)2 nanosheets via an acid-assisted hydrothermal strategy, which exhibited a high specific capacitance of 2537.4 F/g at 1 A/g in 6 mol/L KOH.
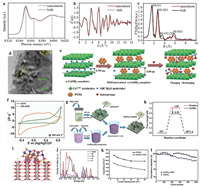
|
Download:
|
| Fig. 5. (a) Ni K-edge XANES spectra. (b) Ni K-EXAFS oscillation functions k2x(k). (c) Fourier transforms of α-Ni(OH)2 nanosheets and bulk counterparts. Reproduced with permission [72]. Copyright 2014, Nature. (d) TEM of 2D MoSe2Ni(OH)2 nanohybrid. Reproduced with permission [77]. Copyright 2019, Elsevier. (e) Schematic representation of DV-intercalated Co(OH)2 and the possible charge storage mechanism. (f) Comparative CV measurements for ACH and DV-ACH electrodes in 1 mol/L Na2SO4. Reproduced with permission [80]. Copyright 2020, Elsevier. (g) Schematic of the synthesis of Ni(OH)2CoQD on NF. (h) The adsorption free energies, (i) charge density difference, (j) total and partial density of states for the CoQD-attached Ni(OH)2 (110) surface. (k) Capacitances as a function of current density. (l) Cycling performance at 15 mA/cm2 for Ni(OH)2CoQD and Ni(OH)2 electrodes. Reproduced with permission [81]. Copyright 2018, The Royal Society of Chemistry. | |
Gao et al. [74] proposed a new concept to optimize the properties of SCs based on the atomic thickness adjustment of β-Co(OH)2 nanosheets. Five atomic layers of Co(OH)2 with preferred (001) plane orientation can be prepared by an oriented-attachment strategy, which was verified by the results of XRD pattern and TEM image. The electrode benefited from 100% exposed hydrogen atoms, demonstrating an excellent capacitance performance of 2028 F/g at 1 A/g in 2 mol/L KOH. The final fabricated all-solid-state ASC with a wide cell voltage of 1.8 V delivered a fairly high energy density of 98.9 Wh/kg at an extremely high power density of 17, 981 W/kg, comparable to that of Li-ion batteries.
3.2.2. 2D TMH-based composite materialsAlthough the single-component TMHs have achieved good results, the issue of capacitance decay at high current densities after long cycles still exists due to the slow ion transfer rate and moderate electronic conductivity [75]. Mixing them with some electrochemical active materials can be selected to address this issue, such as carbonaceous materials, metal selenides, polyoxometalates and other TMHs.
Ling et al. [76] found the rate capacitance deteriorated with the increase of electrode thickness based on the theoretical analysis and experiments of Co(OH)2 nanosheets without the addition of carbon nanotube. Meanwhile, the role of additive carbon nanotubes was further clarified. On the one hand, the charge distribution was optimized to increase the intrinsic electrode performance, and on the other hand, the rate-limiting resistance was transferred from electrode to electrolyte at high current densities to eliminate the thickness-dependent effect. Kirubasankar et al. [77] prepared a new 2D MoSe2-Ni(OH)2 nanohybrid through a simple one-step hydrothermal method. There were no MoSe2 nanoparticles found in the TEM image (Fig. 5d), indicating the presence of main MoSe2 nanoflake morphology and their random distribution onto Ni(OH)2 nanosheets. This nanohybrid possessed a unique perpendicularly oriented structure that effectively inhibited the stacking of Ni(OH)2 nanosheets, increased the accessible surface area, and shortened the ion diffusion distance. The hybrid electrode showed a higher specific capacitance and more stable cycling performance than those of pristine Ni(OH)2 due to the synergistic effect.
Recently, polyoxometalates (POMs) as a family of nanometric cluster compounds composing of early transition metals (V, Mo, W, Ta and Nb) with highest oxidation states and oxygen species exhibit special property, such as rapid and reversible multiple electron transfer without any obvious structural change [78]. For example, a chemical solution deposition strategy was carried out to build 2D layered mesoporous architecture of Ni(OH)2 intercalated with polyoxovanadate (POV) anions in 2018 [79]. The layer-by-layer ordered morphology and intercalation effects enabled the Ni(OH)2–POV electrode with improved ionic conductivity and diffusion, thus providing remarkable electrochemical activity and stability. Similarly, Kumar et al. [80] incorporated decavanadate (DV) anions into the interlayer space between α-Co(OH)2 nanoplates (ACH), constructing ion-buffering reservoirs to enhance the pseudocapacitive behavior. As shown in Fig. 5e, the introduction of DV between ACH layers boosted the in-and-out mobility of ions and provided extra pseudocapacitance under swift faradic transitions. Such pseudocapacitive contribution of DV can be confirmed by the existing multiple redox peaks in the DV-ACH voltammograms (Fig. 5f). The ASC based on DV-ACH nanoplates finally achieved a maximum energy density of 27.67 Wh/kg at a power density of 544.9 W/kg, suggesting the high efficiency of POMs modulation.
Moreover, the hybridization between different TMHs can provide abundant redox reactions and improve electrode activity. Shi et al. [81] anchored Co(OH)2 quantum dots (CoQDs) on Ni(OH)2 nanosheets by a convenient room temperature dipping technology to boost the electrode capacitance behavior (Fig. 5g). DFT calculations were performed to elucidate the decrease of OH− absorption free energy after the addition of CoQDs as a surface modifier (Fig. 5h). Bader charge method was further employed to reveal the transfer of extra electrons from the attached CoQDs into Ni(OH)2 layers. The charge density difference was calculated to shown the main accumulation of many electrons at the interface of Ni(OH)2 (110) surface and CoQDs (Fig. 5i). The result of total and partial density states (Fig. 5j) manifested the contribution of Co 3d orbitals to form the conduction band maximum (CBM) and enhance the density of states near the Fermi level, thus promoting the adsorption of OH−. Consequently, Ni(OH)2-CoQD showed better results than bare Ni(OH)2 in terms of specific capacitance, rate performance and cycling stability (Figs. 5k and l).
3.2.3. 2D LDHs and their composite materialsAmong many reported 2D electrode materials, layered double hydroxides (LDHs) with a general formula of [M1-xM′x(OH)2]x+An−x/n·mH2O (abbreviate to M1-xM′x-LDH), where M, M′ and An− represent bivalence, trivalence metallic cations and an interlayer charge-balancing anion, respectively, have received special attention owing to reversible redox kinetics and abundant chemical composition [82-84]. Ge et al. [85] systematically employed a series of chemical exfoliation, dispersion and self-assembly by co-feeding technologies to fabricate true Ni-Al LDH/rGO superlattice according to electrostatic interactions (Fig. 6a). XRD patterns (Fig. 6b) presented the diffraction peaks of (002) at approximately 16.2°, confirming the formation of stacked superlattice structure unambiguously for both Ni–Al LDH/GO and Ni–Al LDH/rGO. The superlattice electrode showed a high capacity of 129 Ah/kg at 8 min discharge, which was larger than that of Ni-Al LDH significantly. The ASC based on Ni-Al LDH/rGO with high mass loading dual electrodes still exhibited good cycling stability, that was retaining 67.4% of initial capacitance after 10, 000 cycles. Li et al. [86] synthesized ultrathin Ni-Al LDH nanosheet-assembled multi-level nanotube by an atomic layer deposition (ALD) technique followed by a simple hydrothermal treatment. SEM and TEM images both showed the tube-like NiAl LDH nanotubes with perpendicularly grafted nanoflakes (Figs. 6c and d). Such hierarchical structure offered a synergistic effect that 2D nanosheets would create accessible ion transport channels and 1D nanotube was able to avoid the agglomeration issues during prolonged charging/discharging process. Besides, the cycle number of ALD can control the morphology of final products finely, such as thickness of nanosheets and diameter of nanotubes, which was vital to the eventual electrochemical performances. Thereinto, the optimized electrodes delivered a maximum specific capacitance of 2123.7 F/g at 0.5 A/g and maintained 91.9% of the initial capacitance after 10, 000 cycles at 5 A/g (Figs. 6e and f). In another representative work, petal-like ultrathin Ni-Al LDHs nanosheets (3–5 nm) were vertically coated on the graphene/polypyrrole (GP) support [87]. This hybrid architecture can maximum the exposure of active materials and shorten the transport path of electron/electrolyte. For the working electrode derived from this specially structured material, a high specific capacitance of 2395 F/g at 1 A/g in 6 mol/L KOH can be achieved.

|
Download:
|
| Fig. 6. (a) Schematic of the synthetic process for Ni–Al LDH/rGO superlattice materials. (b) XRD patterns of Ni–Al LDH/rGO and Ni–Al LDH/GO superlattice structures. Reproduced with permission [85]. Copyright 2016, Elsevier. (c) SEM and (d) TEM images of 20NiAl-LDH. (e) Rate capabilities, (f) cycling performance of 5-, 10- and 20NiAl-LDH. Reproduced with permission [86]. Copyright 2017, Elsevier. (g) Schematic diagram for the preparation of C60–LDH nanocomposites. (h) FT-IR spectra. Reproduced with permission [88]. Copyright 2019, The Royal Society of Chemistry. | |
Kim et al. [88] also mixed 2D fullerene (C60) nanosheets with Ni–Fe-layered double hydroxide (LDH) to yield a composite of C60–LDH based on the electrostatically-induced self-assembly (Fig. 6g). The change of FT-IR peaks between 1200 cm−1 and 1500 cm−1 (Fig. 6h) indicated the homogeneous hybridization between two components. The largest specific capacitance can reach 2697 F/g at 1 A/g in 1 mol/L KOH, higher than that of the electrode without C60. The improvement of capacitance performance can be attributed to the improved porous structure, the involvement of the redox process from C60 and the enhancement of ion transfer kinetics. Due to the abundant zinc resources and its excellent Faradaic pseudocapacitance, Pan et al. [89] employed a homogeneous precipitation method to in situ crystallize 2D well-defined ZnCo1.5(OH)4.5Cl0.5·0.45H2O nanosheets (~30 nm) on the Ni foam. Synchrotron XRPD data analysis successfully revealed the complexed structure feature of this lamellar compound. The positive [ZnCo1.5(OH)4.5]0.5+ cation layer is the principal part, and the charge is conserved by Cl− anion between the layers. This electrode displayed a remarkable specific capacitance as high as 3946.5 F/g at 3 A/g in 1 mol/L KOH. Accordingly, the all-solid-state ASC delivered a striking energy density of 114.8 Wh/kg at an average power density of 643.8 W/kg, surpassing many reported relative devices. Furthermore, Elgendy et al. [90] also reported the successive ionic layer adsorption and reaction method to synthesize a ternary Ni-Zn-Fe LDH for the application in SCs firstly. The assembled ASC finally displayed a high energy density of 14.9 Wh/kg, showing an ideal application prospect.
3.3. 2D transition metal chalcogenides (TMCs) electrodes 3.3.1. 2D TMC materialsTMCs, constituting another essential category of 2D layered architecture, have been applied as electrodes for SCs due to large surface area, many edge sites, ultrathin thickness and diverse oxidation states [91-93]. Intrinsically, the extraordinary thinness of TMCs derives from the central metal atoms interacted with the layers of chalcogen atoms via the strong covalent bond and the existence of weak van der Waal's forces between individual layers. Among all TMCs, MoS2 is very voguish, which not only can provide pseudocapacitance by faradaic redox reactions in the Mo center but also electrical double layer capacitance at the interface of electrodes and electrolytes [48, 57, 94, 95]. For example, Huang et al. [96] reported a simple hydrothermal method to synthesis several nanometers thickness of 2D MoS2 with a uniform flower-like structure. The unique morphology of MoS2 can be directly characterized by SEM and TEM (Figs. 7a and b), indicating the great hallmark of large specific surface area. The MoS2 nanosheet electrode exhibited a highest specific capacitance of 129.2 F/g at 1 A/g in 1 mol/L Na2SO4 (Fig. 7c). It was also found that the water layer on both sides of metallic phase MoS2 nanosheets prepared by hydrothermal method could construct nanoscale channels with a distance of 1.18 nm for ion diffusion and improve the cyclic stability [97].
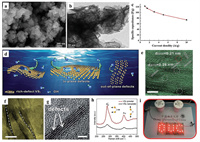
|
Download:
|
| Fig. 7. (a) SEM and (b) TEM iamges of MoS2 nanosheets. (c) Specific capacitance as a function of current density for MoS2 nanosheet electrode. Reproduced with permission [96]. Copyright 2014, Elsevier. (d) Schematic illustration of rich-defect VS2 nanoplates. (e) In-plane defects; (f) the distorted lattice stripes; (g) the defects in the side edge of VS2 nanoplates. (h) Raman spectra of VS2 powder and VS2 nanoplates. (i) Two ASCs were connected in series to light a LED. Reproduced with permission [98]. Copyright 2018, The Royal Society of Chemistry. | |
VS2, partially filled energy band (EF) at the Fermi level, has been examined as an electrochemical active material for SCs. Guo et al. [98] developed a mild colloidal chemical synthesis approach to prepare ultrathin VS2 nanoplates with rich defects (Fig. 7d). The in-plane and out-of-plane defects can be found as marked using dotted signs in the top (Fig. 7e) and side (Figs. 7f and g) view TEM image, respectively. Compared with the bulk VS2, two weaker peaks of nanoplates at 278 cm−1 and 403 cm−1 in the Raman spectra further confirmed the presence of rich defects (Fig. 7h). As a consequent, the improved resulting electrode exhibited an ultrahigh specific capacitance of 2200 F/g at 1 A/g in 1 mol/L KOH. The energy density of the fabricated ASC was 66.54 Wh/kg at a power density of 0.75 kW/kg. At the same time, the two connected devices in series were also able to successfully light the LED panel for more than 10 min (Fig. 7i), holding great promise as a good electrode material.
3.3.2. 2D TMC-based composite materialsAlthough the specific capacitance of 2D TMCs is good, their poor electric conductivity and few accessible active sites result in deteriorated cycling performance during consecutive charging and discharging process. And most TMC-based SCs only can work well under a low electrode mass loading. In this respect, considerable work has been devoted to improving the conductivity by integrating other active materials. Feng et al. [99] developed a new polymer-direct-intercalation strategy to incorporate N-doped carbon materials into the MoS2 interlayers with the assistance of carbonization (Fig. 8a). This method endowed the resulting composites with 9.8 Å MoS2 interlayers (Fig. 8b), 74% MoS2 contents and high +6 Mo valences (Fig. 8c), which can boost pseudocapacitive energy storage and rapid charge transfer. As shown in Fig. 8d, kinetics analysis was taken based on the plots of peak current density (Ip) versus square root of sweep rate (ν1/2), where the slope close to 0.5 indicated the contribution of predominant diffusion-controlled ion intercalation effect. Consequently, 4144 F/g at 1 A/g in 6 mol/L KOH was achieved for the typical MoS2/NC electrode (Fig. 8e), surpassing that of MoS2/C and pure MoS2 electrodes. Chodankar et al. [100] grew a high mass loading (7.2 mg/cm2) of MoS2 on carbon fibers (CF) by a hydrothermal method. The vertically arranged network structure was displayed in the SEM image (Fig. 8f), which was conductive to the access of electrolytes and the intercalation of ions. The specific b values between 0.91 and 0.94 at different potential was illustrated in Fig. 8g, suggesting the main involvement of surface faradaic and non-faradaic reactions. The negative electrode of MoS2@CF exhibited a maximum areal capacitance of 495 mF/cm2 (75 F/g) at 6 mA/cm2 in 1 mol/L Na2SO4 and an extremely high 95% of the initial capacitance retention at 18 mA/cm2 after 15, 000 cycles (Figs. 8h and i), which were comparable to the recently reported MoS2-based work with low mass loading of active materials.
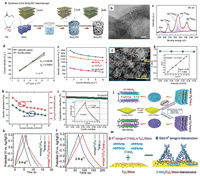
|
Download:
|
| Fig. 8. (a) The schematic of the polymer-direct-intercalation strategy for the synthesis of MoS2/NC. (b) TEM image and (c) XPS spectra of Mo 3d. (d) Dependence of the peak current density on square root of scan rate of MoS2/NC. (e) Specific capacitances as a function of current density for MoS2 nanosheets, MoS2/C and MoS2/NC. Reproduced with permission [99]. Copyright 2019, Nature. (f) SEM images of MoS2@CF. (g) Plot of b values versus the potential. (h) Capacitance versus current density and (i) capacitance-retention plot for MoS2@CF electrode. Reproduced with permission [100]. Copyright 2020, Wiley-VCH. (j) Schematic of the synthetic process for the preparation of 1T-MoS2/Ti3C2 MXene and 2H-MoS2/Ti3C2 MXene. (k) GCD curves of 2H-MoS2, Ti3C2 MXene, and 2H-MoS2/Ti3C2 MXene. (l) GCD curves 1T-MoS2, Ti3C2 MXene and 1T-MoS2/Ti3C2 MXene. (m) Schematic illustrating H+ ion storage. Reproduced with permission [101]. Copyright 2020, Wiley-VCH. | |
Importantly, since 1T-MoS2 (metastable 1T phase) has been shown to be more suitable for the use in SCs than 2H-MoS2 (semiconducting 2H phase), Wang et al. [101] designed the 2D/2D 1T-MoS2/Ti3C2 MXene heterostructure by a magneto-hydrothermal method (Fig. 8j). The specific capacitance of highly ambient-stable 1T-MoS2/Ti3C2 MXene electrode at 2 A/g in 1 mol/L H2SO4 was prominently higher than that of 2H-MoS2/Ti3C2 MXene, 1T-MoS2 and Ti3C2 MXene electrodes (Figs. 8k and l). This notable performance was primarily attributable to synergistically interplayed effect between 1T-MoS2 and Ti3C2 MXene, which constructed enough interwoven space for charge storage (Fig. 8m). Additionally, hierarchical Ni3S2 and Co3S4 were deposited on a reduced graphene oxide hydrogel@Ni foam by a two-step hydrothermal protocol [102], which both showed excellent energy storage performances. Structural integrity is particularly important for the stability of composites. Choudhary et al. developed a "one-body" WO3/WS2 core/shell structure by sequential oxidation and sulfurization on W foil current collectors, where 1D WO3 nanowire arrays were integrated with conformal 2D WS2 layers seamlessly [103]. By virtue of this integrated structure, the hybrid exhibited an exceptional cycling stability of zero loss over ultralong 30, 000 cycles.
3.3.3. 2D ternary TMCs and their composite materialsMoreover, ternary TMCs with low electronegativity and rich redox reactions have been greatly studied as a notable class of pseudocapacitive candidates in the search for advanced electrode materials [104, 105]. For example, uniform and smooth NiCo2S4 sheets can be prepared using a controllable two-step anion-exchange protocol [106]. The interwoven network was intergraded by the even nanotubes in multiple different directions. This particular morphology brought virtues of both a low contact resistance and improved porosity for the sufficient infiltration of electrolytes. Consequently, the capacitance performance can reach 1780 F/g at 1 A/g in 3 mol/L KOH, maintaining 79% of this value at 10 A/g. Strikingly, 92.4% of capacitance retention and 91.6% of Coulombic efficiency value can be obtained after a continuous 10, 000 cycles, demonstrating the marvelous stability of NiCo2S4 electrode material. Shen et al. [107] reported a hydrothermal method followed by a sulfidation process to grow NiCo2S4 nanosheets on nitrogen-doped carbon foams (NCF) with robust adhesion. The resultant binder-free NiCo2S4/NCF electrode presented an impressively enhanced specific capacitance of 877 F/g at 20 A/g in 6 mol/L KOH and possessed the merits of flexibility, lightweight and high energy density simultaneously. In another work, Chen et al. [108] investigated the synergistic effect of nickel and cobalt species in NixCo3-xS4/rGO nanocomposites by regulating their composition. By analyzing the phase structures, metal valent states and electrochemical behaviors of active materials, it can be concluded that Ni provided most of the capacitance due to its variable valence while Co increased the conductivity owing to its lower valence state. The optimized Ni1.5Co1.5S4/rGO electrode with unique sandwich-like structure exhibited high specific capacity of 347.5 mAh/g at 0.5 A/g in 2 mol/L KOH based on the mass of metal sulfides. It was also suggested in previous literature that Co was a promoted phase in TMCs. Therefore, Yang et al. [109] reported a ternary-binary compound system via a one-step hydrothermal process, where Co3S4/CoMo2S4 nanosheets (CMS) were vertically grown on reduced graphene oxide (rGO). CoMo2S4 originating from the sulfurization of Na2MoO4 first nucleated on the graphene substrate and then evolved into a sheet structure. The excess Co source further formed Co3S4 nanoparticles on CoMoS4 nanosheets, generating semicoherent interfaces. Such nanointerfaces with abundant defects left void reservoir for ions transport and buffer volume expansions during charge and discharge process. The CMS-rGO hybrid electrode exhibited a high specific capacitance of 1457.8 F/g at 1 A/g in 3 mol/L KOH, and demonstrated a nice cycling stability of maintaining 97% after 2000 cycles.
In addition, defect engineering has also been conducted to enhance the electrochemical properties of ternary metal sulfides, including increasing active sites and improving electrical conductivity. For instance, Li et al. [110] designed the introduction of sulfur vacancies using the NaBH4 treatment in CoNi2S4 nanosheets. The XPS spectra of S 2p showed the higher 2p1/2 content for r-CoNi2S4 (57.7%) than that of pristine CoNi2S4 (43.3%), reflecting the formation of more sulfur vacancies after reduction. Theoretical calculation based on the first-principle DFT analysis was carried out to reveal the stronger hybridization between Co and Ni d states and closer S p states to the Fermi level for r-CoNi2S4 nanosheets, which was in good agreement with the experimental results. Therefore, the resulting r-CoNi2S4 electrode exhibited an ideal specific capacitance of 1117 C/g at 2 A/g in 6 mol/L KOH. The ASC also presented a superior energy density of 55.4 Wh/kg at the power density of 8 kW/kg.
3.4. 2D novel MXene electrodes 3.4.1. 2D MXene materialsAn emerging family of 2D transition metal carbides or nitrides named as MXenes with a general formula of Mn+1XnTx (n = 1–4) were discovered by Y. Gogotsi in 2011 [111], where M represents an early transition metal (Ti, Cr, V, etc.), X denotes C and/or N, Tx is the surface functional group (-OH, =O, -Cl and -F). It should be noted that these materials can be developed by the selective etching of A from MAX phases, where A is mostly group 13–15 elements of the periodic table. As a type of SC electrode materials with excellent performance, MXenes possess the unique advantages of high electrical conductivity, low ionic diffusion coefficient, large surface area to volume ratio, good hydrophilicity and high surface activity [112, 113]. Electrolyte ions are favorably accessible into the 2D nanochannels between MXenes sheets, providing opportunities for highly reversible redox reactions on the active surface of the transition metal atomic layer and thereby supplying intercalative pseudocapacitance. Besides, the carbon/nitrogen atomic layers in MXenes can rapidly transfer electrons, so that great rate performance can come true even under a high charging/discharging current density.
On this account, Gogotsi's group pioneered a series of excellent work on MXene-based SCs. For example, a method using LiF and HCl to prepare Ti3C2Tx was proposed (Fig. 9a) [114]. The resulting material expanded in volume when combined with water, which can be shaped like clay and dried into a highly conductive solid (1500 S/cm) or can be rolled into a film with tens of microns thick. As shown in Fig. 9b, the additive free Ti3C2 'clay' exhibited superior cyclability and rate performance, and displayed a volume capacitance of up to 900 F/cm3 at 2 mV/s in 1 mol/L H2SO4, which was almost double the values of their previously reported work. Recently, they also reported a novel, simple and controllable concentrated H2SO4 oxidation method (Fig. 9c), in which Ti3C2Tx nanocrystals can be partially etched without affecting or destroying the crystal structure of the unetched part [115]. The utilization of electrochemically inactive by-products such as TiO2 suppressed the re-stacking of the Ti3C2Tx film, and led to an increase in the spacing between atomic layers at the same time. The Ti3C2Tx film with hierarchically porous ion channels (Fig. 9d) increased the interlayer spacing and reduced the flake size, enabling it to combine ultra-high rate performance (64% of capacitance retention at 10, 000 mV/s) with high volumetric capacitance at a practical high mass-loading (12 mg/cm2) as shown in Fig. 9e. Additionally, Orangi et al. [116] constructed all-solid-state micro-SCs (MSCs) through a 3D printing of water-based, viscoelastic and highly concentrated Ti3C2Tx MXene ink without binders on various substrates (Fig. 9f), including flexible polymer films and papers. The cross-sectional SEM image (Fig. 9g) displayed the horizontally aligned morphology of MXene flakes, which endowed the peculiar electrode with high electrical conductivity. By adjusting the number of deposited layers and electrode height, the MSC eventually exhibited a maximum areal capacitance of 1035 mF/cm2 at 2 mV/s and highest areal energy density of 51.7 µWh/cm2 (Figs. 9h and i).
|
Download:
|
| Fig. 9. (a) Schematic of the synthesis for MXene clay and the electrode preparation process. (b) Comparison of rate performances between Ti3C2Tx clay and previous HF-produced MXene. Reproduced with permission [114]. Copyright 2014, Nature. (c) Schematic illustrations of preparation processes for fabricating pristine and etched Ti3C2Tx films. (d) Schematic diagram of the ion pathway improvement in the etched Ti3C2Tx film comparing with the pristine film. (e) Gravimetric rate performance of etched Ti3C2Tx films. Reproduced with permission [115]. Copyright 2020, Wiley-VCH. (f) Schematic illustration of the 3D printing of MSCs. (g) Cross-sectional SEM image of the MSC electrode. (h) Areal capacitance as a function of sweep rate for different MSCs. (i) Ragone plots. Reproduced with permission [116]. Copyright 2020, American Chemical Society. (j) Digital photographs of Li-V2CTx films. (k) The charge density difference after Li insertion. Reproduced with permission [121]. Copyright 2019, Wiley-VCH. | |
As mentioned, the terminated -F or/and -OH groups may prevent the transport of electrolyte ions, leading to a higher diffusion barrier and decreasing the conductivity. Surface nitrogen-modified strategy was usually adopted to enhance the electrochemical performance effectively [117]. Herein, Wen et al. [118] for the first time put forward the inclusion of N atoms into Ti3C2Tx MXenes to enlarge the interlayer spacing and increase the pseudocapacitance. The post-etch annealing treatment in NH3 atmosphere endowed the products with 1.7–20.7 at% surface nitrogen species. The N doped Ti3C2Tx electrodes showed a 460% increase in gravimetric capacitance (192 F/g at 1 mV/s) compared to the pristine Ti3C2Tx (34 F/g at 1 mV/s) in 1 mol/L H2SO4. Since ammonia as a nitrogen source requires a great number of raw materials to achieve high doping levels, Yoon et al. [119] adopted a step-by-step strategy to obtain nitrogen-doped Ti2CTx with cyanamide. In this process, the formation of intermediate polymeric carbon nitride (p-C3N4) is particularly significant, which can cause Ti2CTx nanosheets to be delaminated while doping with heteroatoms. Based on the above modification, N-doped Ti2CTx delivered remarkable characteristics as a SC electrode, such as a notably increased specific capacitance of 327 F/g at 1 A/g (234 F/g for pristine-Ti2CTx) and stable cyclic performance of 96.2% retention at 5 A/g over 5000 cycles.
The self-pseudocapacitance of MXenes often suffers from poor processability and low intrinsic surface area. Ion intercalation and interspace engineering strategies are always adopted to address these issues. For one instance, the energy storage performance and electromagnetic interference shielding of MXenes can be enhanced by the cation intercalation through the hybridization of Mn 3d and O 2p orbitals [120]. Furthermore, Mohammadi et al. [121] also added another 2D chemically stable MXene composition of V2CTx beyond Ti3C2Tx through a cation-driven assembly method. XRD and XPS data of the assembled C-V2CTx flakes (where C represents Li, Na or Mg) showed no significant change after one month, both implying the suppression of oxidation in the ambient conditions. Macroscopically, the Li-V2CTx film kept invariable for one week and one month (Fig. 9j). This was concerned with the charge transferred from the inserted alkali ions to the terminating groups of MXene layers, which can be verified by the DFT calculation (Fig. 9k). A significant decrease in charge density can be found clearly near Li atoms. Bader charge analysis further showed that each Li atom lost 0.8 eV, while each -F terminating atom gained about 0.2 eV and -O terminal groups obtained approximately 0.06 eV because the charge was predominantly transferred into MXene layers. As a result, the best electrode showed an extraordinary specific capacitance value of ~1315 F/cm3 (~420 F/g) at 5 mV/s in 3 mol/L H2SO4 and unprecedented stability of ~77% capacitance retention after one million cycles. Pinto et al. [122] recently reported one kind of previously unexplored MoxV4-xC3 MXenes in a solid solution state, which can be applied as positive electrode materials in SCs compared with other MXenes. By tuning the ratio of Mo: V, the chemical environment of surface terminations and electrical conductivity can be optimized. Thereby, the optimal Mo2.7V1.3C3 electrode exhibited a high volumetric capacitance of 860 F/cm3 at 5 mV/s in 1 mol/L H2SO4 and excellent electrical conductivity of 830 S/cm at room temperature. The emergence of this novel material provides a possibility for the construction of all-MXenes SCs.
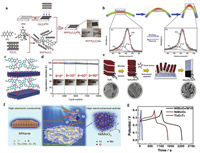
3.4.2. 2D MXene-based composite materialsIn order to further improve the performance, MXenes can be combined with organic and inorganic materials to construct heterogeneous structures, so that they can complement each other and produce a synergistic effect. Combining MXenes with conjugated polymers will provide a set of intriguing properties, such as adjustable band gap, controllable exciton and charge transport and improved water solubility. Wu et al. [123] employed 2, 6-diaminoanthraquinone (DAQ), tetrakis(4-bromophenyl) methane (TM) and Ti3C2Tx nanosheets as raw materials to prepare homogeneous PDT/Ti3C2Tx electrode films based on the Buchwald-Hartwig coupling (Fig. 10a). XPS presented the basically unchanged N 1s spectra of PDT/Ti3C2Tx electrode before and after bending test (Fig. 10b), suggesting the occurrence of excellent hybridization and the potential for good cycling stability. The atomic-scale schematic (Fig. 10c) illustrated the strong hydrogen bonds interaction between N-H groups of decentralized PDT chains and -OH or -F terminal groups of Ti3C2Tx sheets, which can offer highways for charge-carrier transfer. Meanwhile, the introduction of PDT backbones can enhance the mechanical property of this composite and inhibit the volume expansion and contraction effectively during the charging/discharging process, resulting in the negligible capacitance loss for 10, 000 cycles under different static bending angles (Fig. 10d). In another work, one-step co-electrodeposition method was carried out to use Ti3C2Tx with hydrophilic functional groups as a core polymer (Fig. 10e), promoting the gradual polymerization of pyrrole monomer radical cations and forming the MXene/PPy composite films successfully [124]. Boota et al. [125] also focused on 2D Ti3C2Tx with the modification of polyfluorene derivatives (PFDs). The polymer with charged lateral chains had a greater impact on increasing the layer spacing of MXenes than the other two polymers with nonpolar and polar side chains. This organic-inorganic hybrid electrode showed an overall improvement in electrochemical performances, including gravimetric capacitance, volumetric capacitance and capacitance retention. These researches further supported the contribution of conductive polymer to improving the electrochemical performance of MXene-based electrodes.
|
Download:
|
| Fig. 10. (a) Schematic for the preparation of PDT/Ti3C2Tx film electrode. (b) XPS spectra of N 1s for the SC under the bending and recover states. (c) Schematic of decentralized PDT chains with Ti3C2Tx. (d) Capacitance retention of the SC for 10, 000 cycles under different static bending angles. Reproduced with permission [123]. Copyright 2019, Elsevier. (e) Schematic of the electrochemical synthesis process for the PPy/MXene composite film on the ITO-coated glass. Reproduced with permission [124]. Copyright 2019, Elsevier. (f) Schematic of special ion channels and unique interaction between NiMoO4 and Ti3C2Tx. (g) Comparative GCD curves of NiMoO4/Ti3C2Tx, NiMoO4 and Ti3C2Tx electrodes. Reproduced with permission [126]. Copyright 2019, Elsevier. | |
Moreover, NiMoO4 as a promising inorganic electrode material was also hybridized with Ti3C2Tx through hydrothermal method and post-calcination to fabricate an interconnected porous 2D/2D heterostructure (Fig. 10f) [126]. Beneficial from high electronic conductivity and rich redox chemistry of two components, the resulting electrode exhibited a specific capacity of 545.5 C/g at 0.5 A/g in 3 mol/L H2SO4, which was obviously higher than that of individual component (Fig. 10g). Consequently, the assembled ASC device with an operating voltage of 1.6 V showed a desirable energy density of 33.36 Wh/kg at 400.08 W/kg. Besides, Wang et al. [127] incorporated graphite oxide (GO) as a binder into the surface and interlayers of MXenes firstly and then break the C-O bonds in GO nanosheets with the aid of CH4 plasma excitation to exfoliate MXene flakes. The graphene wrapped MXene electrode achieved a maximum areal specific capacitance of 54 mF/cm2 at 0.2 A/cm2, low equivalent series resistance of 13.6 Ω and outstanding stability of 100% capacitance retention in each 1000 individual cycles, all of which were superior to those of the pure MXene electrode obviously. Owing to the high capacity of sulfides, Luo et al. [128] incorporated Ni-S nanostructure into delaminated Ti2C3 nanosheets (d-Ti2C3). The coupling with high-conductivity d-Ti2C3 could efficiently alleviate volume expansion of battery-type Ni-S materials during charging/discharging process. The synergistic effect of both active materials made the nanohybrid electrode possess an increased specific capacity of 840.4 C/g at 1 A/g in 6 mol/L KOH.
3.5. 2D metal-organic frameworks (MOFs) electrodes 3.5.1. 2D MOF materialsSince the concept of metal organic frameworks (MOFs) as highly crystalline hybrid porous materials were firstly proposed by Yaghi and his collaborators in 1995, tremendous attention has been aroused in potential applied field, involving catalysis, sensing, gas storage and separation, energy storage and conversion [129-131]. Currently, 2D MOFs as a new family of researched objects show distinctive hallmarks of abundant exposed redox active sites, large specific surface areas and strong quantum confinements, allowing us to prepare promising electrodes with high storage capacity for SCs [29]. This 2D geometrical configurations are also beneficial for the adequate utilization of inside ion channels compared with conventional bulk MOFs.
In this section, we mainly focus on pristine and composite MOF materials, while MOF derivatives (metal oxides and carbon materials) are not in the scope of discussion. Neat MOFs of Ni3(2, 3, 6, 7, 10, 11-hexaiminotriphenylene)2 (Ni3(HITP)2) without any conductive additives or binders were entirely used as active electrode materials for the first time in 2017 [132]. As shown in Fig. 11a, Ni3(HITP)2 consisted of stacked π-conjugated 2D sheets and open cylindrical channels with a diameter of 1.5 nm, which was further determined by N2 adsorption isotherm. The schematic diagram (Fig. 11b) showed spacious compatibility between pores and large electrolyte ions (TEA+, TEA+·7ACN, BF4− and BF4−·9ACN). The anodic and cathodic sweep CV curves (Fig. 11c) presented a working potential of 1.0 V and EDLC behavior for Ni3(HITP)2. Meanwhile, ex situ XAS (Fig. 11d) was carried out to probe the quasi-reversible faradaic phenomenon occurred at about 0.7 V (vs. Ag/AgCl), revealing the center of redox reaction was on the HITP ligand rather than Ni. The symmetric device with neat Ni3(HITP)2 can obtain a capacitance normalized to specific surface area (630 m2/g) of 18 µF/cm2, which was higher than that of most carbon materials except holey graphene. In another work, 2D Ni3(HITP)2 nanosheets were also loaded on nickel foam substrates to be explored as active materials in SCs [133]. The constructed device had an impressive areal specific capacitance of 15.69 mF/cm2 at 0.1 mA/cm2 and a prominent capacitance retention of 84% over ultralong 100, 000 cycles.

|
Download:
|
| Fig. 11. (a) Molecular structure of Ni3(HITP)2. (b) Relative size of pores and electrolytes. (c) CV curves of Ni3(HITP)2 powder in a three-electrode system. (d) Ni K-edge XANES of Ni3(HITP)2 electrode. Reproduced with permission [132]. Copyright 2017, Nature. (e) Molecular structure of Cu-/Ni-HAB MOFs. (f) 2D GIXD pattern of a Cu-HAB thin film. (g) CV curves of Ni-HAB. (h) Comparison of the volumetric and areal capacitance of Ni-HAB with other materials. Reproduced with permission [134]. Copyright 2018, Nature. | |
Feng et al. [134] reported a coordination reaction in alkaline solution under the air atmosphere to synthesize robust and conductive 2D M-HAB (M = Ni or Cu, HAB = hexaaminobenzene) MOFs (Fig. 11e). Grazing-incidence X-ray diffraction (GIXD) clearly showed 2D distinct face-on orientation (Fig. 11f). The CV curves of Ni-HAB exhibited a pair of predominantly broad peaks in an almost mirror image relationship (Fig. 11g), indicating the pseudocapacitive behavior. Accordingly, the gravimetric capacitance and areal capacitance of Ni-HAB electrode with an areal density of 4 mg/cm2 were 420 F/g and 1.6 F/cm2 in 1 mol/L KOH, respectively. The assembled device based on freestanding additive-free Ni-HAB pellets with 190 µm thick showed an exceptionally high volumetric capacitance of 760 F/cm3 and areal capacitance of 13.7 F/cm2, which both outperformed the reported carbon electrodes (Fig. 11h). Wechsler and Amir [135] also developed an electrophoretic deposition method to fabricate the pristine Ni3(HAB)2 MOF as SC electrodes. The Ni3(HAB)2-based symmetric SC exhibited outstanding performances with an areal capacitance of 13.64 mF/cm2 and 81% capacity retention over continuous 50, 000 cycles. Zheng et al. [136] synthesized 2D Co2(OH)2BDC (BDC = 1, 4-benzenedicarboxylate) nanosheets through a mild surfactant-assisted one-pot hydrothermal route. This ultrathin MOF acted as a superior charge storing electrode material with a specific capacitance of up to 1159 F/g at 0.5 A/g in 3 mol/L KOH and remarkable stability of 96.7% capacitance retention after 6000 cycles.
3.5.2. 2D MOF-based composite materialsNonetheless, there are still some intrinsic shortcomings of MOFs that limit their practical application, including low conductivity and unsatisfactory chemical stability. In this case, the composite use of MOFs with different types of materials (such as conductive polymers, carbon materials) is beneficial to resolve these limitations. Yao et al. [137] developed a simple in situ chemical oxidation technology to grow polypyrrole (PPy) on 2D Cu-TCPP (TCPP = 5, 10, 15, 20-tetrakis(4-carboxyphenyl)porphyrin) nanosheets (Fig. 12a). FT-IR spectra (Fig. 12b) displayed the appearance of peak shift, corroborating the polymerization of PPy on the surface of Cu-TCPP. GCD profiles (Fig. 12c) revealed the introduction of an appropriate content of PPy could improve the electrochemical performance. The optimal electrode exhibited an excellent charge storage capacity of 496 F/g at 1 A/g in 0.5 mol/L H2SO4, higher than that of neat Cu-TCPP (245 F/g). Wang et al. [138] prepared 2D Ni2[CuPc(NH)8] nanosheets with high crystallinity and a p-type semiconducting behavior by the virtue of ball milling mechanical exfoliation method (Fig. 12d). The MSCs based on various mass ratio of graphene/Ni2[CuPc(NH)8] would presented different integral areas of CV curves (Fig. 12e) and discrepant capacity performances. The optimized device delivered a competitive areal capacitance of 18.9 mF/cm2 at 0.04 mA/cm2 (Fig. 12f). Bai et al. [139] proposed the concept of xD–2D hybrid nanostructures to obtain 1D–2D M-TCPP nanofilm/CNT ((M = Cu, Co and Ni)) and 2D–2D M-TCPP nanosheet/GO. The best specific capacitance of 2280 F/g at 5 A/g in 6 mol/L KOH can be achieved for Ni-TCPP nanofilm/CNT electrodes, confirming the significance of mixing individual components.

|
Download:
|
| Fig. 12. (a) Schematic of the synthetic process of Cu-TCPP@PPy. (b) FT-IR spectra. (c) GCD profiles. Reproduced with permission [137]. Copyright 2018, Elsevier. (d) Schematic illustration of the exfoliation for 2D Ni2[CuPc(NH)8]. (e) CV curves of MSCs based on EG and Ni2[CuPc(NH)8]/EG. (f) Capacitances as a function of current density for Ni2[CuPc(NH)8]/EG-2-based MSC. Reproduced with permission [138]. Copyright 2020, Wiley-VCH. | |
Besides the 2D materials discussed above, some other layered materials that have not been exploited on a large scale are still worthy of attention due to their intriguing electrochemical properties and high specific surface area. Nickel phosphate with the open structure of abundant channels and cavities can supply rich redox active sites and high charge storage capacity [30]. The current main work is focused on the synthesis of 1D nickel phosphate, and Yamauchi et al. successfully prepared a 2D crumpled sheet-like amorphous nickel phosphate for the first time. Nickel glycerate particles were first self-deconstructed to form nickel hydrogen phosphate nanotubes, and then underwent a self-weaving process into this 2D special morphology. The amorphous nature obtained after calcination can further improve the electrochemical performance. The ASC based on the optimal nickel phosphate material displayed a maximum energy density of 50 Wh/kg at an average power density of 362 W/kg.
As the second most abundant element in the earth's crust, silicon plays an important role in current microelectronic devices. 2D layered silicon-based nanosheets, as one kind of graphene-like Si material, was widely used as anodes of batteries due to its unparalleled specific capacity, but its application in SCs has been less reported. Based on this, Gao et al. [140] from the University of Tsukuba, reported an electrode composed of layered silicon-based nanosheets (TSNs) with excellent performance. By the reaction of CaSi2 with hydrochloric acid solution at 20 ℃ to remove Ca2+, they obtained a layered Si with Si6 ring, and then modified the Si skeleton with functional groups, including hydrogen and hydroxyl species. The SCs based on TSNs electrode can provide a high capacitance of up to 8.29 mF/cm2 and a maximum energy density of 7.65 mWh/cm3. The specific capacitance still can remain 90.6% of its initial value after 12, 000 cycles.
Based on the analysis of experimental and theoretical studies, antimonene with a puckered lamellar structure, displays great electrical conductivity (1.6 × 104 S/m), high environmental stability and ideal interlayer channer size (3.73 Å). These fascinating properties make antimonene an attractive candidate for storage energy applications. Impressively, Martínez-Periñán et al. [32] employed a modified liquid-phase exfoliation technique to prepare few-layer antimonene. This electrode showed an outstanding specific capacitance of 1578 F/g with a rather high current density of 14 A/g. Under the same conditions, the electrode can still retain about 65% of its initial value after 10, 000 cycles at 14 A/g, and the attenuation mainly occurred in the first 1000 cycles.
4. Summary and outlookWe have sorted a systematic overview of few recently reported non-carbon 2D materials such as TMOs, TMHs, TMCs, MXenes and MOFs. for current energy storage in the aqueous electrolyte-based SCs. Comparative electrochemical performances of all above mentioned 2D electrode materials are listed in Table 1. Since the charge storage mechanism near the electrode surface mainly determines the overall performance, designing the ideal electrode material with suitable pore size distribution and tunable surface characteristic is a critical factor for optimizing the function of SCs. Non-carbon 2D materials are one kind of promising candidates in improving many aspects, including adjustable conductivity, enhanced ion absorption affinity, facilitated electrochemical kinetics and effective interfacial electronic interaction. The resultant controllable 2D surface property not only guarantees the inherent advantage of quick charging and discharging process for SCs, but also offers a large number of redoxable components to achieve broad voltage window and impressive specific capacitance. Several nanomaterial engineering strategies have been applied to design advanced electrodes rationally, including the construction of core-shell architecture, fabrication of heterogeneous structure, chemical substitution, cations intercalation, defects modification, surface anchoring and so on. Considering the above approaches, the low electrical conductivity of TMOs, TMHs and MOFs is thus increased effectively. MXenes and TMCs also exhibit rich redox activity, stable cycling life and improved charge/mass transport property.
|
|
Table 1 Various 2D materials for SC electrodes. |
Nevertheless, it is of noteworthy that there are still certain bottlenecks alongside with this research field, and some reasonable suggestions should be adopted enthusiastically for further improvements. (1) SCs are used in the different energy storage sites compared with batteries and are unlikely to replace the latter, because their energy density is still considerably lower than batteries (100–300 Wh/kg) especially for aqueous electrolyte-based SCs. Considerable research advancements have been made, such as selecting electrode materials with high electrochemical activity, combining various materials, and designing nanometer multi-level structures. Taking some strategies to extend the voltage window of aqueous SCs to 2.6 V or higher is also feasible, such as electrochemical cyclic modification [62], introduction of "water in salt" [141-143], electrode modification to repel H+ and OH−. (2) The choice of negative electrode materials is relatively rare. When assembling a full device, the commonly used negative electrode material is activated carbon [144], but its specific capacitance is only about 200 F/g. In order to achieve the charge balance between two electrodes, the loading of activated carbon is much larger than that of the positive electrode active material, which will degrade the energy density. Another common negative material is Fe2O3 [145, 146], which owns high specific capacity but poor rate and cycling performance, making it unable to match the positive material perfectly. (3) In contrast to areal capacitance (F/cm2) and volumetric capacitance (F/cm3), gravimetric capacitance (F/g) in the most literature is used to evaluate the performance of electrodes. In general, carbon-based electrode materials with porous structures display high specific surface area and can provide high gravimetric capacitance, but their low packing density (g/cm3) results in low volumetric capacitance, which hinders their development in demanding industrial applications. At present, the proper balance between gravimetric and volumetric capacitance, which takes into account both packing density and resistance, has become a prime challenge [147]. (4) The self-discharge characteristic of SCs is an important factor in evaluating their performance and commercial specifications. The charged capacitor is in a high free energy state relative to the discharge state, so there is a pseudo driving force of self-discharge. But SCs have low persistence and high self-discharge rate, about 10%−40% per day, which is considered to be the main obstacle in practical applications [148-150]. (5) In order to bind the active materials together with current collectors, binders are indispensable components in most current electrodes. Among them, polytetrafluoroethylene (PTFE) and polyvinylidene fluoride (PVDF) as insulating polymers, are the most commonly used binders owing to their electrochemical stability and great binding ability [151]. However, there are still some hindering limitations in the use of binders. Firstly, these polymers have poor electrical conductivity, which will increase the internal resistance and are adverse to the rate performance of materials. Secondly, the binder usually accounts for 10%~20% of the electrode mass but providing a little capacitance will degrade the energy density of SCs. Thirdly, some porous structures may be blocked, causing local "dead pores" and reducing the available surface area. Last, the toxic fluorine-containing binders can also raise some environmental concerns which are not conducive to sustainable development. Seriously, this conventional slurry treatment based on polymer binders can destroy the ordered structures of 2D materials, leading severe self-aggregation as well as diminishing the redox reactions [57]. With this context, integrating the electrodes directly on metal current collectors without any binders will ensure mechanically and chemically stable interfaces [151]. Therefore, there will be a great deal of space for the rational design of the ordered freestanding structure growing over the current collectors in the future, which will effectively address the "dead mass" issues and boost the electron transport kinetics, especially for 2D materials [93, 103]. (6) Most 2D materials suffer from large irregularities in the lateral size and crystallite shape, which has an adverse effect on the formation of uniform pore channels inside the sheet. It is essential to design a dependable and reproducible synthesis method for the obtaining of monodisperse 2D nanosheets through minimizing the lattice strain that causes the fracture of 2D nanocrystals. (7) Most monolayer 2D nanosheets require to be synthesized stepwise, including the preparation of original lamellar materials and subsequent multistep stripping process, which demands tremendous amount of time and efforts. The corresponding one-pot synthetic methodology is only suitable for a few materials. Hence, it has become an urgent requirement to exploit a universal one-pot synthesis protocol applicable for a variety of 2D novel materials. (8) There are still enormous rooms to employ the merits of superlattice hybrid structure with face-to-face contact between layers for enriching the functionality of hybrid electrodes. Therefore, an orderly superlattice heterostructure should be formed to maximize the interfacial electronic coupling. (9) Although macroscopic performance results can be obtained through electrochemical tests, the mechanism of various reaction processes on the electrode surface still requires to be understood in-depth. It is necessary to exert systematic spectroscopic investigations combining with theoretical calculations to clarify the operating mechanism of these 2D nanostructured electrodes with high activities. Besides, a proper understanding of surface chemistry, such as chemical composition, functional groups and defect sites, will contribute to the construction of excellent electrode materials. Classical XPS [152], XRD [153] and XAS [154] technologies are difficult to meet the above requirements. In-situ spectroscopic analyses including XRD, Raman, FT-IR and XAS are efficient characterization tools to probe the transport characteristics of metal ions and the variation of surface crystalline phase under working conditions. In addition, it is very crucial to control the structural and morphological characteristics of layered materials while designing high performance devices. In-situ techniques like SEM, TEM, AFM and scanning electrochemical microscope (SECM) can be used to study various surfaces and overall reaction processes of SCs, such as morphology change, interface evolution, volume expansion/shrinkage. Recently, the in-situ piezoelectric electrochemical spectroscopy method based on nanometer depth and ultra-fast speed (femtosecond) research can provide the specific evidence of piezoelectric electrochemical phenomena in the energy conversion and storage process of SCs [155]. By combining various in-situ and ex-situ technologies, deep multi-dimensional and multi-modal information of various interface reactions occurring in EES devices can be realized, which will enable us to adjust parameters for developing smart devices. (10) Nowadays, a great deal of efforts has been devoted into developing flexible energy storage devices with stable electrochemical performance, excellent electrical conductivity, high capacity and cyclic stability. 2D layered materials are suitable candidates for the design of flexible SCs due to the presence of persistent van der Waals forces within the stacked layers, which makes these materials relatively soft and elastic. In one instance, 2D MOFs as flexible charge storage materials are in the early development stage owing to their special surface area, abundant porous architecture and unique waving nature, providing great applicable room for miniaturized and flexible SCs [156].
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsY. Hu acknowledges the financial support from National Natural Science Foundation of China (No. 21671173), the Independent Designing Scientific Research Project of Zhejiang Normal University (No. 2020ZS03) and Zhejiang Provincial Ten Thousand Talent Program (No. 2017R52043).
| [1] |
L. Yan, Z. Xu, W. Hu, et al., Nano Energy 82 (2021) 105710. DOI:10.1016/j.nanoen.2020.105710 |
| [2] |
S. Kumar, G. Saeed, L. Zhu, K, et al., Chem. Eng. J. 403 (2021) 126352. DOI:10.1016/j.cej.2020.126352 |
| [3] |
H. Wang, Y. Yang, Q. Li, et al., Sci. China Mater. 64 (2020) 840-851. |
| [4] |
H.C. Jin, S. Xin, C.H. Chuang, et al., Science 370 (2020) 192-197. DOI:10.1126/science.aav5842 |
| [5] |
H.F. Wang, K.F. Zhang, Y.Q. Song, et al., Carbon 146 (2019) 420-429. DOI:10.1016/j.carbon.2019.02.035 |
| [6] |
J. Yan, Q. Wang, T. Wei, Z. Fan, Adv. Energy Mater. 4 (2014) 1300816. DOI:10.1002/aenm.201300816 |
| [7] |
D.P. Dubal, N.R. Chodankar, D.H. Kim, P. Gomez-Romero, Chem. Soc. Rev. 47 (2018) 2065-2129. DOI:10.1039/c7cs00505a |
| [8] |
P. Kulkarni, S.K. Nataraj, R.G. Balakrishna, D.H. Nagaraju, M.V. Reddy, J. Mater. Chem. A 5 (2017) 22040-22094. DOI:10.1039/C7TA07329A |
| [9] |
F. Wang, X. Wu, X. Yuan, et al., Chem. Soc. Rev. 46 (2017) 6816-6854. DOI:10.1039/C7CS00205J |
| [10] |
M. Yu, Z. Wang, Y. Han, et al., J. Mater. Chem. A 4 (2016) 4634-4658. DOI:10.1039/C5TA10542K |
| [11] |
W. Ye, H. Wang, J. Ning, Y. Zhong, Y. Hu, J. Energy Chem. 57 (2021) 219-232. DOI:10.1016/j.jechem.2020.09.016 |
| [12] |
X. Ma, D. Gao, ChemSusChem 11 (2018) 1048-1055. DOI:10.1002/cssc.201702457 |
| [13] |
T.H. Gu, N.H. Kwon, K.G. Lee, X.Y. Jin, S.J. Hwang, Coord. Chem. Rev. 421 (2020) 213439. DOI:10.1016/j.ccr.2020.213439 |
| [14] |
K.S. Kumar, N. Choudhary, Y. Jung, J. Thomas, ACS Energy Lett. 3 (2018) 482-495. DOI:10.1021/acsenergylett.7b01169 |
| [15] |
Y. Liu, X. Peng, Appl. Mater. Today 8 (2017) 104-115. DOI:10.1016/j.apmt.2017.05.002 |
| [16] |
G. Yuan, S. Yu, J. Jie, et al., Chin. Chem. Lett. 31 (2020) 1941-1945. DOI:10.1016/j.cclet.2019.12.034 |
| [17] |
F. Bonaccorso, L. Colombo, G.H. Yu, et al., Science 347 (2015) 1246501. DOI:10.1126/science.1246501 |
| [18] |
H. Wang, W. Ye, Y. Yang, Y. Zhong, Y. Hu, Nano Energy 85 (2021) 105942. DOI:10.1016/j.nanoen.2021.105942 |
| [19] |
S.R.C. Vivekchand, C.S. Rout, K.S. Subrahmanyam, A. Govindaraj, C.N.R. Rao, J. Chem. Sci. 120 (2008) 9-13. DOI:10.1007/s12039-008-0002-7 |
| [20] |
C. Atzeri, L. Marchio, C.Y. Chow, et al., Chem. Eur. J. 22 (2016) 6482-6486. DOI:10.1002/chem.201600562 |
| [21] |
M.L. Guan, Q.W. Wang, X. Zhang, et al., Front. Chem. 8 (2020) 390. DOI:10.3389/fchem.2020.00390 |
| [22] |
S.M. Oh, S.J. Hwang, J. Korean Ceram. Soc. 57 (2020) 119-134. DOI:10.1007/s43207-020-00023-2 |
| [23] |
B. Raj, A.K. Padhy, S. Basu, M. Mohapatra, J. Electrochem. Soc. 167 (2020) 136501. DOI:10.1149/1945-7111/abb40d |
| [24] |
Y. Ibrahim, A. Mohamed, A.M. Abdelgawad, et al., Nanomater. Basel 10 (2020) 1916. DOI:10.3390/nano10101916 |
| [25] |
W.W. Zhao, J.L. Peng, W.K. Wang, et al., Coord. Chem. Rev. 377 (2018) 44-63. DOI:10.1016/j.ccr.2018.08.023 |
| [26] |
M. Guan, Q. Wang, X. Zhang, et al., Front. Chem. 8 (2020) 390. DOI:10.3389/fchem.2020.00390 |
| [27] |
L. Lin, W. Lei, S. Zhang, et al., Energy Storage Mater. 19 (2019) 408-423. DOI:10.1016/j.ensm.2019.02.023 |
| [28] |
S. Yuan, S.Y. Pang, J. Hao, Appl. Phys. Rev. 7 (2020) 021304. DOI:10.1063/5.0005141 |
| [29] |
B. Xu, H.B. Zhang, H. Mei, D.F. Sun, Coord. Chem. Rev. 420 (2020) 213438. DOI:10.1016/j.ccr.2020.213438 |
| [30] |
N.L. Wulan Septiani, Y.V. Kaneti, K.B. Fathoni, et al., Nano Energy 67 (2020) 104270. DOI:10.1016/j.nanoen.2019.104270 |
| [31] |
K. Krishnamoorthy, P. Pazhamalai, S.-.J. Kim, Energy Environ. Sci. 11 (2018) 1595-1602. DOI:10.1039/C8EE00160J |
| [32] |
E. Martínez-Periñán, M.P. Down, C. Gibaja, et al., Adv. Energy Mater. 8 (2018) 1702606. DOI:10.1002/aenm.201702606 |
| [33] |
Y.G. Wang, Y.F. Song, Y.Y. Xia, Chem. Soc. Rev. 45 (2016) 5925-5950. DOI:10.1039/C5CS00580A |
| [34] |
M. Winter, R.J. Brodd, Chem. Rev. 104 (2004) 4245-4269. DOI:10.1021/cr020730k |
| [35] |
Y.L. Shao, M.F. El-Kady, J.Y. Sun, et al., Chem. Rev. 118 (2018) 9233-9280. DOI:10.1021/acs.chemrev.8b00252 |
| [36] |
E. Raymundo-Pinero, F. Leroux, F. Beguin, Adv. Mater. 18 (2006) 1877-1882. DOI:10.1002/adma.200501905 |
| [37] |
X.T. Zhang, Z.Y. Sui, B. Xu, et al., J. Mater. Chem. 21 (2011) 6494-6497. DOI:10.1039/c1jm10239g |
| [38] |
R.L. Liu, L. Wan, S.Q. Liu, et al., Adv. Funct. Mater. 25 (2015) 526-533. DOI:10.1002/adfm.201403280 |
| [39] |
X.Y. Feng, W.F. Chen, L.F. Yan, Nanoscale 7 (2015) 3712-3718. DOI:10.1039/C4NR06897A |
| [40] |
Z.X. Zhang, H. Wang, Y.X. Zhang, et al., Chem. Eng. J. 325 (2017) 221-228. DOI:10.1016/j.cej.2017.05.045 |
| [41] |
Y. Gogotsi, R.M. Penner, ACS Nano 12 (2018) 2081-2083. DOI:10.1021/acsnano.8b01914 |
| [42] |
C. Costentin, T.R. Porter, J.M. Saveant, ACS Appl. Mater. Interfaces 9 (2017) 8649-8658. DOI:10.1021/acsami.6b14100 |
| [43] |
T. Brezesinski, J. Wang, S.H. Tolbert, B. Dunn, Nat. Mater. 9 (2010) 146-151. DOI:10.1038/nmat2612 |
| [44] |
H. Lindstrom, S. Sodergren, A. Solbrand, et al., J. Phys. Chem. B 101 (1997) 7717-7722. DOI:10.1021/jp970490q |
| [45] |
B.E. Conway, J. Electrochem. Soc. 138 (1991) 1539-1548. DOI:10.1149/1.2085829 |
| [46] |
B.E. Conway, V. Birss, J. Wojtowicz, J. Power Sources 66 (1997) 1-14. DOI:10.1016/S0378-7753(96)02474-3 |
| [47] |
W.F. Wei, X.W. Cui, W.X. Chen, D.G. Ivey, Chem. Soc. Rev. 40 (2011) 1697-1721. DOI:10.1039/C0CS00127A |
| [48] |
P. Shang, J. Zhang, W. Tang, Q. Xu, S. Guo, Adv. Funct. Mater. 26 (2016) 7766-7774. DOI:10.1002/adfm.201603504 |
| [49] |
C. Guan, J.L. Liu, Y.D. Wang, et al., ACS Nano 9 (2015) 5198-5207. DOI:10.1021/acsnano.5b00582 |
| [50] |
D.W. Wang, F. Li, H.M. Cheng, J. Power Sources 185 (2008) 1563-1568. DOI:10.1016/j.jpowsour.2008.08.032 |
| [51] |
X.X. Pan, X.M. Chen, Y. Li, Z.N. Yu, Electrochim. Acta 182 (2015) 1101-1106. DOI:10.1016/j.electacta.2015.10.035 |
| [52] |
T. Zhai, L. Wan, S. Sun, et al., Adv. Mater. 29 (2017) 1604167. DOI:10.1002/adma.201604167 |
| [53] |
Z. Ma, G. Shao, Y. Fan, et al., ACS Appl. Mater. Interfaces 8 (2016) 9050-9058. DOI:10.1021/acsami.5b11300 |
| [54] |
S.X. Wu, K. San Hui, K.N. Hui, Carbon 132 (2018) 776-784. DOI:10.1016/j.carbon.2017.12.051 |
| [55] |
H.C. Gao, F. Xiao, C.B. Ching, H.W. Duan, ACS Appl. Mater. Interfaces 4 (2012) 2801-2810. DOI:10.1021/am300455d |
| [56] |
Z.M. Li, Y.F. An, Z.G. Hu, et al., J. Mater. Chem. A 4 (2016) 10618-10626. DOI:10.1039/C6TA03358J |
| [57] |
F. Li, M. Xue, X. Zhang, et al., Adv. Energy Mater. 8 (2018) 1702794. DOI:10.1002/aenm.201702794 |
| [58] |
F. Jiang, W. Li, R. Zou, et al., Nano Energy 7 (2014) 72-79. DOI:10.1016/j.nanoen.2014.04.007 |
| [59] |
H. Zhang, C. Lu, C. Chen, et al., ChemElectroChem 4 (2017) 1990-1996. DOI:10.1002/celc.201700253 |
| [60] |
T. Ling, P.F. Da, X.L. Zheng, et al., Sci. Adv. 4 (2018) eaau6261. DOI:10.1126/sciadv.aau6261 |
| [61] |
P. Gao, P. Metz, T. Hey, et al., Nat. Commun. 8 (2017) 14559. DOI:10.1038/ncomms14559 |
| [62] |
N. Jabeen, A. Hussain, Q. Xia, et al., Adv. Mater. 29 (2017) 1700804. DOI:10.1002/adma.201700804 |
| [63] |
S. Zhu, L. Li, J. Liu, et al., ACS Nano 12 (2018) 1033-1042. DOI:10.1021/acsnano.7b03431 |
| [64] |
Z.M. Hu, X. Xiao, H.Y. Jin, et al., Nat. Commun. 8 (2017) 15630. DOI:10.1038/ncomms15630 |
| [65] |
I. Hussain, T. Hussain, C. Lamiel, K.L. Zhang, J. Power Sources 480 (2020) 228873. DOI:10.1016/j.jpowsour.2020.228873 |
| [66] |
X. Zhao, L. Mao, Q.H. Cheng, et al., Chem. Eng. J. 387 (2020) 124081. DOI:10.1016/j.cej.2020.124081 |
| [67] |
Y. Li, Y. Shan, H. Pang, Chin. Chem. Lett. 31 (2020) 2280-2286. DOI:10.1016/j.cclet.2020.03.027 |
| [68] |
X. Zhang, F. Yang, H. Chen, et al., Small 16 (2020) e2004188. DOI:10.1002/smll.202004188 |
| [69] |
C.L. Xiao, X.Y. Zhang, D.R. MacFarlane, Electrochim. Acta 280 (2018) 55-61. DOI:10.1016/j.electacta.2018.05.112 |
| [70] |
Y.S. Zhou, L. Chen, Y.T. Jiao, Z.L. Li, Y.M. Gao, Electrochim. Acta 299 (2019) 388-394. DOI:10.1016/j.electacta.2018.12.186 |
| [71] |
K.Z. Li, B.C. Zhao, J. Bai, et al., Small 16 (2020) 2001974. DOI:10.1002/smll.202001974 |
| [72] |
Y.Q. Zhu, C.B. Cao, S. Tao, et al., Sci. Rep. 4 (2014) 5787. DOI:10.1038/srep05787 |
| [73] |
X.W. Hu, S. Liu, C.H. Li, et al., Nanoscale 8 (2016) 11797-11802. DOI:10.1039/C6NR02912D |
| [74] |
S. Gao, Y.F. Sun, F.C. Lei, et al., Angew. Chem. Int. Ed. 53 (2014) 12789-12793. DOI:10.1002/anie.201407836 |
| [75] |
L.X. Zheng, L.T. Guan, J.L. Song, H.J. Zheng, Appl. Surf. Sci. 480 (2019) 727-737. DOI:10.1016/j.apsusc.2019.02.243 |
| [76] |
Z. Ling, A. Harvey, D. McAteer, et al., Adv. Energy Mater. 8 (2018) 1702364. DOI:10.1002/aenm.201702364 |
| [77] |
B. Kirubasankar, P. Palanisamy, S. Arunachalam, V. Murugadoss, S. Angaiah, Chem. Eng. J. 355 (2019) 881-890. DOI:10.1016/j.cej.2018.08.185 |
| [78] |
Y.D. Zhang, B.P. Lin, Y. Sun, et al., Electrochim. Acta 188 (2016) 490-498. DOI:10.2175/193864716821122865 |
| [79] |
J.L. Gunjakar, A.I. Inamdar, B. Hou, et al., Nanoscale 10 (2018) 8953-8961. DOI:10.1039/C7NR09626G |
| [80] |
D. Kumar, A.K. Tomar, G. Singh, R.K. Sharma, Electrochim. Acta 363 (2020) 137238. DOI:10.1016/j.electacta.2020.137238 |
| [81] |
D.W. Shi, L.Y. Zhang, N.D. Zhang, et al., Nanoscale 10 (2018) 10554-10563. DOI:10.1039/C8NR01186A |
| [82] |
G.C. Liu, X.Z. Song, S.P. Zhang, et al., J. Power Sources 465 (2020) 228239. DOI:10.1016/j.jpowsour.2020.228239 |
| [83] |
S.J. Patil, N.R. Chodankar, R.B. Pujari, Y.K. Han, D.W. Lee, J. Power Sources 466 (2020) 228286. DOI:10.1016/j.jpowsour.2020.228286 |
| [84] |
L. Wan, D.Q. Chen, J.X. Liu, et al., J. Power Sources 465 (2020) 228293. DOI:10.1016/j.jpowsour.2020.228293 |
| [85] |
X. Ge, C.D. Gu, Z.Y. Yin, et al., Nano Energy 20 (2016) 185-193. DOI:10.1016/j.nanoen.2015.12.020 |
| [86] |
X.Y. Li, L. Yu, G.L. Wang, et al., Electrochim. Acta 255 (2017) 15-22. DOI:10.1016/j.electacta.2017.09.155 |
| [87] |
D. Du, X. Wu, S. Li, et al., J. Mater. Chem. A 5 (2017) 8964-8971. DOI:10.1039/C7TA00624A |
| [88] |
Y.H. Kim, X.Y. Jin, S.J. Hwang, J. Mater. Chem. A 7 (2019) 10971-10979. DOI:10.1039/c9ta01532a |
| [89] |
Z. Pan, Y. Jiang, P. Yang, et al., ACS Nano 12 (2018) 2968-2979. DOI:10.1021/acsnano.8b00653 |
| [90] |
A. Elgendy, N.M. El Basiony, F. El-Taib Heakal, A.E. Elkholy, J. Power Sources 466 (2020) 228294. DOI:10.1016/j.jpowsour.2020.228294 |
| [91] |
J. Theerthagiri, K. Karuppasamy, G. Durai, et al., Nanomater. Basel 8 (2018) 256. DOI:10.3390/nano8040256 |
| [92] |
W. Lu, Y. Yang, T. Zhang, et al., J. Colloid Interface Sci. 590 (2021) 226-237. DOI:10.1016/j.jcis.2021.01.050 |
| [93] |
T. Deepalakshmi, T.T. Nguyen, N.H. Kim, K.T. Chong, J.H. Lee, J. Mater. Chem. A 7 (2019) 24462-24476. DOI:10.1039/c9ta08677c |
| [94] |
J. Zhou, M. Guo, L.L. Wang, et al., Chem. Eng. J. 366 (2019) 163-171. DOI:10.1016/j.cej.2019.02.079 |
| [95] |
H. Jeon, J.M. Jeong, H.G. Kang, et al., Adv. Energy Mater. 8 (2018) 1800227. DOI:10.1002/aenm.201800227 |
| [96] |
K.J. Huang, J.Z. Zhang, G.W. Shi, Y.M. Liu, Electrochim. Acta 132 (2014) 397-403. DOI:10.1016/j.electacta.2014.04.007 |
| [97] |
X. Geng, Y. Zhang, Y. Han, et al., Nano Lett. 17 (2017) 1825-1832. DOI:10.1021/acs.nanolett.6b05134 |
| [98] |
Z.L. Guo, L. Yang, W. Wang, L.X. Cao, B.H. Dong, J. Mater. Chem. A 6 (2018) 14681-14688. DOI:10.1039/C8TA03812K |
| [99] |
N. Feng, R.J. Meng, L.H. Zu, et al., Nat. Commun. 10 (2019) 1372. DOI:10.1038/s41467-019-09384-7 |
| [100] |
N.R. Chodankar, S.J. Patil, G.S.R. Raju, et al., ChemSusChem 13 (2020) 1582-1592. DOI:10.1002/cssc.201902339 |
| [101] |
X. Wang, H. Li, H. Li, et al., Adv. Funct. Mater. 30 (2020) 1910302. |
| [102] |
D. Ghosh, C.K. Das, ACS Appl. Mater. Interfaces 7 (2015) 1122-1131. DOI:10.1021/am506738y |
| [103] |
N. Choudhary, C. Li, H.S. Chung, et al., ACS Nano 10 (2016) 10726-10735. DOI:10.1021/acsnano.6b06111 |
| [104] |
S.G. Mohamed, I. Hussain, J.J. Shim, Nanoscale 10 (2018) 6620-6628. DOI:10.1039/C7NR07338K |
| [105] |
L. Mei, T. Yang, C. Xu, et al., Nano Energy 3 (2014) 36-45. DOI:10.1016/j.nanoen.2013.10.004 |
| [106] |
J. Wu, X. Shi, W. Song, et al., Nano Energy 45 (2018) 439-447. DOI:10.1016/j.nanoen.2018.01.024 |
| [107] |
L.F. Shen, J. Wang, G.Y. Xu, et al., Adv. Energy Mater. 5 (2015) 1400977. DOI:10.1002/aenm.201400977 |
| [108] |
T. Chen, Y.F. Tang, W.F. Guo, et al., Electrochim. Acta 212 (2016) 294-302. DOI:10.1016/j.electacta.2016.07.023 |
| [109] |
X. Yang, H. Sun, P. Zan, L. Zhao, J. Lian, J. Mater. Chem. A 4 (2016) 18857-18867. DOI:10.1039/C6TA07898B |
| [110] |
Z.P. Li, D. Zhao, C.Y. Xu, et al., Electrochim. Acta 278 (2018) 33-41. DOI:10.1016/j.electacta.2018.05.030 |
| [111] |
M. Naguib, M. Kurtoglu, V. Presser, et al., Adv. Mater. 23 (2011) 4248-4253. DOI:10.1002/adma.201102306 |
| [112] |
P. Dutta, A. Sikdar, A. Majumdar, et al., Carbon 169 (2020) 225-234. DOI:10.1016/j.carbon.2020.07.041 |
| [113] |
Z.R. Zhang, Z.P. Yao, X. Zhang, Z.H. Jiang, Electrochim. Acta 359 (2020) 136960. DOI:10.1016/j.electacta.2020.136960 |
| [114] |
M. Ghidiu, M.R. Lukatskaya, M.Q. Zhao, Y. Gogotsi, M.W. Barsoum, Nature 516 (2014) 78-U171. DOI:10.1038/nature13970 |
| [115] |
J. Tang, T. Mathis, X.W. Zhong, et al., Adv. Energy Mater. (2020) 2003025. |
| [116] |
J. Orangi, F. Hamade, V.A. Davis, M. Beidaghi, ACS Nano 14 (2020) 640-650. DOI:10.1021/acsnano.9b07325 |
| [117] |
Y. Tian, W. Que, Y. Luo, et al., J. Mater. Chem. A 7 (2019) 5416-5425. DOI:10.1039/c9ta00076c |
| [118] |
Y. Wen, T.E. Rufford, X. Chen, et al., Nano Energy 38 (2017) 368-376. DOI:10.1016/j.nanoen.2017.06.009 |
| [119] |
Y. Yoon, M. Lee, S.K. Kim, et al., Adv. Energy Mater. 8 (2018) 1703173. DOI:10.1002/aenm.201703173 |
| [120] |
X. Feng, J. Ning, B. Wang, et al., Nano Energy 72 (2020) 104741. DOI:10.1016/j.nanoen.2020.104741 |
| [121] |
A.V. Mohammadi, M. Mojtabavi, N.M. Caffrey, M. Wanunu, M. Beidaghi, Adv. Mater. 31 (2019) 1806931. DOI:10.1002/adma.201806931 |
| [122] |
D. Pinto, B. Anasori, H. Avireddy, et al., J. Mater. Chem. A 8 (2020) 8957-8968. DOI:10.1039/d0ta01798a |
| [123] |
X.M. Wu, B. Huang, R.Y. Lv, Q.G. Wang, Y. Wang, Chem. Eng. J. 378 (2019) 122246. DOI:10.1016/j.cej.2019.122246 |
| [124] |
X. Jian, M. He, L. Chen, et al., Electrochim. Acta 318 (2019) 820-827. DOI:10.1016/j.electacta.2019.06.045 |
| [125] |
M. Boota, M. Pasini, F. Galeotti, et al., Chem. Mater. 29 (2017) 2731-2738. DOI:10.1021/acs.chemmater.6b03933 |
| [126] |
Y.N. Wang, J.W. Sun, X.Y. Qian, et al., J. Power Sources 414 (2019) 540-546. DOI:10.3390/mi10080540 |
| [127] |
K.L. Wang, B.C. Zheng, M. Mackinder, et al., Energy Storage Mater 20 (2019) 299-306. DOI:10.1016/j.ensm.2019.04.029 |
| [128] |
Y. Luo, C. Yang, Y. Tian, et al., J. Power Sources 450 (2020) 227694. DOI:10.1016/j.jpowsour.2019.227694 |
| [129] |
O. Yaghi, Acta Crystallogr. A 58 (2002) C42. |
| [130] |
Z.X. Li, B.L. Yang, L.J. Kong, M.L. Yue, H.H. Duan, Carbon 144 (2019) 540-548. DOI:10.1016/j.carbon.2018.12.061 |
| [131] |
Q. Xu, H. Pang, H. Xue, Q. Li, S. Zheng, Nat. Sci. Rev. 7 (2020) 305-314. DOI:10.1093/nsr/nwz137 |
| [132] |
D. Sheberla, J.C. Bachman, J.S. Elias, et al., Nat. Mater. 16 (2017) 220-224. DOI:10.1038/nmat4766 |
| [133] |
D.K. Nguyen, I.M. Schepisi, F.Z. Amir, Chem. Eng. J. 378 (2019) 122150. DOI:10.1016/j.cej.2019.122150 |
| [134] |
D.W. Feng, T. Lei, M.R. Lukatskaya, et al., Nat. Energy 3 (2018) 30-36. DOI:10.1038/s41560-017-0044-5 |
| [135] |
S.C. Wechsler, F.Z. Amir, ChemSusChem 13 (2020) 1491-1495. DOI:10.1002/cssc.201902691 |
| [136] |
Y. Zheng, S.S. Zheng, Y.X. Xu, et al., Chem. Eng. J. 373 (2019) 1319-1328. DOI:10.1016/j.cej.2019.05.145 |
| [137] |
H. Yao, F. Zhang, G.W. Zhang, et al., Chem. Eng. J. 334 (2018) 2547-2557. DOI:10.1016/j.cej.2017.12.013 |
| [138] |
M.C. Wang, H.H. Shi, P.P. Zhang, et al., Adv. Funct. Mater. 30 (2020) 2002664. DOI:10.1002/adfm.202002664 |
| [139] |
W.S. Bai, S.J. Li, J.P. Ma, W. Cao, J.B. Zheng, J. Mater. Chem. A 7 (2019) 9086-9098. DOI:10.1039/c9ta00311h |
| [140] |
R.S. Gao, J. Tang, X.L. Yu, et al., Adv. Funct. Mater. 30 (2020) 2002200. DOI:10.1002/adfm.202002200 |
| [141] |
Q.Y. Dou, S.L. Lei, D.W. Wang, et al., Energy Environ. Sci. 11 (2018) 3212-3219. DOI:10.1039/c8ee01040d |
| [142] |
H. Wang, Y. Deng, J. Qiu, et al., ChemSusChem 14 (2021) 632-641. DOI:10.1002/cssc.202002236 |
| [143] |
C.Y. Yang, J. Chen, X. Ji, et al., Nature 569 (2019) 245-250. DOI:10.1038/s41586-019-1175-6 |
| [144] |
W. Lu, J.L. Shen, P. Zhang, et al., Angew. Chem. Int. Ed. 58 (2019) 15441-15447. DOI:10.1002/anie.201907516 |
| [145] |
H.W. Wang, Z.J. Xu, H. Yi, et al., Nano Energy 7 (2014) 86-96. DOI:10.1016/j.nanoen.2014.04.009 |
| [146] |
P.H. Yang, Y. Ding, Z.Y. Lin, et al., Nano Lett. 14 (2014) 731-736. DOI:10.1021/nl404008e |
| [147] |
I. Khan, N. Baig, S. Ali, et al., Energy Storage Mater. 35 (2021) 443-469. DOI:10.1016/j.ensm.2020.11.033 |
| [148] |
H. Li, T. Lv, N. Li, et al., Nanoscale 9 (2017) 18474-18481. DOI:10.1039/C7NR07424G |
| [149] |
J.F. Zang, C.Y. Cao, Y.Y. Feng, J. Liu, X.H. Zhao, Sci. Rep. 4 (2014) 6492. |
| [150] |
Y.X. Xu, Z.Y. Lin, X.Q. Huang, et al., Adv. Mater. 25 (2013) 5779-5784. DOI:10.1002/adma.201301928 |
| [151] |
B. Xu, H. Wang, Q. Zhu, et al., Energy Storage Mater. 12 (2018) 128-136. DOI:10.1016/j.ensm.2017.12.006 |
| [152] |
V. Shutthanandan, M. Nandasiri, J.M. Zheng, et al., J. Electron. Spectrosc. 231 (2019) 2-10. DOI:10.1016/j.elspec.2018.05.005 |
| [153] |
J. Nelson, S. Misra, Y. Yang, et al., J. Am. Chem. Soc. 134 (2012) 6337-6343. DOI:10.1021/ja2121926 |
| [154] |
T. Bartsch, A.Y. Kim, F. Strauss, et al., Chem. Commun. 55 (2019) 11223-11226. DOI:10.1039/c9cc04453a |
| [155] |
X.Q. Yu, Y.C. Lyu, L. Gu, et al., Adv. Energy Mater. 4 (2014) 1300950. DOI:10.1002/aenm.201300950 |
| [156] |
M.S. Javed, N. Shaheen, S. Hussain, et al., J. Mater. Chem. A 7 (2019) 946-957. DOI:10.1039/c8ta08816k |
 2021, Vol. 32
2021, Vol. 32