b Jiangxi Key Laboratory of Organic Chemistry, Jiangxi Science and Technology Normal University, Nanchang 330013, China;
c Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China
Organic and organometallic light-emitting materials are widely applied in many areas including optical devices, bioimaging and photoelectronic devices [1-12]. Especially, mechanochromic luminescence materials sensitive to external mechanical force have attracted considerable interest in both fundamental and applied research fields [13-19]. This type of stimulus-responsive smart materials can exhibit changes in emission color under mechanical stress. In general, mechanical force-responsive luminescence is expected to be triggered by chemical or physical change. Compared with chemical structural change, physical structural change is considered to be more advantageous for achieving reversible solid-state luminescence. It is well-known that the solid-state luminescence of luminophors relies strongly on their molecular packings and intermolecular interactions [20, 21]. Therefore, different luminescence may be obtained from the same emissive molecule by adjusting the corresponding molecular stacking without involving the variation of chemical structure. Indeed, intermolecular non-covalent interactions, which contain halogen bonds, hydrogen bonds, C-H•••π stacking, π-π stacking, etc., may be effectively manipulated via different types of external stimuli such as grinding, pressing, rubbing, or hydrostatic pressure, enabling the exploitation of highly-efficient mechanoluminochromic luminophors. Over the last two decades, a large number of mechanochromic luminophors have been discovered, and the majority of these reported mechano-responsive luminescent molecules are proved to be organic compounds. Meanwhile, a number of metal-organic complexes exhibiting mechanoluminochromic characteristics have also been reported.
Owing to the existence of attractive aurophilic interactions induced by the synergy of relativistic effects and dispersion forces, luminophors involving gold(I) complexes have aroused increasing attention [22-33]. To date, a series of gold(I) complexes possessing various photophysical properties have been reported. For example, in 2012, Luo et al. reported aggregation-induced emission (AIE)-active gold(I)-thiolate complexes [34]. Subsequently, in 2013, Liu et al. reported a gold(I) complex simultaneously exhibiting AIE and thermochromic luminescence properties [35]. Bright solid-state emission and mechanochromic luminescence with high contrast are two vital factors for high-performance mechanical force-responsive smart materials. Gold(I) complexes are expected to exhibit excellent AIE or strong aggregative-state luminescence feature triggered by intriguing aurophilic Au•••Au interactions, which is beneficial for the preparation of high-efficiency mechanochromic luminophors. In addition, given the fact that intramolecular and intermolecular aurophilic Au•••Au interactions may be controlled by the method of external mechanical stress [36], introducing gold(I)-containing structural units into suitable molecule skeletons is a rational strategy for the development of mechanoluminochromic metal complexes. Motivated by the effective design idea, a variety of mechanochromic luminescent gold(I) complexes with various auxiliary ligands have been synthesized. Although the corresponding examples about the detailed applications of these gold(I) complexes are still rather rare, it is no doubt that these mechanically responsive luminescence gold(I) complexes are promising candidates for the development of mechanical sensors and molecular switches. To date, there is no relevant review summarizing AIE and non-AIE-active mechanochromic luminescence gold(I) complexes in the last two decades. Indeed, it is significative for the development of stimuli-responsive gold(I) chemistry to summarize and discuss the researches related to AIE and non-AIE-active gold(I)-bearing luminophors with mechanochromism.
In this review, we will provide a comprehensive summary of progress in mechanoluminochromic gold(I) complexes (Scheme 1). More specifically, the review can be divided into three sections according to the number of Au(I) units in the molecular structures. The mechanochromic luminescence of reported AIE and non-AIE-active gold(I) complexes including mononuclear, binuclear, and multinuclear gold(I) units will be summarized. Meanwhile, their mechanochromic mechanisms of these gold(I) complexes will be also discussed. Finally, this review is expected to provide a valuable reference for designing high-efficient mechanochromic gold(I)-containing luminophors.
|
Download:
|
| Scheme 1. Mechanochromic luminescence of gold(I) complexes. | |
2. Mononuclear Au(I) complexes with mechanochromic luminescent (MCL) characteristics
With the rapid development of mechano-responsive gold(I) complexes, many mononuclear MCL gold(I) complexes with AIE or non-AIE effect have been reported. For AIE-active mononuclear MCL gold(I) complexes, crystalline-to-amorphous (CTA) phase transition is responsible for their observed MCL phenomena, while as for non-AIE-active mononuclear gold(I) complexes, their MCL phenomena are attributed to CTA phase transition, crystalline-to-crystalline (CTC) phase transition or uncertain mechanisms.
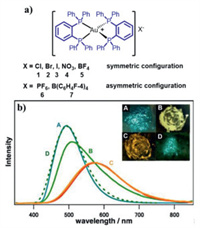
2.1. Mononuclear MCL Au(I) complexes without AIE effect 2.1.1. The MCL phenomena resulting from CTA phase transitionOsawa et al. designed a series of tetrahedral Au(I) complexes 1–7 to study the effect of counter anions on their mechanochromic behaviors (Fig. 1a) [37]. Indeed, the types of counter anions could directly affect their mechanochromic properties. Compared with complexes 6 and 7, complexes 1–5 possessed smaller counter anions, and these complexes showed intense blue phosphorescence. Complexes 1–7 exhibited obvious MCL behaviors. For example, as shown in Fig. 1b, the strong blue phosphorescence of microcrystalline sample of 5 was finally converted to yellow-orange luminescence with a yellow-emitting intermediate state after continuous grinding for an hour. Upon treating the yellow-orange-emitting sample with diethyl ether, the initial blue luminescence was observed again. The powder X-ray diffraction (PXRD) measurements of 5 in various solid states demonstrated that its MCL phenomenon was attributed to the morphological interconersion between the crystalline and amorphous states. Similarly, complexes 1, 2, 3, 6 and 7 also displayed MCL responses resulting from CTA phase transition. Notably, for MCL complex 4, "a mechanical equilibrium" of crystalline and amorphous states could be achieved after consecutive grinding.
|
Download:
|
| Fig. 1. (a) Molecular structures of complexes 1–7. (b) Corrected emission spectra (λexc = 350 nm) and the insets show photographic images of 5 in various states under UV irradiation. Reproduced with permission [37]. Copyright 2010, Wiley-VCH (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
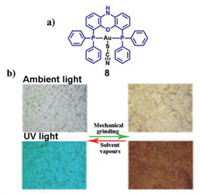
Baranyai et al. reported a mononuclear gold(I)-diphosphine complex [38]. Interestingly, the complex displayed both mechanochromic and MCL characteristics. Upon mechanical grinding, complex 8 changed its color from white to yellow. Meanwhile, its blue luminescence was transformed into the red luminescence (Fig. 2). By analyzing the obtained PXRD results, the authors discovered that the crystallinity of 8 gradually decreased with the increasing of grinding time, and an amorphous state was obtained after 13 min of ball-milling. Furthermore, the original crystalline state was restored upon treating with methanol solvent. Therefore, the reversible mechanochromic and MCL behaviors could be attributed to the CTA and amorphous-to-crystalline phase transitions.
|
Download:
|
| Fig. 2. (a) The molecular structure of complex 8. (b) Photographs of complex 8 in different states (before grinding, after grinding, and after treating with dichloromethane) under ambient light and 365 nm UV light. Reproduced with permission [38]. Copyright 2015, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
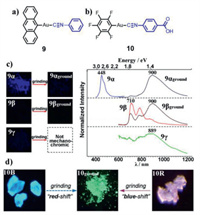
Seki et al. described a mononuclear gold(I) isocyanide complex 9 with an anthryl unit (Fig. 3a) [39]. Excitingly, the authors obtained three polymorphs 9α,9β and 9γ with different luminescence properties of 9. For polymorphs 9α and 9β, the emission maxima were bathochromically shifted to 900 nm in response to mechanical grinding, exhibiting mechano-responsive IR-emissive feature (Fig. 3c). Moreover, polymorph 9γ with IR-emissive behavior was also obtained, which was beneficial to the deep understanding of the rare mechanochromic IR emission. In 2018, Seki et al. synthesized an arylgold (arylisocyanide) complex 10, and recrystallization of complex 10 from dichloromethane/methanol provided two polymorphs 10B and 10R [40]. Interestingly, polymorph 10B exhibited MCL behavior with bathochromic spectral shift, while polymorph 10R exhibited MCL behavior with hypsochromic spectral shift (Fig. 3d). It was speculated that the variations of their intermolecular aurophilic interactions were opposite after grinding 10B and 10R. For 10B, intermolecular aurophilic interactions were formed upon grinding, which resulted in red-shifted luminescence. While for 10R, intermolecular aurophilic interactions became weaker upon grinding, which caused blue-shifted luminescence.
|
Download:
|
| Fig. 3. Molecular structures of complexes 9 (a) and 10 (b). (c) Photoluminescence photographs and emission spectra of 9α/9β/9γ before and after grinding, excitation wavelength: 365 nm. Reproduced with permission [39]. Copyright 2017. American Chemical Society. (d) Photoluminescence photographs of 10B and 10R before and after grinding, excitation wavelength: 365 nm. Reproduced with permission [40]. Copyright 2018, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Dong et al. reported five mononuclear gold(I) isocyanide complexes 11a–11e with alkyl chains of different lengths [41]. The solid-state emission behaviors of these complexes could be affected by the alkyl chain length. Indeed, 11a–11e displayed various solid-state luminescence (Fig. 4a), and these complexes showed different luminescent spectra in the solid state (Fig. 4b). Furthermore, upon grinding, 11a–11e all exhibited mechanochromic luminescence quenching properties. Taking complex 11a as a representative example, as shown in Fig. 4c, after grinding, the green emission band with the maximal emission peak at 480 nm almost disappeared, indicating that 11a possessed a force-induced emission quenching feature. The formation of strong intermolecular interactions and conformation planarization after grinding were responsible for luminescence quenching phenomena. On the other hand, the PXRD results demonstrated that the mechanical force-responsive luminescence quenching processes of 11a–11e involved the CTA morphological transition.
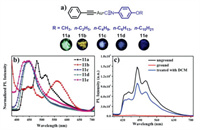
|
Download:
|
| Fig. 4. (a) Molecular structures of complexes 11a–11e and photographic images of solid samples 11a–11e under 365 nm UV light. (b) Photoluminescence spectra of 11a–11e in the solid state, excitation wavelength: 365 nm. (c) Photoluminescence spectra of 11a in various solid states, excitation wavelength: 365 nm. Reproduced with permission [41]. Copyright 2018, Elsevier Ltd. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Chen, Liu and coworkers designed and synthesized a series of triphenylamine, carbazole or tetraphenylethylene-based MCL mononuclear gold(I) complexes. For example, the as-synthesized complexes 12a and 12b displayed reversible bathochromic or hypsochromic MCL behavior respectively, whereas the pristine complex 12c displayed irreversible hypsochromic MCL phenomenon [42]. For complex 13, it showed self-reversible MCL phenomenon involving color change from yellow to yellow-green [43]. As for complexes 14–16, they exhibited contrasting mechano-responsive emissive behaviors [44]. More specifically, 14 showed luminescence on-off MCL phenomenon with yellow luminescence disappeared after grinding (Fig. 5). 15 also showed switchable MCL phenomenon between yellow–green and colorless. In contrast, no MCL phenomenon was observed for 16. The noticed mechanical force-responsive characteristics of 12–15 were ascribed to CTA phase transition.
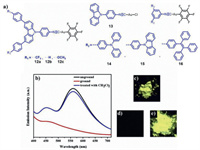
|
Download:
|
| Fig. 5. (a) Molecular structures of complexes 12–16. (b) PL spectra of complex 14 in various solid states, excitation wavelength: 365 nm. Photographic images of complex 14 in various solid states under 365 nm UV light: (c) the unground sample, (d) the ground sample, (e) the sample after treatment with dichloromethane. Reproduced with permission [44], Copyright 2018. Elsevier Ltd. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Wang et al. synthesized an aryl gold(I) isocyanide complex 17 [45]. As can be seen in Fig. 6, after heavy grinding, green light-emitting solid of 17 was changed into yellow light-emitting solid with the CTA transformation process, and the recrystallization of ground solid 17 could restore its initial green luminescence. Interestingly, the crystalline phase was also recovered when ground solid 17 was placed at room temperature for 10 h, and thus complex 17 showed an unusual self-reversible MCL feature. It was speculated that the mechanically triggered amorphous state could be restored to the initial crystalline state over a lower energy barrier at room temperature via an effective synergistic action of weak intermolecular halogen bonds and π•••π stacking interactions.

|
Download:
|
| Fig. 6. (a) Molecular structure of complex 17. (b) Solid-state emission spectra of complex 17 in various states. (c) Photoluminescence image of unground complex 17 under 365 nm UV irradiation. (d) Photoluminescence image of ground complex 17 under 365 nm UV irradiation. Reproduced with permission [45]. Copyright 2019, American Chemical Society (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Espinet et al. reported two MCL gold(I) isocyanide complexes 18 and 19 [46]. Upon grinding, the blue luminescence of solid 18 changed to a bright yellow luminescence (Fig. 7), and the green luminescent solid 19 was also converted to the similar yellow light-emitting solid. PXRD measurements demonstrated that blue light-emitting sample 18 and green light-emitting sample 19 lost crystalline nature after grinding. It was possible that different molecular packings of unground solids 18 and 19 were transformed into almost identical molecular stackings accompanied by shorter Au•••Au distances upon grinding, which were responsible for the observed mechanically induced red-shifted luminescence of complexes 18 and 19.

|
Download:
|
| Fig. 7. (a) Molecular structures of complexes 18 and 19. (b) Photoluminescence image of unground complex 18 under 365 nm UV irradiation. (c) Photoluminescence image of ground complex 18 under 365 nm UV irradiation. Reproduced with permission [46]. Copyright 2019, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
CTC transformation caused by a mechanical stimulus is a very significative phenomenon, and it provides a molecular-level understanding of mechanochromic process. The single-crystal-to-single-crystal (SCSC) transformation of a gold(I) isocyanide complex 20 (Fig. 8) was firstly observed by Ito et al. in 2013 [47]. The authors obtained two polymorphs 20b and 20y, which emitted blue luminescence and yellow luminescence respectively, by means of rapid crystallization or slow crystallization from hexane/CH2Cl2. Interestingly, for polymorph 20b, upon mechanical stimulus, the emission color was changed from blue to yellow with a SCSC molecular domino transformation, and the molecular packing of the resulting yellow light-emitting crystal was similar to that of polymorph 20y. The observed SCSC transformation involving a conversion from C-H•••π to intermolecular gold-gold interactions was characterized by single crystal X-ray analysis. The phase transition was accompanied by an obvious emission color change, which allowed a clear visualization of phase transition.

|
Download:
|
| Fig. 8. Molecular structures of complexes 20–26. | |
Similarly, Ito et al. synthesized the methyl-substituted analogue of complex 20 [48], which formed polymorph 21g with green emission and polymorph 21b with blue emission. Notably, the whole single crystal 21g could be converted to a blue-emitting single crystal 21b via a weak mechanical stimulation-induced SCSC phase transition. Furthermore, as shown in Fig. 9, for 21g, it showed no change of luminescent color after heavy grinding [49]. However, a prominent crystalline structural change was noticed. Conversely, 21b exhibited an obvious change of luminescent color, but it retained its initial crystalline structure upon strong grinding [49].
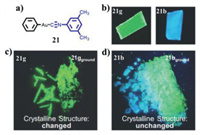
|
Download:
|
| Fig. 9. (a) Molecular structure of complex 21. (b) Photoluminescence images of polymorphs 21g and 21b. (c) Photoluminescence images of unground 21g and ground 21g. (d) Photoluminescence images of unground 21b and ground 21b. Reproduced with permission [49]. Copyright 2015, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
In 2017, Ito et al. prepared a thienyl gold(I) isocyanide complex 22 exhibiting low-temperature-selective mechanochromic behavior [50]. The complex did not display MCL phenomenon at room temperature. In contrast, when cooled below −50 ℃, complex 22 showed notable MCL phenomenon (Fig. 10). As exhibited in Fig. 11, PXRD patterns indicated that the luminescent transformation from blue light-emitting 22RBlue to green light-emitting 22G upon grinding below −50 ℃ was attributed to crystalline structure changes.

|
Download:
|
| Fig. 10. Molecular structure of complex 22, and schematic diagram of low-temperature-selective mechanochromic behavior of 22. Reproduced with permission [50]. Copyright 2017, The Royal Society of Chemistry. | |
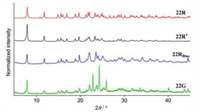
|
Download:
|
| Fig. 11. PXRD patterns of 22R, 22R', and 22G. Reproduced with permission [50]. Copyright 2017, The Royal Society of Chemistry (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.). | |
Ito et al. reported a systematic screening approach to discover mechanochromic compounds exhibiting a CTC phase transition [51]. The authors synthesized 48 para-substituted (R4) phenyl[para-substituted (R5) phenyl isocyanide]gold(I) complexes (Fig. 12). An effective three-step screening program was then performed. Consequently, two gold(I) complexes 20 and 23 showed a CTC phase transition after mechanical stimulation. For complex 23, the PXRD pattern of its ground sample also displayed many intense diffraction peaks, which were different from those in the PXRD pattern of its unground sample. This implied that complex 23 undergone a CTC phase transition upon applying mechanical stimulation.
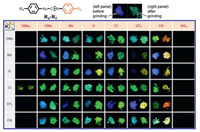
|
Download:
|
| Fig. 12. Photographs of the 48 gold(I) complexes before and after grinding under 365 nm UV light. Reproduced with permission [51]. Copyright 2016, American Chemical Society. | |
Ito et al. prepared a mononuclear gold(I) complex 24 [52]. Upon ball milling of its crystals 24B with blue luminescence, a CTC phase transition occurred, and a yellow-emitting powder 24YG was obtained. Notably, both the luminescent characteristics and morphological structure of 24YG were similar to those of crystals 24Y obtained upon photoirradiation of 24B. This is the first report of mechanical force promoting a phase transition to a photo-accessible crystalline phase.
In 2017, Ito et al. reported a gold(I) complex 25 containing a biphenyl unit, which afforded two types of crystals that involved chiral crystal 25ch and achiral crystal 25ac (Fig. 13) [53]. Upon grinding, the chiral crystal 25ch was converted into achiral crystal 25gROUND, which possessed a crystal structure similar to 25ac. It is the first example of mechanical-stimulation-triggered chiral-crystal-to-achiral-crystal phase transition with a clear luminescence change. Subsequently, in 2018, Ito et al. reported a MCL gold(I) isocyanide complex 26 with a mechanical-cutting-triggered SCSC phase transition feature [54]. More interestingly, the complex firstly achieved the reverse phase transition of the obtained crystalline phase after mechanical stimulus via the method of vapor-induced SCSC phase transition.

|
Download:
|
| Fig. 13. Photographs of, and 25ac recorded under UV irradiation, and the schematic diagram of phase transitions. Reproduced with permission [53]. Copyright 2017, American Chemical Society. | |
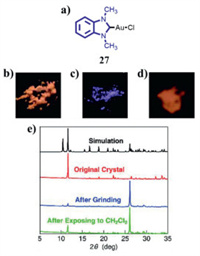
In 2018, Hisano et al. reported a MCL gold(I) N-heterocyclic carbene complex 27 (Fig. 14a) [55]. Aurophilic interactions were considered to be the key factor for the observed MCL behavior of 27. As can be seen in Fig. 14b, the initial crystals 27 obtained by recrystallization emitted orange luminescence due to the presence of intermolecular gold-gold interactions. Upon grinding, the gold-gold interactions were destroyed and the orange luminescence changed into the blue luminescence (Fig. 14c). According to the PXRD pattern of ground crystals 27 (Fig. 14e), some sharp diffraction peaks were noticed, which implied that the ground crystals 27 was also crystalline phase state. The orange luminescence could be restored completely in the presence of dichloromethane vapor (Fig. 14d). However, the corresponding PXRD pattern recovered only partially (Fig. 14e), which was due to the possibility that morphological structure recovered only occurred in the surface of the crystal.
|
Download:
|
| Fig. 14. (a) Molecular structure of complex 27. Photoluminescence images of complex 27 in various states under 365 nm UV light: (b) unground, (c) ground, (d) vapor-exposure. (e) PXRD patterns of simulation based on single crystal 27 and single crystal 27 in various states. Reproduced with permission [55]. Copyright 2018, Springer (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
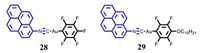
Liu et al. prepared two pyrene-based gold(I) complexes 28 and 29 (Fig. 15) [56]. 28 and 29 exhibited similar reversible MCL phenomena between blue and green emissions, and the green luminescence came from mechano-triggered excimer emission, which was induced by the amorphization of crystalline samples 28 and 29. Furthermore, the MCL behavior of 29 was self-reversible.
|
Download:
|
| Fig. 15. Molecular structures of complexes 28 and 29. | |
Laguna et al. reported a gold(I)-containing heterodimetallic complex {Tl[Au(C6Cl5)2]}n (30, Fig. 16) [57]. After gentle crushing, the emission maximum of its crystals exhibited a blue shift from 560 nm to 547 nm at 77 K. Deák et al. prepared a heterodimetallic coordination polymer Me3Sn[Au(CN)2] (31, Fig. 16) [58], and its pink emission was converted into the blue luminescence upon grinding. However, the MCL mechanisms of 30 and 31 are still unknown.
|
Download:
|
| Fig. 16. Molecular structures of complexes 30 and 31. | |
Chen et al. synthesized seven carbazole-containing gold(I) isocyanide complexes 32–38 (Fig. 17), and these complexes possessed alkyl chains of different lengths [59]. All these obtained gold(I) complexes displayed typical AIE and reversible MCL characteristics. The MCL mechanism of 32–38 was ascribed to CTA phase transition. Meanwhile, the observed AIE and MCL phenomena were associated with the formation or alteration of intermolecular C-H•••F, π•••π, and aurophilic interactions.

|
Download:
|
| Fig. 17. Molecular structures of complexes 32–41. | |
In 2016, Chen et al. reported three mononuclear gold(I) complexes 39–41 (Fig. 17) [60]. These complexes showed excellent AIE feature involving luminescent color transformation from colorless to green upon aggregation. Furthermore, 39–41 also showed reversible MCL behaviors. For example, the yellow-green luminescence of the as-prepared complex 40 was converted into the green luminescence after grinding, and the original yellow-green luminescence could be restored completely when the ground powder of 40 was fumigated with dichloromethane vapor for 1 min (Fig. 18).

|
Download:
|
| Fig. 18. (a) Emission spectra of complex 40 (2.0 × 10−5 mol/L) in DMF-H2O mixtures with different water contents (0–90%), excitation wavelength: 350 nm. Photographic images of complex 40 in various solid states under 365 nm UV light: (b) the unground sample, (c) the ground sample, (d) the sample after treatment with dichloromethane. Reproduced with permission [60]. Copyright 2016, Elsevier Ltd. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
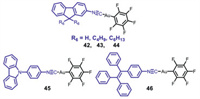
Chen et al. synthesized three AIE-active fluorene-based mononuclear gold(I) isocyanide complexes 42–44 (Fig. 19) [61], and complex 42 without containing alkyl chains exhibited reversible MCL phenomenon involving interconversion between green and faint yellow luminescence, and its MCL behavior was resulted from CTA phase transition. In contrast, complexes 43 and 44 with various alkyl chains did not show any MCL effect.
|
Download:
|
| Fig. 19. Molecular structures of complexes 40–44. | |
In 2017, Chen et al. reported a carbazole-based AIE-active gold(I) isocyanide complex 45 [62], and this complex could emit long-lived room-temperature phosphorescence with a luminescence lifetime up to 86.84 ms in the solid state. Upon grinding, its bright yellow phosphorescence was changed into yellow-green luminescence, and thus exhibiting MCL behavior (Fig. 20). In addition, 45 also showed vapochromic luminescence behavior. It is the first report of an AIE-active gold(I) complex possessing persistent room-temperature phosphorescence and multistimuli-responsive properties.

|
Download:
|
| Fig. 20. (a) Solid-state emission spectra of complex 45 in various solid states, excitation wavelength: 365 nm. Photographic images of complex 45 in various solid states under 365 nm UV light: (b) the unground sample, (c) the ground sample, (d) the sample after treatment with dichloromethane. Reproduced with permission [62]. Copyright 2017, Elsevier Ltd. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Yuan et al. reported a tetraphenylethene-containing mononuclear gold(I) complex 46, which exhibited aggregation induced phosphorescence nature [63]. Owing to the synergistic effects of conformation planarization and the formation of intermolecular aurophilic interactions, the phosphorescent color of solid sample 46 changed from blue to green after grinding, and this observed MCL phenomenon was associated with CTA phase transition.
3. Mechanochromic luminescence of binuclear gold(I) complexesTo date, many binuclear gold(I) complexes have been found to be able to show MCL behavior. Consistent with the source of mechanochromic luminescence of mononuclear gold(I) complexes, the MCL mechanism of a majority of binuclear gold(I) complexes is also attributed to either CTA phase transition or CTC phase transition. Meanwhile, the MCL mechanisms of several binuclear gold(I) complexes are relevant with chemical reactions or are still unknown. In the following portion, a series of binuclear gold(I) complexes with or without AIE effect in response to mechanical stimulation will be introduced.
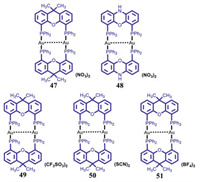
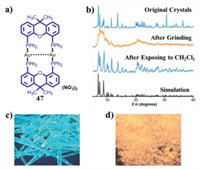
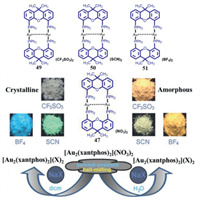
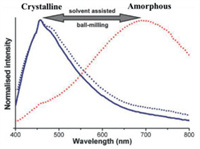
3.1. Binuclear MCL Au(I) complexes without AIE effect 3.1.1. The MCL phenomena resulting from CTA phase transitionDeák and coworkers reported a series of binuclear three-coordinate [Au2(xantphos)2](X)2 (X = NO3, CF3SO3, SCN, BF4) and [Au2(nixantphos)2](NO3)2 (Fig. 21). As shown in Fig. 22, upon grinding, blue light-emitting crystalline 47 was converted into red luminescent powder with a large red-shift of 200 nm in emission [64]. Moreover, the initial blue emission could be recovered upon fuming the ground red light-emitting powder with dichloromethane vapor. PXRD experimental results confirmed that this observed reversible MCL phenomena were resulted from the mutual transformation between crystalline and amorphous states. The MCL behavior of gold(I) complex 48 was also checked, and the solid-state luminescent color of 48 could be switched effectively between ochre and dark red, which was attributed to the reversible transformation of crystalline and amorphous phases [65]. In 2014, Deák et al. prepared crystalline and amorphous dinuclear gold(I) helicates 49–51 with different anions via a high-efficiency solvent-assisted mechanochemical method [66]. Interestingly, the solid-state luminescence of crystalline 49–51 were different from their respective amorphous forms (Fig. 23). The crystalline helicates 49 and 51 were amorphized upon mechanical milling in the presence of water, and thus helicates 49 and 51 showed MCL features, while the helicate 50 still retained its crystalline nature even after mechanical milling for one hour. As can be seen in Fig. 24, helicate 51 exhibited a reversible MCL property with a high contrast between blue and orange-red colours, and the maximum emission wavelength exhibited a large reversible shift of 235 nm via the solvent-assisted ball-milling method.
|
Download:
|
| Fig. 21. Molecular structures of complexes 47–51. | |
|
Download:
|
| Fig. 22. (a) Molecular structure of complex 47. (b) PXRD patterns of crystals 47 in various states and simulation based on single crystal 47. Photoluminescence images of blue light-emitting crystals 47 before and after grinding under 365 nm UV light: (c) before grinding, (d) after grinding. Reproduced with permission [64]. Copyright 2014, The Royal Society of Chemistry (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.). | |
|
Download:
|
| Fig. 23. Molecular structures of complexes 49–51 and photoluminescence images under 365 nm UV light of crystalline and amorphous 49–51 obtained from solvent-assisted ball-milling. Reproduced with permission [66]. Copyright 2014, The Royal Society of Chemistry. | |
|
Download:
|
| Fig. 24. Luminescence spectra of crystalline helicate 51, amorphous helicate 51 obtained from crystalline helicate 51 via a water-assisted ball-milling, and crystalline helicate 51 obtained from amorphous helicate 51 via a dichloromethane-assisted ball-milling. Reproduced with permission [66]. Copyright 2014, The Royal Society of Chemistry. | |
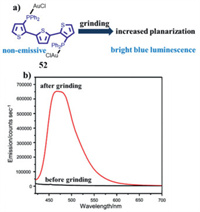
Wolf et al. synthesized a binuclear gold(I) complex based on a terthienyl diphosphine ligand [67]. This gold(I) complex 52 was non-emissive in the crystalline state, and an intense blue luminescence was observed when the initial sample was ground (Fig. 25), which was ascribed to the amorphization of as-prepared microcrystalline sample 52. Meanwhile, the mechanically triggering blue emission was associated with increased planarization of a conjugated terthienyl ligand.
|
Download:
|
| Fig. 25. (a) Molecular structure of complex 52 and the MCL schematic diagram of 52. (b) Emission spectra of 52 before and after grinding. Reproduced with permission [67]. Copyright 2009, The Royal Society of Chemistry. | |
López-de-Luzuriaga et al. reported a yellow light-emitting [Au2Ag2(4-C6F4I)4]n (53) (Fig. 26a) [68], and its emissive spectrum displayed an emission maximum at 577 nm. The initial yellow luminescence (Fig. 26b) could be switched to an intense orange luminescence (Fig. 26c) with a λmax at 617 nm by grinding, and this observed MCL behavior of 53 was related with partial amorphization of crystalline sample 53.

|
Download:
|
| Fig. 26. (a) Molecular structure of complex 53. Photoluminescence images under 365 nm UV light of before grinding (b) and after grinding (c). Reproduced with permission [68]. Copyright 2010, American Chemical Society (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
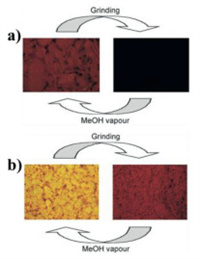
In 2015, Deák et al. reported five MCL [Au2(nixantphos)2](X)2 (X = NO3, 48; CF3COO, 54; CF3SO3, 55; [Au(CN)2], 56; BF4, 57) (Fig. 27) [65]. Crystals 54 displayed switchable MCL quenching feature, and complex 55 displayed reversible MCL phenomenon involving the interconversion of yellow luminescence and red luminescence (Fig. 28). As shown in Fig. 29, crystals 56 exhibited MCL property accompanied with emission color changes from orange to red, and this MCL process was reversible. Complex 57 also exhibited reversible MCL switching between orange and dark red. The MCL phenomena of 48 and 54–57 were resulted from the CTA phase transition. In addition, variations in the N-H•••X hydrogen-bonding interactions between the [Au2(nixantphos)2]2+ cations and X−counteranions were also important factors for the formation of their MCL phenomena of 48 and 54–57.

|
Download:
|
| Fig. 27. Molecular structures of complexes 54–57. | |
|
Download:
|
| Fig. 28. (a) Photoluminescence images under 365 nm UV light of stimuli-responsive luminescence on–off switching of crystals 54. (b) Photoluminescence images under 365 nm UV light of stimuli-responsive luminescence of 55. Reproduced with permission [65]. Copyright 2015, Wiley-VCH (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |

|
Download:
|
| Fig. 29. (a) Photoluminescence images under 365 nm UV light of stimuli-responsive luminescence of crystals 56. (b) Photoluminescence images under 365 nm UV light of stimuli-responsive luminescence of 57. Reproduced with permission [65]. Copyright 2015, Wiley-VCH (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Penney et al. reported two reversible MCL dinuclear N-heterocyclic carbene gold(I) complexes 58 and 59 (Fig. 30) [69], which contained the bidentate benzimidazol-2-ylidene scaffold with alkyl spacers of different length. As presented in Fig. 31, the as-prepared solids 58 and 59 exhibited blue emission, which quickly changed into green luminescence upon grinding, and the mechanically induced solid-state green luminescence of 58 and 59 were reversed by exposure of the ground solids 58 and 59 to methanol or ethanol vapor. The observed MCL phenomena resulted from amorphization. Meanwhile, there may be a close relationship between bromide coordination and aurophilic interaction, and thus alterations in bromide coordination caused the corresponding changes in intramolecular aurophilic interactions, which possibly triggered mechano-responsive luminescence behaviors of 58 and 59.
|
Download:
|
| Fig. 30. Molecular structures of complexes 58 and 59. | |

|
Download:
|
| Fig. 31. (a) Photoluminescence images under 365 nm UV light of complex 58 in various solid states. (b) Photoluminescence images under 365 nm UV light of complex 59 in various solid states. Reproduced with permission [69]. Copyright 2016, American Chemical Society (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
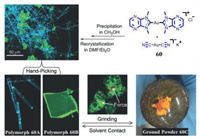
Liu et al. prepared a complex double salt [Au(NHC)2][Au(CN)2] (NHC is referring to 1, 3-dimethylimidazo[4, 5-b]pyrazin-2-ylidene) by the method of co-precipitation of [Au(NHC)2]Cl and K[Au(CN)2] [70], which generated two distinct polymorphic crystals 60A and 60B that exhibited cyan and green luminescence, respectively. As shown in Fig. 32, for polymorph 60B, upon small mechanical stimulus, the orange luminescent spots were noticed in where the mechanical force applied, and an orange light-emitting amorphous powder 60C was observed by thoroughly grinding the crystals of polymorph 60B. Similarly, cyan-emitting polymorph 60A was also converted to orange luminescent powder 60C. Interestingly, upon treatment of 60C prepared by grinding the polymorph 60B with CH3OH solvent, the luminescent color of powder was restored to green, while the initial cyan luminescence could not be obtained upon treatment of 60C prepared by grinding the polymorph 60A with CH3OH solvent, and the green luminescence of 60B was seen.
|
Download:
|
| Fig. 32. Molecular structure of gold(I) double salt 60, and the schematic diagrams of its polymorphism and mechanochromic luminescence. Reproduced with permission [70]. Copyright 2018, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
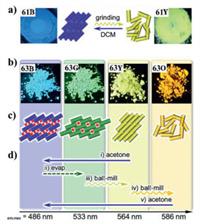
Ito et al. reported reversible mechanochromic luminescence of a binuclear gold(I) isocyanide complex 61 (Fig. 33) [71]. In 2013, Kawaguchi et al. prepared a MCL cholesterol-functionalized binuclear gold(I) complex 62, and it formed two different microscopic structures through a cholesterol-aided self-organization process (Fig. 34) [72]. More specifically, self-organized semicrystalline fiber and noncrystalline globule were obtained by the method of vapor-diffusion of a poor solvent (acetone or methanol) into a dichloromethane solution containing complex 62. Notably, the blue-emitting semicrystalline fibrous precipitates displayed MCL effect involving luminescent color changes of blue to green, which was similar with that of powder 62. However, the green luminescent amorphous precipitates exhibited no MCL behavior. This work provided a deeper understanding of structural and MCL properties of a gold(I) complex. For complex 61, its as-synthesized solid showed blue luminescence. Upon gentle grinding of sample 61, a new emission band was observed, and a strong yellow luminescence was noticed under 365 nm UV light (Fig. 35a). The yellow light-emitting powder reverted back to the initial blue luminescence by the addition of several solvents involving ethyl acetate, diethyl ether and chloroform, or exposure to dichloromethane solvent vapor. Upon grinding, the crystalline state of the as-prepared sample 61 was converted into the amorphous state, which was confirmed by PXRD measurements. Yellow luminescence of ground sample 61 was possibly ascribed to the emergence of intermolecular aurophilic interactions after grinding.
|
Download:
|
| Fig. 33. Molecular structures of complexes 61–64 and complexes 65a–65e. | |

|
Download:
|
| Fig. 34. Schematic diagram of solvent-assisted self-organization of 62 into two different microscopic structures. Reproduced with permission [72]. Copyright 2013, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
|
Download:
|
| Fig. 35. (a) Photographs of complex 61 in various solid states under 365 nm UV light, and schematic diagram of the mechanism of its reversible MCL phenomenon. (b) Photographs of four different solid structures formed by complex 63 under 365 nm UV light. (c) Schematic diagram of molecular arrangements of four different solid structures formed by complex 63. (d) Specific procedures for the interconversion of various emission colors of complex 63. Reproduced with permission [73]. Copyright 2015, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
Subsequently, in 2015, Seki et al. synthesized a multistimuli-responsive gold(I) complex 63 [73], which could afford four solid structures with four different luminescence (Fig. 35b). Upon different external stimuli, these various emission colors were interconvertible through structural changes (Figs. 35c and d). In particular, the green luminescence of 63 was changed into the orange luminescence by means of long ball-milling, and this mechanochromic process involved the CTA phase transition.
In 2018, Seki et al. prepared a meta-diisocyanide benzene-based binuclear gold(I) complex 64 [74], and this complex could form 64a–64g with different crystal structures and various luminescent colors through effective crystallization procedures. All of the resulting solid-state structures of complex 64 showed clear MCL characteristics based on CTA phase transitions.
Liang et al. reported five binuclear gold(I) complexes 65a–65e with different diisocyano bridges [75]. The five complexes displayed various mechanical-force-responsive behaviors. Complexes 65a–65c with either methylsubstituted phenyl bridges or a diphenylmethane bridge exhibited MCL phenomena resulting from CTA phase transitions, while complexes 65d and 65e showed no MCL properties.
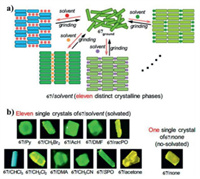
Seki et al. prepared two types of crystals with racemic or homochiral form from a gold(I) isocyanide complex possessing a binaphthyl unit (Fig. 36), and crystals of (rac)−66 and (S)−66 displayed different luminescence [76]. Upon mechanical grinding, their luminescence was converted into the similar emission colors due to CTA phase changes. In the same year, Seki and Ito et al. described an aryl gold isocyanide complex 67 with a biphenyl moiety [77]. Interestingly, the authors obtained 11 types of 67/solvent samples possessing various emission properties via the addition of a variety of solvents, and thus 67 may be applied as a detector for different volatile organic compounds. Furthermore, single crystals of all these 67/solvent samples and solvent-free 67/none were successfully prepared (Fig. 37b). The 11 types of 67/solvent samples showed distinct crystalline structures, and the crystalline phases and amorphous phases of these 67/solvent samples could be interconverted by alternatingly applying mechanical grinding and solvent fuming (Fig. 37a).
|
Download:
|
| Fig. 36. Molecular structures of complexes (rac)−66, (S)−66, and 67. | |
|
Download:
|
| Fig. 37. (a) Schematic diagram of molecular arrangements of complex 67 caused by mechanical grinding and solvent addition. (b) Photographs of various single crystals of 67 under UV irradiation. Reproduced with permission [77]. Copyright 2016, American Chemical Society. | |
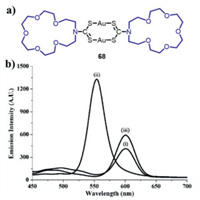
Tzeng and Chao obtained a dinuclear complex 68•2 CH3CN [78], and the molecular structure of 68 is shown in Fig. 38a. The as-prepared powder samples of 68•2 CH3CN emitted red luminescence with an emission maximum at 602 nm (Fig. 38b, spectrum i), while the powder samples showed a new green emission band with a maximum at 553 nm upon drying under vacuum (Fig. 38b, spectrum ii). Moreover, the red luminescence could be recovered through further grinding with CH3CN (Fig. 38b, spectrum iii). The variations of intermolecular aurophilic interactions in different conditions were responsible for these observed interesting phenomena.
|
Download:
|
| Fig. 38. (a) Molecular structure of complex 68. (b) Photoluminescence spectra (excitation wavelength: 380 nm) of 68•2 CH3CN in various states: (i) the as-prepared samples, (ii) the dry powder samples, (iii) the dry powder samples after grinding with CH3CN. Reproduced with permission [78], Copyright 2015, Wiley-VCH. | |
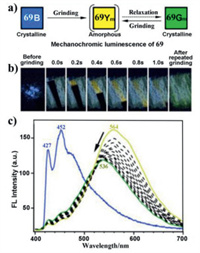
Ito and coworkers synthesized three MCL dinuclear Au(I) isocyanide complexes 69–71 with flexible side chains (Fig. 39). For complex 69, its MCL characteristics involved an unusual CTC phase transition mediated by a transient amorphous phase (Fig. 40a) [79]. When the as-prepared blue light-emitting sample 69B was ground with a spatula, a yellow light-emitting temporary 69Ym was formed, and the observed yellow luminescence was automatically converted to the initial blue luminescence within seconds. Notably, a green light-emitting 69Gm was obtained by repeated grinding (Fig. 40b). Similarly, complexes 70 and 71 also exhibited rare MCL phenomena mediated by transient phases [80]. Moreover, the lifetimes of the transient amorphous phases of 69–71 were closely related with the structures of the terminal substituents of their side chains. Among them, complex 71 with the flexible hexyl units showed blue luminescence, and mechanical stimulation of blue light-emitting 71B provided the transient amorphous 71Y exhibiting yellow luminescence. Intriguingly, the yellow luminescence was immediately changed into the initial blue luminescence within 1 s.
|
Download:
|
| Fig. 39. Molecular structures of complexes 69–71. | |
|
Download:
|
| Fig. 40. (a) Schematic diagram of MCL behavior of complex 69. (b) The photoluminescence photographs of 69 in various conditions. The left photograph shows the as-synthesized solid sample 69. Photographs labeled from "0.0 s" to "1.0 s" show the transient after grinding. The right photograph shows the formed by repeated grinding. (c) Blue line: emission spectrum of 69 before grinding. Progression of yellow to green lines: time-dependent emission spectra measured at 0.2 s intervals of 69 after grinding. Reproduced with permission [79]. Copyright 2016, American Chemical Society (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.). | |
Eisenberg and Lee reported two three-coordinate binuclear gold(I) thiouracilate complexes 72 and 73, which were non-emissive [81]. However, blue luminescence for ground solid 72 and cyan luminescence for ground solid 73 were observed. The PXRD measurements indicated that no obvious phase changes for 72 and 73 could be seen before and after grinding. The MCL effect of 72 or 73 was triggered by molecular structure variation involving a solid-state chemical reaction. An infinite helical nonemissive 72 or 73 with weak intermolecular gold-gold interactions was rearranged into the corresponding aurophilically linked dimeric structure. Furthermore, this process was reversible in the existence of CF3COOH vapor (Fig. 41).
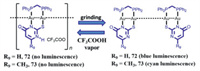
|
Download:
|
| Fig. 41. Schematic diagram of MCL phenomena of binuclear complexes 72 and 73. | |
In 2002, Assefa et al. prepared a linearly coordinated gold(I) complex 74 (Fig. 42) simultaneously possessing thermochromic luminescence and MCL characteristics, which is the first example of MCL gold(I) complex [82]. Crystals 74 showed no luminescence, while a green luminescence was clearly observed after grinding. Catalano and Horner reported a three-coordinate binuclear gold(I) complex 75 (Fig. 42), and the complex exhibited strong intramolecular aurophilic interaction with short Au•••Au distance of 2.8261 Å [83]. Upon crushing, its orange-emitting crystals were converted to the blue luminescent powder. In 2008, Lee et al. reported three binuclear gold(I) complexes 76–78 (Fig. 42), and their three-coordinate gold(I) centres were closely connected through aurophilic interactions of 2.90607 Å for 76, 2.8861 Å for 77, 2.8807 Å for 78 [84]. Interestingly, upon gentle grinding, the blue luminescence of 76–78 changed into the luminescence with higher energies. As stated above, gold(I) complexes 74–78 displayed notable MCL behaviors. However, it is regrettable that the MCL mechanisms of 74–78 still have not been reported.

|
Download:
|
| Fig. 42. Molecular structures of complexes 74–78. | |
Liang et al. synthesized three diisocyano-based donor-acceptor (D-A) type gold(I) complexes 79a–79c (Fig. 43), and these complexes showed remarkable AIE phenomena [85]. In addition, 79a–79c also exhibited unusual "off-on" switchable MCL behaviors, which were driven by CTA morphological transition. Furthermore, the repeatabilities of their MCL phenomena were outstanding.

|
Download:
|
| Fig. 43. Molecular structures of complexes 79a–79c and 80–84. | |
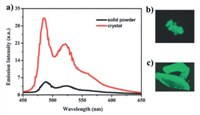
In 2015, Chen et al. reported a fluorene-based AIE-active dinuclear gold(I) isocyanide complex 80 [86]. The complex displayed strong yellow luminescence in the aggregation state. In the solid state, complex 80 emitted green luminescence, and its green luminescence was changed into yellow luminescence upon grinding the powder sample of 80. The observed mechanical force-induced yellow luminescence reverted back to the green emission after treatment with fuming dichloromethane vapor. On the other hand, luminogen 80 also showed crystallization-induced emission enhancement feature (Fig. 44). In the same year, Chen et al. reported three constitutional isomeric dinuclear gold(I) complexes 81–83 [87]. Although luminogens 81–83 exhibited similar AIE characteristics, the different mechano-responsive behaviors of 81–83 were noticed. For ortho-isomer 81, it showed reversible MCL conversion between blue and green emission colors. For meta-isomer 82, it showed switchable mechanical stimulation-induced luminescence enhancement behavior. As for para-isomer 83, it displayed negligible MCL phenomenon. In 2018, Chen et al. described a bipyridine-based binuclear gold(I) complex 84 with AIE and self-assembly characteristics [88]. This complex also showed reversible phosphorescent mechanochromism, and its mechanochromic process involved the CTA phase transition.
|
Download:
|
| Fig. 44. (a) Emission spectra of solid powder and crystals of complex 80. Photographic images of solid powder (b) and crystals (c) of complex 80 under 365 nm UV light. Reproduced with permission [86]. Copyright 2015, The Royal Society of Chemistry (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
To date, some MCL multinuclear gold(I) complexes have been discovered, and the MCL phenomena of these multinuclear gold(I) complexes are attributed to CTA phase transition. In the following portion, several multinuclear MCL gold(I) complexes with or without AIE effect will be introduced.
4.1. Trinuclear AIE-active Au(I) complexes with MCL phenomena resulting from CTA phase transitionChen et al. synthesized three triisocyano-based constitutional isomers 85–87 containing trinuclear gold(I) units (Fig. 45a) [89], and these gold(I) complexes displayed similar AIE and mechanoresponsive luminescence behaviors. Taking complex 85 as a representative example, as presented in Figs. 45b and c, 85 was nearly nonemissive when its diluted DMF solution (1.0 × 10−5 mol/L) was photoexcited. In contrast, a strong green emission band appeared and the bright green luminescence was observed upon aggregation, indicating its typical AIE feature. Furthermore, upon grinding, luminogen 85 displayed "off-on" green luminescence, which was caused by CTA morphological transformation.

|
Download:
|
| Fig. 45. (a) Molecular structures of complexes 85–87. (b) Photographic images of 85 in pure DMF as well as 90% water fraction under 365 nm UV light. (c) Emission spectra of 85 in DMF-H2O mixtures with various water fractions, excitation wavelength: 330 nm. Reproduced with permission [89]. Copyright 2015, Elsevier Ltd. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
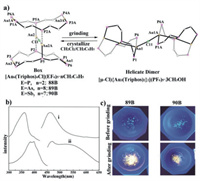
Walters et al. prepared three gold(I) complexes 88B–90B with a boxlike skeleton, and their molecular structures were crystallographically characterized [90]. After mechanical grinding, blue light-emitting crystals containing the same cation [Au6(Triphos)4Cl]5+ of 88B–90B were converted into the corresponding amorphous green light-emitting solids possessing the bridged helicate cation [μ-Cl{Au3(Triphos)2}2]5+ (Fig. 46). On the other hand, the authors confirmed that a triphosphine ligand exhibiting no luminescence could be applied to construct a luminescent container cation containing six gold(I) ions.
|
Download:
|
| Fig. 46. (a) Schematic diagram of reversible mechanochromic phenomena of 88B–90B. Triphos = bis(2-diphenylphosphinoethyl)phenylphosphine). The solvent molecules, phenyl rings, and five hexafluorophosphate, hexafluoroarsenate or hexafluoroantimonate anions are omitted. (b) Excitation (i, left) and emission (i, right) spectra at 22 ℃ of unground crystals of 90B; Excitation (ii, left) and emission (ii, right) spectra at 22 ℃ of ground crystals of 90B (c) Photographic images of unground 89B, 90B (top) and ground 89B, 90B (bottom) at 22 ℃ under UV irradiation. Reproduced with permission [90]. Copyright 2018, American Chemical Society (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.). | |
In 2011, Koshevoy and Chou synthesized an octanuclear gold(I) cluster complex 91 containing alkynyl-diphosphine ligands (Fig. 47), and its crystals of 91 emitted red luminescence [91]. After fully grinding of the crystals, the emission band with a λmax at 730 nm almost completely disappeared, and a new yellowish-green-emitting emission band with a λmax at 560 nm appeared. The emission band of ground crystals 91 was similar to that of 91 in CH2Cl2 solution. This MCL effect of 91 was ascribed to CTA phase transition. For crystals 91, as demonstrated by extended X-ray absorption fine structure (EXAFS) data, upon grinding, some Au-Au bond distances might become closer, which resulted in the increase of coordination number of Au-Au, and the amorphous structure with the loss of lattice energy after grinding might be similar to that of 91 in CH2Cl2 solution.

|
Download:
|
| Fig. 47. Molecular structure of gold(I) cluster complex 91. | |
In this review, an overview of the research progress on MCL gold(I) complexes has been presented. The reported MCL gold(I) systems involve mononuclear, binuclear, and multinuclear gold(I) complexes. Furthermore, some MCL gold(I)-containing complexes have been proved to be able to display typical AIE characteristics. Single crystal X-ray diffraction and PXRD measurements provide significant information for sources of the observed MCL phenomena of these gold(I) complexes. On the whole, their MCL mechanisms are resulted from either CTA phase transition or CTC phase transition. Meanwhile, the mechanoluminochromic mechanisms of several gold(I) complexes are still unknown. Nevertheless, in our opinion, it is speculated that the MCL phenomena of these gold(I) complexes are possibly attributed to CTA phase transition. During their MCL processes, alterations in multiple intermolecular weak interactions (C-H•••π, π•••π, C-H•••O, C-H•••N, C-H•••F, C-H•••S, etc.) and intermolecular aurophilic interactions play important roles in promoting effective mechanical force-responsive luminescent phenomena. Although a series of MCL gold(I)-based luminophors have been discovered, the number of high-contrast MCL gold(I) complexes, especially the AIE-active high-contrast mechanically responsive gold(I) complexes, is still inadequate because of the lacking of effective guidelines for preparing the corresponding smart molecules. AIE-active luminophors can exhibit strong luminescence upon aggregation, which is beneficial for achieving high-contrast mechanoluminochromic phenomena. Therefore, AIE-active MCL gold(I) complexes may become an important source of high-performance gold(I)-containing mechanoresponsive luminescent materials in the future. Further systematic investigation on structures-properties relationships of MCL gold(I) complexes is still a meaningful and challenging subject. Meanwhile, the related research of the potential applications of MCL gold(I) complexes in the fields of sensors, anti-counterfeiting and luminescent switches remains to be explored. We enthusiastically expect that this review can provide a valuable reference for scientists in this fascinating area of research.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThe authors acknowledge financial support from the National Natural Science Foundation of China (Nos. 22061018, 21702079 and 21772054), and the startup funding from South-Central University for Nationalities (No. YZZ19005).
| [1] |
A.C. Grimsdale, K.L. Chan, R.E. Martin, P.G. Jokisz, A.B. Holmes, Chem. Rev. 109 (2009) 897-1091. DOI:10.1021/cr000013v |
| [2] |
M.M. Islam, Z. Hu, Q. Wang, C. Redshaw, X. Feng, Mater. Chem. Front. 3 (2019) 762-781. DOI:10.1039/c9qm00090a |
| [3] |
E. Cariati, E. Lucenti, C. Botta, et al., Coord. Chem. Rev. 306 (2016) 566-614. DOI:10.1016/j.ccr.2015.03.004 |
| [4] |
S. Kang, J.S. Huh, J.J. Kim, J. Park, J. Mater. Chem. C 8 (2020) 11168-11176. DOI:10.1039/d0tc01811b |
| [5] |
Z. Zhang, Y. Li, X. Wu, W. Chu, S. Yin, J. Mater. Chem. C 8 (2020) 11239-11251. DOI:10.1039/d0tc02148b |
| [6] |
T. Sudyoadsuk, P. Chasing, C. Chaiwai, et al., J. Mater. Chem. C 8 (2020) 10464-10473. DOI:10.1039/d0tc02131h |
| [7] |
C.L. Wong, M. Ng, E.Y.H. Hong, et al., J. Am. Chem. Soc. 142 (2020) 12193-12206. DOI:10.1021/jacs.0c03057 |
| [8] |
S. Sonalin, A. Mishra, A.K. Sahu, et al., J. Phys. Chem. C 124 (2020) 13053-13062. DOI:10.1021/acs.jpcc.0c03924 |
| [9] |
H.T. Mao, G.F. Li, G.G. Shan, X.L. Wang, Z.M. Su, Coord. Chem. Rev. 413 (2020) 213283. DOI:10.1016/j.ccr.2020.213283 |
| [10] |
L. Tu, Y. Xu, Q. Ouyang, X. Li, Y. Sun, Chin. Chem. Lett. 30 (2019) 1731-1737. DOI:10.1016/j.cclet.2019.05.022 |
| [11] |
Q. Lin, Z. Li, Q. Yuan, Chin. Chem. Lett. 30 (2019) 1547-1556. DOI:10.1016/j.cclet.2019.06.016 |
| [12] |
D. Li, W. Chen, S.H. Liu, X. Chen, J. Yin, Chin. Chem. Lett. 31 (2020) 2891-2896. DOI:10.1016/j.cclet.2020.02.047 |
| [13] |
F. Ciardelli, G. Ruggeri, A. Pucci, Chem. Soc. Rev. 42 (2013) 857-870. DOI:10.1039/C2CS35414D |
| [14] |
P. Xue, J. Ding, P. Wang, R. Lu, J. Mater. Chem. C 4 (2016) 6688-6706. DOI:10.1039/C6TC01503D |
| [15] |
Z. Yang, Z. Chi, Z. Mao, et al., Mater. Chem. Front. 2 (2018) 861-890. DOI:10.1039/C8QM00062J |
| [16] |
J. Zhao, Z. Chi, Z. Yang, et al., Mater. Chem. Front. 2 (2018) 1595-1608. DOI:10.1039/C8QM00130H |
| [17] |
S. Xue, X. Qiu, Q. Sun, W. Yang, J. Mater. Chem. C 4 (2016) 1568-1578. DOI:10.1039/C5TC04358A |
| [18] |
Z. Chen, J.H. Tang, W. Chen, et al., Organometallics 38 (2019) 4244-4249. DOI:10.1021/acs.organomet.9b00544 |
| [19] |
Y. Yin, Z. Chen, R.H. Li, et al., Inorg. Chem. (2021) 10.1021/acs.inorgchem.1c00233. DOI:10.1021/acs.inorgchem.1c00233 |
| [20] |
Z. He, W. Li, G. Chen, Y. Zhang, W.Z. Yuan, Chin. Chem. Lett. 30 (2019) 933-936. DOI:10.1016/j.cclet.2019.03.015 |
| [21] |
B. Lu, S. Liu, D. Yan, Chin. Chem. Lett. 30 (2019) 1908-1922. DOI:10.1016/j.cclet.2019.09.012 |
| [22] |
N. Mirzadeh, S.H. Privér, A.J. Blake, H. Schmidbaur, S.K. Bhargava, Chem. Rev. 120 (2020) 7551-7591. DOI:10.1021/acs.chemrev.9b00816 |
| [23] |
Z. Chen, D. Wu, X. Han, et al., Chem. Commun. 50 (2014) 11033-11035. DOI:10.1039/C4CC04469J |
| [24] |
H. Schmidbaur, H.G. Raubenheimer, Angew. Chem. Int. Ed. 59 (2020) 14748-14771. DOI:10.1002/anie.201916255 |
| [25] |
Y. Dong, F. Zhu, Z. Chen, J. Yin, S.H. Liu, Mater. Chem. Front. 3 (2019) 1866-1871. DOI:10.1039/c9qm00274j |
| [26] |
H. Schmidbaur, A. Schier, Chem. Soc. Rev. 41 (2012) 370-412. DOI:10.1039/C1CS15182G |
| [27] |
X.Y. Wang, Y.X. Hu, X.F. Yang, et al., Org. Lett. 21 (2019) 9945-9949. DOI:10.1021/acs.orglett.9b03875 |
| [28] |
G.J. Hutchings, M. Brust, H. Schmidbaur, Chem. Soc. Rev. 37 (2008) 1759-1765. DOI:10.1039/b810747p |
| [29] |
A. Pinto, N. Svahn, J.C. Lima, L. Rodríguez, Dalton Trans. 46 (2017) 11125-11139. DOI:10.1039/C7DT02349A |
| [30] |
E.R.T. Tiekink, Coord. Chem. Rev. 275 (2014) 130-153. DOI:10.1016/j.ccr.2014.04.029 |
| [31] |
S. Sculfort, P. Braunstein, Chem. Soc. Rev. 40 (2011) 2741-2760. DOI:10.1039/c0cs00102c |
| [32] |
X.S. Xiao, C. Zou, X. Guan, et al., Chem. Commun. 52 (2016) 4983-4986. DOI:10.1039/C5CC09571A |
| [33] |
J.G. Yang, K. Li, J. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 6915-6922. DOI:10.1002/anie.202000792 |
| [34] |
Z. Luo, X. Yuan, Y. Yu, et al., J. Am. Chem. Soc. 134 (2012) 16662-16670. DOI:10.1021/ja306199p |
| [35] |
J. Liang, Z. Chen, J. Yin, G.A. Yu, S.H. Liu, Chem. Commun. 49 (2013) 3567-3569. DOI:10.1039/c3cc00157a |
| [36] |
A.L. Balch, Angew. Chem. Int. Ed. 48 (2009) 2641-2644. DOI:10.1002/anie.200805602 |
| [37] |
M. Osawa, I. Kawata, S. Igawa, et al., Chem. Eur. J. 16 (2010) 12114-12126. DOI:10.1002/chem.201001908 |
| [38] |
P. Baranyai, G. Marsi, C. Jobbágy, et al., Dalton Trans 44 (2015) 13455-13459. DOI:10.1039/C5DT01795E |
| [39] |
T. Seki, N. Tokodai, S. Omagari, et al., J. Am. Chem. Soc. 139 (2017) 6514-6517. DOI:10.1021/jacs.7b00587 |
| [40] |
T. Seki, K. Kobayashi, T. Mashimo, H. Ito, Chem. Commun. 54 (2018) 11136-11139. DOI:10.1039/c8cc06490c |
| [41] |
Y.B. Dong, Z. Chen, L. Yang, et al., Dyes Pigm. 150 (2018) 315-322. DOI:10.1016/j.dyepig.2017.12.030 |
| [42] |
Z. Chen, Y. Yin, S. Pu, S.H. Liu, Dyes Pigm. 184 (2021) 108814. DOI:10.1016/j.dyepig.2020.108814 |
| [43] |
Z. Chen, G. Liu, R. Wang, S. Pu, RSC Adv. 7 (2017) 15112-15115. DOI:10.1039/C7RA00913E |
| [44] |
Z. Chen, G. Liu, S. Pu, S.H. Liu, Dyes Pigm. 159 (2018) 499-505. DOI:10.1016/j.dyepig.2018.07.016 |
| [45] |
M.J. Wang, Z.Y. Wang, P. Luo, et al., Cryst. Growth Des. 19 (2019) 538-542. DOI:10.1021/acs.cgd.8b01495 |
| [46] |
V. Conejo-Rodríguez, M.N. Peñas-Defrutos, P. Espinet, Dalton Trans. 48 (2019) 10412-10416. DOI:10.1039/c9dt01618j |
| [47] |
H. Ito, M. Muromoto, S. Kurenuma, et al., Nat. Commun. 4 (2013) 2009-2013. DOI:10.1038/ncomms3009 |
| [48] |
T. Seki, K. Sakurada, H. Ito, Angew. Chem. Int. Ed. 52 (2013) 12828-12832. DOI:10.1002/anie.201307672 |
| [49] |
T. Seki, K. Sakurada, H. Ito, Chem. Commun. 51 (2015) 13933-13936. DOI:10.1039/C5CC04609B |
| [50] |
T. Seki, K. Kobayashi, H. Ito, Chem. Commun. 53 (2017) 6700-6703. DOI:10.1039/C7CC03190D |
| [51] |
T. Seki, Y. Takamatsu, H. Ito, J. Am. Chem. Soc. 138 (2016) 6252-6260. DOI:10.1021/jacs.6b02409 |
| [52] |
K. Sakurada, T. Seki, H. Ito, CrystEngComm 18 (2016) 7217-7220. DOI:10.1039/C5CE02565F |
| [53] |
M. Jin, T. Seki, H. Ito, J. Am. Chem. Soc. 139 (2017) 7452-7455. DOI:10.1021/jacs.7b04073 |
| [54] |
M. Jin, T. Sumitani, H. Sato, T. Seki, H. Ito, J. Am. Chem. Soc. 140 (2018) 2875-2879. DOI:10.1021/jacs.7b12455 |
| [55] |
A. Sathyanarayana, S.Y. Nakamura, K. Hisano, et al., Sci. China Chem. 61 (2018) 957-965. DOI:10.1007/s11426-018-9318-9 |
| [56] |
X.Y. Wang, J. Zhang, J. Yin, S.H. Liu, Chem. Asian J. 14 (2019) 2903-2910. DOI:10.3390/app9142903 |
| [57] |
E.J. Fernández, J.M. López-de-Luzuriaga, M. Monge, et al., J. Am. Chem. Soc. 125 (2003) 2022-2023. DOI:10.1021/ja028734u |
| [58] |
A. Deák, T. Tunyogi, G. Pálinkás, J. Am. Chem. Soc. 131 (2009) 2815-2817. DOI:10.1021/ja809067t |
| [59] |
Z. Chen, L. Yang, Y. Hu, et al., RSC Adv. 5 (2015) 93757-93764. DOI:10.1039/C5RA19378H |
| [60] |
Z. Chen, Z. Li, F. Hu, et al., Dyes Pigm. 125 (2016) 169-178. DOI:10.1016/j.dyepig.2015.10.038 |
| [61] |
Z. Chen, Y. Nie, S.H. Liu, RSC Adv. 6 (2016) 73933-73938. DOI:10.1039/C6RA17806E |
| [62] |
Z. Chen, G. Liu, S. Pu, S.H. Liu, Dyes Pigm. 143 (2017) 409-415. DOI:10.1016/j.dyepig.2017.05.003 |
| [63] |
W.B. Li, W.J. Luo, K.X. Li, W.Z. Yuan, Y.M. Zhang, Chin. Chem. Lett. 28 (2017) 1300-1305. DOI:10.1016/j.cclet.2017.04.008 |
| [64] |
C. Jobbágy, M. Molnár, P. Baranyai, et al., CrystEngComm 16 (2014) 3192-3202. DOI:10.1039/C3CE42474J |
| [65] |
A. Deák, C. Jobbágy, G. Marsi, et al., Chem. Eur. J. 21 (2015) 11495-11508. DOI:10.1002/chem.201501066 |
| [66] |
C. Jobbágy, M. Molnár, P. Baranyai, A. Deák, Dalton Trans. 43 (2014) 11807-11810. DOI:10.1039/C4DT01214C |
| [67] |
A.M. Kuchison, M.O. Wolf, B.O. Patrick, Chem. Commun. (2009) 7387-7389. DOI:10.1039/b915089g |
| [68] |
A. Laguna, T. Lasanta, J.M. López-de-Luzuriaga, et al., J. Am. Chem. Soc. 132 (2010) 456-457. DOI:10.1021/ja909241m |
| [69] |
A.A. Penney, V.V. Sizov, E.V. Grachova, et al., Inorg. Chem. 55 (2016) 4720-4732. DOI:10.1021/acs.inorgchem.5b02722 |
| [70] |
Q. Liu, M. Xie, X. Chang, et al., Chem. Commun. 54 (2018) 12844-12847. DOI:10.1039/c8cc05210g |
| [71] |
H. Ito, T. Saito, N. Oshima, et al., J. Am. Chem. Soc. 130 (2008) 10044-10045. DOI:10.1021/ja8019356 |
| [72] |
K. Kawaguchi, T. Seki, T. Karatsu, et al., Chem. Commun. 49 (2013) 11391-11393. DOI:10.1039/c3cc47162d |
| [73] |
T. Seki, T. Ozaki, T. Okura, et al., Chem. Sci. 6 (2015) 2187-2195. DOI:10.1039/C4SC03960B |
| [74] |
T. Seki, K. Ida, H. Ito, Mater. Chem. Front. 2 (2018) 1195-1200. DOI:10.1039/C8QM00074C |
| [75] |
J. Liang, F. Hu, X. Lv, et al., Dyes Pigm. 95 (2012) 485-490. DOI:10.1016/j.dyepig.2012.06.014 |
| [76] |
M. Jin, T. Seki, H. Ito, Chem. Commun. 52 (2016) 8083-8086. DOI:10.1039/C6CC03541H |
| [77] |
T. Seki, M. Jin, H. Ito, Inorg. Chem. 55 (2016) 12309-12320. DOI:10.1021/acs.inorgchem.6b02107 |
| [78] |
B.C. Tzeng, A. Chao, Chem. Eur. J. 21 (2015) 2083-2089. DOI:10.1002/chem.201405466 |
| [79] |
S. Yagai, T. Seki, H. Aonuma, et al., Chem. Mater. 28 (2016) 234-241. DOI:10.1021/acs.chemmater.5b03932 |
| [80] |
T. Seki, K. Kashiyama, S. Yagai, H. Ito, Chem. Lett. 46 (2017) 1415-1418. DOI:10.1246/cl.170482 |
| [81] |
Y.A. Lee, R. Eisenberg, J. Am. Chem. Soc. 125 (2003) 7778-7779. DOI:10.1021/ja034560k |
| [82] |
Z. Assefa, M.A. Omary, B.G. McBurnett, et al., Inorg. Chem. 41 (2002) 6274-6280. DOI:10.1021/ic025784r |
| [83] |
V.J. Catalano, S.J. Horner, Inorg. Chem. 42 (2003) 8430-8438. DOI:10.1021/ic030200m |
| [84] |
J. Schneider, Y.A. Lee, J. Pérez, et al., Inorg. Chem. 47 (2008) 957-968. DOI:10.1021/ic701763x |
| [85] |
J. Liang, Z. Chen, L. Xu, et al., J. Mater. Chem. C 2 (2014) 2243-2250. DOI:10.1039/c3tc31638f |
| [86] |
Z. Chen, J. Zhang, M. Song, et al., Chem. Commun. 51 (2015) 326-329. DOI:10.1039/C4CC08087D |
| [87] |
Z. Chen, Z. Li, L. Yang, et al., Dyes Pigm. 121 (2015) 170-177. DOI:10.1016/j.dyepig.2015.05.021 |
| [88] |
Z. Chen, G. Liu, S. Pu, S.H. Liu, Dyes Pigm. 152 (2018) 54-59. DOI:10.1016/j.dyepig.2018.01.036 |
| [89] |
Z. Chen, P.S. Huang, Z. Li, et al., Inorgan. Chim. Acta 432 (2015) 192-197. DOI:10.1016/j.ica.2015.04.016 |
| [90] |
D.T. Walters, R.B. Aghakhanpour, X.B. Powers, et al., J. Am. Chem. Soc. 140 (2018) 7533-7542. DOI:10.1021/jacs.8b01666 |
| [91] |
I.O. Koshevoy, C.L. Lin, A.J. Karttunen, et al., Chem. Commun. 47 (2011) 5533-5535. DOI:10.1039/c1cc11352f |
 2021, Vol. 32
2021, Vol. 32 

