b Shenzhen Key Laboratory of Kidney Diseases, Department of Nephrology; Department of Radiation Oncology, Shenzhen People's Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, China;
c School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Guangzhou 510000, China;
d Health Science Center, Shenzhen University, Shenzhen 518060, China
Drug delivery systems (DDS) are used to transport therapeutic drugs to a specific site in the body at a specific time [1]. These systems are usually designed to meet people's expectations, either improving the pharmacological activity of drugs or alleviating toxic and side effects with the help of drug carriers. Recent years, nanotechnology has shown great potential in the treatment of diseases [2, 3]. A large number of nanocarriers with different characteristics have been developed and applied to drug delivery, such as liposomes [4], micells [5], polymers, quantum dots (QDs) [6, 7], black phosphorus [8], metal oxide nanoparticles [9], mesoporous silica nanoparticles (MSNs) [10]. Among these, mesoporous silica nanoparticles have gained extensive attention from researchers because of their simple synthesis [11], uniform morphology, high surface area and pore volume [12, 13], tunable mesopore size and pore shape, controllable diameter and high biocompatibility [14]. The high surface area and pore volume provide the ability to load different kinds of drug molecules and MSNs with diverse particle sizes have been studied as nanocarriers for the delivery of small drug molecules and antigen [15-17]. In addition, MSNs can be modified by different functional groups through the silane chemistry [18, 19]. Most importantly, MSNs possess commendable biocompatibility not merely in vitro but also in vivo, and they are proved to be safe when circulating into the blood [20]. The degradability and clearance of mesoporous silica nanoparticles are in a high level, which make them one of the promising candidates for the biomedicine application [21, 22].
Based on the pore system, mesoporous silica nanoparticles (MSNs) can realize on-command drug delivery and the limitation of premature drug release before arriving at the target sites can be avoided. The loaded cargoes can be released in a controllable way with the design of the "gate keepers" responding to certain stimuli. Normally, the gate keepers seal the drug molecules in the pore. In the presence of intracellular or extracellular stimuli, the gated materials may undergo size/shape/conformation changes or dissociate to release the drug. Moreover, MSNs can be targeted to specific tissue sites by modifying the outer surface of the gated material with ligands. In this way, the effectiveness of the drug is greatly increased and the damage to the whole body is reduced. Recently, the study of cancer remains a focal point, there have been numerous scientists doing their efforts to solve the toxic and side effects of chemotherapy drugs. In view of the application of mesoporous silica in antineoplastic drug delivery [23, 24], this review aims to summarize the recent progress about the development of mesoporous silica gated materials in nearly few years (2017-2020). In the previous reviews, there have been some reports on mesoporous silica nanoparticles, however, they are more focused on the process of nanoparticles entering the organism, with only a small amount of space devoted to gating, and the classification of materials is not clear [25, 26]. In addition, with the progress of science and technology, some new materials are emerging. Therefore, here we will make a unified statement on the gated materials of MSN, so as to provide more references for the future development of mesoporous silica in drug delivery. We mainly focus on the "gate keepers" of mesoporous silica for drug controlled release and present from the following categories: inorganic gated materials, organic gated materials, self-gated drug molecules, and biological membranes.
2. Mechanism of drug controlled releaseThe ideal mesoporous silica nanocarrier is capable of delivering antitumor drugs to the tumor site and releasing high concentration of the therapeutic cargo on-demand, which is mainly dependent on changes of gate keepers to regulate the "on" and "off" of mesoporous silica pores under specific stimulus, known as the stimulus response. The stimulus responses in tumor site include endogenous stimulus response and exogenous stimulus response.
Unlike normal tissues and cells, tumor tissue has its specific tumor microenvironment and many physiological parameters are different [27]. In general, the pH of cytoplasm, blood, and normal tissues is about 7.0−7.4, while the pH of tumor microenvironment is weakly acidic about 6.5−6.8, and the pH of endosome/lysosomal organelles is about 4−6 [28]. This can induce the cleavage of acid-sensitive molecules. In addition, higher glutathione (GSH) level and lower oxygen level are prominent characteristics of tumors, indicating good conditions for redox reactions. There are also some highly expressed enzymes in tumor sites, such as matrix metalloproteinases (MMPs), proteinases K, cathepsin B, hyaluronidase (HAase) [29], which can hydrolyze the corresponding substrates specifically. All of these endogenous stimuli can be good triggers applying to gated mesoporous silica design.
Apart from endogenous stimuli, exogenous stimuli are also important components, mainly including heat, magnetic field, ultrasonic wave, light, etc. [30, 31]. Compared with endogenous stimulation, exogenous stimulation can achieve safer and more effective controlled release, and it can overcome the different effects caused by internal individual differences. With the aid of exogenous physical stimulations, the physicochemical changes of materials can accelerate the release of drugs and realize spatiotemporal controlled manipulation. Exogenous stimuli not only prevent premature drug release and reduce side effects, but also can cause additional therapeutic effects, such as photothermal therapy, photodynamic therapy.
3. Inorganic gated materialsInorganic materials are a large class of materials, which are commonly used to seal mesoporous silicon nanoparticles. To date, plenty of them have been developed and applied as sealants of MSNs, such as gold nanoparticles, zinc derivatives, copper sulphide, metal polyphenol and calcium materials, and they possess different characteristics and advantages. Most of these materials are pH sensitive or contain breakable chemical bonds to regulate drug release. In addition to serve as gate keepers, some with excellent metallicity can also play roles in promoting synergistic cancer therapy. Due to the excellent biocompatibility plus biodegradability, inorganic gated materials have shown wide range of applications [32, 33].
3.1. Gold nanoparticlesGold nanoparticles are good candidates for mesoporous silica gating because of their following properties. (1) Gold nanoparticles have good biocompatibility, chemical inertness and especially adjustable size, which makes them very befitting for blocking pores [34]. (2) By means of covalent bonds or linkers, gold nanoparticles can be well attached to MSNs surface [35]. (3) Gold nanoparticles exhibit excellent photothermal properties, which can convert the-NIR light into heat, thus realizing the binding of chemotherapy and photothermal therapy, and enhancing the therapeutic effectiveness [36]. Up to now, the preparation of gold nanoparticles has been very mature [37], which makes gold nanoparticles become popular caps of MSNs. Yang et al. [35] have prepared a gold nanoparticles-gated MSNs drug delivery system loading with classic anticancer drug doxorubicin (DOX), which was triggered by redox response. MSN was first modified by mercaptan, and then ultrafine Au nanoparticles were appended to the openings of mesoporous silicon in vitue of Au-S bonds, which, like the disulfide bonds, could be cracked under the condition of high GSH. On account of the significant difference of glutathione concentration in and out of tumor cells [38, 39], drug molecules displayed specific release behavior. Moreover, gold nanoparticles had strong photothermal conversion capability, on the one hand, chemo-photothermal therapy was realized, on the other hand, the killing efficacy of chemotherapy agents on tumor was improved since tumor was more sensitive to chemotherapy drugs under high temperature conditions. In addition to blocking drugs within mesoporous, ligand modification can also be performed on the surface of gold nanoparticles to improve drug targeting. A gold nanoparticle-biotin conjugate was introduced by Eskandari et al. [40] to grafted onto the pore outlets. It was finished with the help of a peptide which can be cleaved by the matrix metalloproteinases, the enzymes with high expression in tumor tissues [41]. Therefore, a multifunctional nanocarrier with both enzymatic responsiveness and active targeting was obtained.
3.2. Zinc derivativesZinc derivatives include zinc oxide (ZnO), zinc sulfide (ZnS) and so on. ZnO quantum dots (QDs) have many merits, such as easy to synthesis, inexpensiveness, acid sensitivity, excellent biocompatibility and biodegradability [42], that is why they are employed as ideal candidates to gate MSN. Liu and his coworkers reported a magnetic mesoporous silica platform gated with zinc oxide, which was used to transmit chemotherapy drug daunomycin [43]. In a neutral environment, MMSN could stably accommodate daunomycin, whereas at low pH of the tumor, zinc oxide rapidly dissolved into zinc ions (Zn2+), which resulted in the release of anticancer drugs. Furthermore, the increased zinc ions concentration enhanced the toxicity towards the tumor cells [44] and realized the chemo-/ions cooperative treatment effect. Besides zinc oxide, zinc sulfide can also act as a gated molecule. The rich physical and chemical properties of ZnS nanomaterials make them widely used, of which the most important is that they are non-toxic [45]. Salinas et al. [46] fabricated a new gate control system based on the ZnS. ZnS was wrapped with phenylboronic acid first of all, after that it was attached to the entrance of MSN through forming pH-sensitive boronate ester bond, which revealed "zero premature cargo release" and biocompatibility (Fig. 1A). Recently, metal organic frameworks have also attracted strong attention in the biomedical field due to their biocompatibility and biodegradability [47-49]. Among them, MOFs containing zinc have great application potential in this field [50, 51]. Chandan et al. recently reported a new strategy to modified the mesoporous silica nanoparticle with metal organic framework [52]. They encapsulated doxorubicin into MSN first, and then combined the MSN with metal organic framework to form complexes. External stimulation both pH and liposome were the triggers used to guide the drug release, the results showed good compatibility and low toxicity, and it opens up a new method for controlled drug delivery.

|
Download:
|
| Fig. 1. (A) Cargo release process of MSNs capped with boronic-acid-coated ZnS nanocrystals. Reproduced with permission [46]. Copyright 2018, American Chemical Society. (B) An example of multifunctional MSN coated with metal-polyphenol complexes. Reproduced with permission [61]. Copyright 2017, Wiley. | |
Copper sulphide (CuS), with its strengths of hypotoxicity [53], low cost, and photostability, is widely used as a photothermal agent owing to its high molar extinction coefficient and excellent photothermal conversion efficiency [54, 55]. Remarkable chemo-photothermal therapy can be achieved by combining copper sulfide with mesoporous silica. To solve the kidney clearance problem, Wei et al. [56] adopted the renal-clearable ultrasmall nanodots (CuSNDs) to seal doxorubicin (DOX)-loaded mesoporous silica NPs for tumor imaging and cancer therapy. Copper sulfide nanoparticles can be grafted onto mesoporous silica surfaces with the aid of cleavable chemical bonds [57] or macromolecules [13]. For example, Yang et al. fabricated a versatile supramolecular drug delivery system, which consisted of pyridinium (Py)-modified hollow mesoporous silica nanoparticles and carboxylatopillar [5] arene (CP5)-functionalized CuS nanoparticles (CP5-CuS) [13]. The CP5 rings and Py stalks can recognize with each other and exist supramolecular host-guest interactions, thus actting as gating entities. The CP5-CuS served as not only a gate keeper for on-demand drug release but also a special agent for NIR-guided photothermal therapy.
3.4. Metal-polyphenol complexesMetal-polyphenol complexes include the natully occurring polyphenols, such as tannic acid (TA), gallic acid (GA)) and metal ions, which have become a multifunctional surface dressing agent for the adhensiveness [9, 58]. With the high content of dihydroxyphenyl and trihydroxyphenyl, the polyphenols can form coordination bonds with different metal ions, thereby crosslinking resulting in three-dimensional stabilized metal-phenolic networks [59]. The method for the metal-polyphenol coating is rapid and low cost, which is simply a mixture in one-pot in the presence of substrates [60]. Of note, the metal polyphenol network provides a pH-sensitive property, which is very beneficial for drug release in the acidic environment of tumors. MSNs with signal-induced guests release were reported by Park et al. [61]. In their design, photoacid generators (PAGs) and polyphenol-metal coordination chemistry were combined for payloads "going out". Upon exposure to UV light, acid was produced by PAGs due to the photolysis, which enabled the channels open by decomposing the the coordination interaction of tannic acid with CuⅡ ions (Fig. 1B). Besides, some metal-polyphenol complexes also have photothermal conversion characteristics. For instance, Yang et al. have fabricated a carrier system combining photothermal therapy and chemotherapy, which possesses both acid and photothermal response [62]. The core-shell nanoparticles composed of a mesoporous silica nanoparticle (MSN) as the porous matrix for accommodating small molecular anti-tumor drug doxorubicin, and the tannic acid-FeⅢ/AlⅢ complex shell coating the pores, which exhibited outstanding effectiveness and superior biocompatibility.
3.5. Calcium materialsCalcium related material is one of the most frequently-used inorganic materials. The mechanism by which calcium materials control drug release is acid sensitivity. Many calcium salts, such as calcium carbonate and calcium phosphate, are highly soluble in acids. They remain intact under physiological conditions, but are easy to react with hydrogen ions (H+) and degrade in tumor lower pH environment., which makes them biologically safe and good activity [63, 64]. In addition to calcium carbonate and calcium phosphate themselves can be good carriers [64], they are also excellent auxiliary materials. Srivastava et al. reported an ATP-decorated mesoporous silica nanoparticle with biomineralization of calcium carbonate [65]. Calcium carbonate was employed as a gatekeeper through the interaction between calcium carbonate and ATP to ensure the no leak of doxorubicin and the stability of ATP during circulation. This novel design significantly reduced the tumor burden and increased survival rate. Calcium phosphate, most commonly used for gene delivery, can apply to the preparation of composite mesoporous silicon nanoparticles and is also a fascinating encapsulating material [66, 67]. Ma et al. prepared calcium phosphate coated MSNs loading chemotherapy drug and photosensitizer at the same time, which realized the synergetic treatment of chemotherapy and photodynamic therapy [67].
4. Organic gated materialsBeyond a variety of inorganic gated materials, organic materials also account for a considerable part, including natural materials, synthetic polymer materials, mussel-inspired material and biomolecular materials. Natural materials like cyclodextrin, chitosan, and hyaluronic acid have potential advantages in biocompatibility due to their natural sources, and synthetic polymer materials have a wide variety because of their structural diversity. Mussel-inspired polydopamine possesses many excellent properties. Biological macromolecules can be targeting ligand simultaneously due to their origin in the body. These gated materials can offer additional functions to MSNs. The interaction between host and guest, acid response, enzyme response, hypoxia response etc. are the main influencing factors to control the gating molecular switch state.
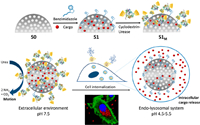
4.1. Natural materials 4-1-1. Cyclodextrins(CDs)Cyclodextrins, a natural compound family of cyclic oligosaccharides connected by α-1, 4-glucosidic bonds, have enjoyed great popularity in the field of drug delivery due to low toxicity and high stability, as well as large molecular cavities. According to the number of glucopyranose units, cyclodextrins (CDs) can be divided into α-, β-, and γ-CD, which contain six, seven, and eight monomers, respectively [68, 69]. Among them, β-cyclodextrin is the most favored by researchers because of its low production cost and good properties. Bulky molecular cavities allow host-guest interactions in a reversible way and are well propitious for stimulation-induced drug release. The binding degree between the host and the guest will decrease under the influence of internal and external environment, which will cause the automatical departure of guest molecules from the CD cavities [70]. In other words, host-guest complex is an environment-regulated switch at the mesoporous silicon gate. Cyclodextrin molecules can be grafted onto mesoporous silicon by covalent bonds or intermolecular interactions. For example, Hu et al. established a β-cyclodextrin-gated self-accelerating nanoplatform through the ROS-cleavable thioketal (TK) linker to encapsulate doxorubicin and ROS production agents α-tocopheryl succinate (α-TOS) [71]. Wu et al. [72] used disulfide bonds to connect cyclodextrin onto mesoporous silicon, constructing an smart system with dual internal redox and external light stimuli. Mesoporous silica nanoparticle can also be functionalization modified first, and then the functional group as guest molecular to interact with cyclodextrin. Sánchez et al. grafted benzimidazole groups to the mesoporous silica outer surface, and capped the entrance by the formation of inclusion complexes between benzimidazole and cyclodextrin-modified urease [73]. Cargo release is only triggered at acidic pH due to the protonation of benzimidazole groups and loss of supramolecular interaction. Moreover, the enzyme-powered nanomotors were self-propelled because the urease units allowed the enhanced Brownian motion of nanoparticles in the existence of urea as biofuel (Fig. 2).
|
Download:
|
| Fig. 2. Schematic illustration of the preparation and performance of enzyme-powered stimuli-responsive nanomotors. Reproduced with permission [73]. Copyright 2019, American Chemical Society. | |
Chitosan and chitin are natural-sourced polysaccharide with high biodegradability, biocompatibility, non-toxicity and other functions [74]. Chitin is synthesized by numerous living organisms and occurs in nature as major structural component in the exoskeleton of arthropods [75]. The main commercial sources of chitin are the shell waste of shrimps, lobsters, krills and crabs. Due to the poor solubility of chitin in common solvents, it is often converted to its deacetylated derivant, chitosan, in the presence of alkali. However, in a recent study, carboxymethyl chitin (CMCH), a soluble derivative, was first applied by Ding et al. to modify hollow mesoporous silica nanoparticle (HMSN) as drug delivery systen for cancer therapy [19]. The HMSN template was chemically modified by thioketal (TK) linker and then gated with CMCH by electrostatic interaction, achieving pH/ROS dual responsive protection. Chitosan is a polyelectrolyte and is preferred generally. It can be protonated under acidic pH, which will lead to depolymerization, making chitosan per se an attractive pH-responsive material and a wider range of applications [76, 77]. Yan et al. developed a chemo- and phototherapy platform by sealing the mesoporous silica pore with chitosan [78]. The nanocarrier showed excellent drug controlled release properties based on the pH-dependent swelling effect of the coating layer. Chen et al. reported a self-targeting and controllable drug delivery system bedecked with a chitosan-based thin film layer cross-linked by N, N'-bis(acryloyl)cystamine (BAC) via disulfide bond [18]. The folium was stable at physiological conditions, but impressible to tumor acidic and high GSH conditions, which prevented the premature release of the drug. Moreover, folic acid (FA) was connected to the chitosan thin film membrane to enhance the recognition of tumor cells [18].
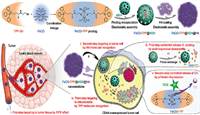
4.1.3. Hyaluronic acid (HA)Hyaluronic acid, a natural polysaccharide, is the main extracellular matrix of connective tissue and is widely used for drug delivery due to its good biocompatibility, non-toxicity, non-immunogenicity and so on [79-81]. It can also serve as a capping agent to prevent drug leakage due to its large molecular size. In tumor tissues rich in hyaluronidase (HAase), it can be degraded into small molecular fragments to facilitate drug release, which also endows it enzyme responsiveness [82]. More importantly, HA can specifically interact with CD44 receptors overexpressed in tumor sites and act as a targeting ligand to actively target the tumor [83]. Recently, Meng et al. [84] proposed a multistage targeted delivery and controlled release strategy. They constructed a multifunctional intelligent nanomedicine named as FeCO-TPP@MSN@HA. The mitochondria-targeted and intramitochondrial micro-environment-responsive prodrug (FeCO-TPP) was encapsulated into mesoporous silica nanoparticle, which was further coated with hyaluronic acid by electrostatic assembly [84]. Combined with the enhanced permeability and retention (EPR) effect of nanoparticle, tumor tissue-cell-mitochondria-targeted multi-stage delivery and controlled release of CO were successfully achieved (Fig. 3).
|
Download:
|
| Fig. 3. Construction and transport of FeCO-TPP@MSN@HA nanomedicine. Reproduced with permission [84]. Copyright 2020, American Association for the Advancement of Science. This article is licensed under a Creative Commons Attribution 4.0 International License. | |
Regarding drug delivery, synthetic polymers are the most popular innovative materials due to their diversities in structure and form, which endow them extensive functions [85-87]. Gated mesoporous silica nanoparticles based on synthetic polymers can realize the sustained and controlled release of drugs in response to different internal or external stimuli, thus improving the fate and therapeutic performance of drugs. These polymers can act individually or in combination, including poly(2-(diethylamino)-ethyl methacrylate) (PDEAEMA) [88], poly(acrylic acid) (PAA) [89], poly(N-isopropyl acrylamide) (PNIPAAm) [90], poly(ethylenimine) (PEI) [91] etc. The main ways in which polymers are connected to mesoporous silica nanoparticles contain "graft to" and coating. For example, Chen et al. Adopted poly(allylamine)-dimethylmaleic anhydride-polyethylene glycol (PAH-DMMA-PEG) and polyethyleneimine (PEI) to fabricate a size and charge dual-transformable mesoporous nanoassembly for enhanced drug delivery and tumor penetration [92]. Li et al. coated the MSN pore with pH-responsive N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer shell, forming a disassembled nanohybrid for anti-tumor therapy [93]. Polymers control drug molecule release mainly depending on three strategies: curl-stretch, swelling and degradation. Mu et al. constructed a novel pH-controlled smart and reversible "on-off " switch system by grafting the poly(l-histidine)-poly(ethylene glycol) (PLH-PEG) to the the surface of MSN [94]. The PLH-PEG could restrain the leakage of drugs under systemic circulation, but upon the nanoparticles accumulated in tumor site, the conformation of PLH changes from curled ("off " state) to stretched ("on" state) as a result of protonation, which enabled drug release. In another research, polyacrylic acid (PAA) coated hollow mesoporous silica nanoparticles (HMSNs) loaded with calcium peroxide (CaO2) were reported by Wu et al. [95] for prostate cancer therapy. This pH-responsive release derived from the distinct swelling behavior of PAA under different pH conditions. Cracking of polymers is also a major cause of drug release. Yan et al. [96] proposed the hypoxia-responsive azobenzene polymer, poly(4, 4′-azodianiline) (pDAB), to block MSN (Fig. 4A). Under the hypoxic condition of the tumor, the azobenzene received electrons and then was destroyed into aniline, which gave rise to the polymer degradation and rapid cargo release. This work enriched the gated materials as well as the family of stimuli-responsive mesoporous silica nanocarriers, and expanded the application of MSNs in pharmaceutical and biomedical areas.

|
Download:
|
| Fig. 4. (A) The schematic diagram shows the preparation process and action mechanism of hypoxia-responsive mesoporous silica nanocarriers (MSNs). Reproduced with permission [96]. Copyright 2019, American Chemical Society. (B) Schematic illustration of doxorubicin-loaded and PDA-coated MSNs-DOX@PDA-TPGS. Reproduced with permission [102]. Copyright 2017, Wiley. | |
Polydopamine, formed by the oxidation of dopamine, is a melanin-like mimic of mussel adhesive protein, which possesses many advantages, such as good biocompatibility, degradability, low toxicity, easy to functionalization and synthesis [97, 98]. Since Lee et al. first reported the synthesis of PDA via simple oxidative self-polymerization of dopamine in 2007, its application is becoming more and more extensive [99]. Under weakly alkaline conditions, the catechin groups of dopamine can be oxidized to quinones, which then further react with other catechins and/or quinones to form a water-insoluble PDA film [100, 101]. It has been shown that PDA has similar structure and superior adhesion to adhesion proteins, indicating it a promising molecule for forming an adhesion layer. PDA can be anchored to a variety of substrates, including mesoporous silica. Because the PDA layer is highly sensitive to pH, it can block drug molecules in a neutral environment and release drugs in an acidic environment, making it an important member of the MSN gate keepers. What is more, functional ligand molecules containing nucleophilic functional groups can be modified to PDA via Michael addition reaction or Schiff base reaction, and physical interactions (van der Waals' force or hydrogen bond) are also useful [100]. In one research, Zeng et al. [102] developed a polydopamine-coated mesoporous silica nanoparticle system for pH-responsive delivery of doxorubicin (DOX) (Fig. 4B). The functional ligand, d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), which has been approved by US FDA as a safe pharmaceutical adjuvant, was introduced to their system (MSNs-DOX@PDA-TPGS). It not only suppressed P-glycoprotein (P-gp) meditated multidrug resistance (MDR) in cancer cells by inhibiting P-gp activity but also prolonged blood circulation time and improved cellular uptake. In addition to acid responsiveness, PDA has excellent photothermal properties and can absorb the near-infrared (NIR) light. When irradiating with 808 nm NIR light, it can convert light into heat to destroy tumor, which manifests its great potential in photothermal therapy [103]. A mesoporous silica nanosystem gated by doxorubicin and polydopamine (PDA) film was designed by Chen and his colleagues to load P-gp siRNA [104]. The surface folic acid endowed it active targeting property. Due to the photothermal conversion capability of the PDA layer, this multifunctional nano-platform realized the synergistic treatment of chemotherapy, gene therapy and photothermal therapy. In vitro and in vivo experiments both demonstrated the higher lethality of the nanoparticles on multidrug resistant tumor.
4.4. BiomacromoleculesApart from various polymers, biomacromolecules are also exciting constituents for surface engineering modification of mesoporous silica, like proteins and nucleic acids. In recent years, biocompatible capping agents have been developed by employing biologically active molecules. Several proteins have been used to coat over the mesoporous silica nanoparticles surface, such as bovine serum albumin (BSA) [105], human serum albumin (HSA) [106], transferrin (Tf) [107], biotin-avidin complex, lectin [108], cytochrome c (CytC) [109]. The selection of protein needs to take charge into account, however in some instances, protein can be fixed directly by covalent bonding. Drug release depends on responsive covalent bond rupture or protease hydrolysis to unbolt the gate. The advantage of using proteins as sealants derives from the specificity, because some proteins can bind to the receptors which are upregulated at the site of lesion, effectively active targeting can be obtained. For example, Chen et al. designed a targeted and controlled drug delivery system based on transferrin decorated mesoporous silica nanoparticles (MSNs) [107]. Transferrin (Tf), an endogenous protein, was modified to the surfaces of MSNs via GSH responsive disulfide bonds, acting as not only a capping agent but also a targeting ligand meanwhile. Moreover, due to the interference caused by the adsorption of some proteins on nanoparticles during transport, protein coating can be a good solution to this problem. In a paper, Zhang et al. [106] probed the interaction between protein and prodrug as well as the influence on drug release behavior. Some proteins also possess therapeutic functions, and the construction of protein-decorated nanocarriers is conducive to the co-delivery of small molecular drugs and biomacromolecule drugs.
Nucleic acids, an admittedly powerful tool, can also act as biotechnological materials for mesoporous silica, usually in the forms of aptamer and fragments of partial nucleotides. They can seal the channels by electrostatic deposition or by forming hybrid chains [110]. Because of the coding role in living cells, substrate or sequence recognition is the most promising, which equips them precise targeting property [111]. Under the hydrolysis of DNAse enzymes, the chain breaks and cargo is released. Alternatively, some fragments may be specifically designed to polymerize with the mRNA when entering cells, thus leading to "gate" open [111, [112]. When the mesoporous silica is loaded with fluorescent molecules, it will be a very good integrated platform for diagnosis and treatment.
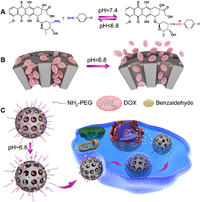
5. Self-gated drug moleculesAmong all the gating methods mentioned above, extra auxiliary materials are used to seal mesoporous silica, and the potential risks of these additives are inevitable. To solve the problem, Zeng et al. [113, 114] proposed a drug-self-gating strategy. As shown in Fig. 5, mesoporous silica nanoparticle was first functionalized with benzaldehyde and then gated by doxorubicin, a representative anticancer drug containing amino groups, through pH-sensitive benzoic-imine covalent bonds. Amino polyethylene glycol was attached to the MSN surface in the same way, which not only increased long circulation, but also promoted cell uptake in tumor acidic environment due to bond breakage [113]. This ingenious design minimized the potential risks of the auxiliary cappers, and could be extended to the self-controlled delivery of other biomolecules, such as nucleic acids, peptides, and proteins, due to their intrinsic amino groups. In another research, drug-self gated MSN was used for the co-delivery of permeability glycoprotein (P-gp) small interfering RNA (siRNA) and chemotherapeutic drugs to defeat multidrug resistant tumors [115]. Besides, the delivery system possessed active targeting characteristic and tumor microenvironment-responsive on-demand drug release, realizing the "four in one" multifunction. In brife, drug gated MSN delivery systems put away complicated sealing agents, meanwhile, it also reach controllable drug release caused by physiological stimuluses.
|
Download:
|
| Fig. 5. Schematic illustration of the drug DOX-self-gated MSNs. (A) The dynamic interaction between DOX and benzaldehyde via pH-sensitive benzoic-imine bond. (B) Drug release process with pH-respon-sive property. (C) Site-specific drug release and cell uptake of the self-gated system. Reproduced with permission [113]. Copyright 2017, Wiley. | |
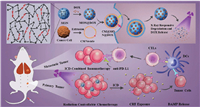
Compared with other gated materials, biological membranes have unique advantages in biocompatibility, which has attracted extensive attention from researchers. In recent years, the use of isolated biofilms to camouflage nanoparticles has aroused great interest in the biomedical field, and has achieved visible results in tumor therapy research [116, 117]. Due to its inherent versatility, biological membrane coating is superior to polymer coating. First, it can avoid being swallowed and attacked by the reticuloendothelial system (RES), extending the circulation time in the blood and increasing the accumulation of drugs in the tumor site. In addition, due to the similarity in the composition of biofilms, these membranes can not only ensure blood compatibility, but also promote entanglement and fusion with tumor biofilms, enhancing tumor targeting and internalization. Furthermore, biofilm bionics can also reduce the adhesion of other free biological blocks and improve the delivery efficiency. Currently, many different sources of biofilms have been used to camouflage mesoporous silica nanoparticles (MSNs), such as red blood cell (RBC) membranes, macrophage cell membranes, and cancer cells [118-120]. For example, Li et al. [118] reported a laser-responsive red blood cell mimicking mesoporous silica nanoparticle with longer blood circulation time and tumor-specific drug release. It co-loaded the photosensitizer chlorin e6 (Ce6) and the chemotherapy drug doxorubicin, which avoided early drug leakage and could be used for tumor imaging. Among diverse cell membranes, the cancer cell membrane stands out for tumor-targeted delivery through its excellent homology and tumor homing ability. In another case, Shao et al. prepared mesoporous organosilica nanoparticles (MONs) which contained X-ray- and reactive oxygen species-responsive diselenide bonds [121]. Doxorubicin (DOX) was loaded in the pore, and the 4T1 breast cancer cell membrane was employed to achieve cancer cell targeting. The combination of DOX-mediated immunogenic cell death and PD-L1 immune checkpoint blocking therapy further enhanced the anti-tumor and anti-metastasis effects (Fig. 6). Although cell membrane coating endows mesoporous silica nanoparticles with prolonged circulation time, targeting property and enhanced internalization, some flaws still remain. The biomimetic MSNs have less structural flexibility than natural cells, which may affect how they interact with cells in the body. Moreover, the rigid structure can affect its deformability, resulting in defects in permeability and transport. It is also worth noting that the size of mesoporous silica nanoparticles also needs to be taken into account when wrapping them in cell membranes. Chen et al. [122] synthesized a series of MSNs ranging from 10 nm to 200 nm and investigated the effect of size on human erythrocyte membrane encapsulation by a hypotonic dialysis-based method. Results showed that effective encapsulation can be achieved when the particle hydrodynamic diameter was less than 30 nm. Despite great progress has been made in the performance of biomimetic mesoporous silicon nanoparticles in vitro and in vivo, it is still in its infancy, and more research is needed on the mechanical properties and interfacial interactions with cells of this composite material.
|
Download:
|
| Fig. 6. Schematic illustration for an example of cancer cell membrane-mimetic mesoporous organosilica nanoparticles containing chemotherapy and immunotherapy. Reproduced with permission [121]. Copyright 2020, Wiley. | |
Mesoporous silica is facinating drug delivery carrier. The design of gated mesoporous silica nanoplatform realizes controlled release of drugs at tumor sites on demand, thus reducing early leakage as well as toxic and side effects on normal tissues. Here, we present the mechanisms of drug controlled release in gated mesoporous silica nanoparticles (MSNs) in short and mainly review the gated materials. The mechanisms that control drug release include internal stimuli caused by differences between tumor cells and normal cells and external physical stimuli artificially applied, which are used to fabricate MSNs delivery system and are the basis for the precise design of gated MSNs. "Gate keepers" including inorganic materials, natural and synthetic polymers, biomacromolecules, drug molecules themselves, and biological membranes are employed to seal mesoporous silica channels to improve anti-cancer effects and avoid unexpected adverse impact. The introduction of these materials not only enhances the effect of chemotherapy, but also can achieve a variety of therapeutic synergies due to the intrinsic characteristics of gated materials themselves.
Gated mesoporous silica nanoparticle delivery systems have been designed to break through the limitations of traditional chemotherapy, however, some challenges still exist. First of all, how to improve the sensitivity of the "gate keepers" to attain zero release in the blood circulation and complete release in the tumor site remains to be solved. Second, surface modification by gated materials will cause changes in physical and chemical properties, and the harm to the body, covering acute injury and chronic toxicity, needs to be verified in more experimental models. Third, in addition to the efficient delivery of therapeutic passenger molecules, the biocompatibility and blood compatibility of the carrier are insurmountable gaps to achieve clinical translation. Since the predominant administration route of MSNs that in laboratory is intravenous route, MSNs should be highly compatible with blood components, which will largely determine its administration capability. Last but not least, it is very important to standardize production procedures to solve the industrial scale production and repeatable synthesis of functional MSNs.
From a general perspective, great progress has been made in the design and development of mesoporous silica for biomedical field, and the join of "gate keepers" adds new functions and vitality to the drug delivery system. It can be anticipated that the cross integration and innovative development of materials science, biomedicine and pharmaceutical science will overcome the predicaments and illuminate the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful for the financial support from the National Natural Science Foundation of China (No. 32071342), Guangdong Special Support Program (No. 2019TQ05Y209), Natural Science Foundation of Guangdong Province (No. 2021A1515010431), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP001), Shenzhen Key Laboratory of Kindey Diseases (No. ZDSYS201504301616234), the Key Project of Basic Research of Shenzhen (No. JCYJ20200109113603854), the International Cooperation Research Project of Shenzhen (No. GJHZ20180418190557102), and the Special Funds of Key Disciplines Construction from Guangdong and Zhongshan Cooperating.
| [1] |
C. Li, J. Wang, Y. Wang, et al., Acta Pharm. Sin. B 9 (2019) 1145-1162. DOI:10.1016/j.apsb.2019.08.003 |
| [2] |
J. Shi, P.W. Kantoff, R. Wooster, O.C. Farokhzad, Nat. Rev. Cancer 17 (2017) 20-37. DOI:10.1038/nrc.2016.108 |
| [3] |
A. Pugazhendhi, T. Edison, I. Karuppusamy, B. Kathirvel, Int. J. Pharm. 539 (2018) 104-111. DOI:10.1016/j.ijpharm.2018.01.034 |
| [4] |
X. Li, A.S. Widjaya, J. Liu, et al., Acta Biomater. 106 (2020) 301-313. DOI:10.1016/j.actbio.2020.02.013 |
| [5] |
X. Wei, L. Liu, X. Li, et al., J. Control. Release 313 (2019) 42-53. DOI:10.1016/j.jconrel.2019.09.021 |
| [6] |
S. Wang, G. Yu, Z. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 14758-14763. DOI:10.1002/anie.201908997 |
| [7] |
K.O. Boakye-Yiadom, S. Kesse, Y. Opoku-Damoah, et al., Int. J. Pharm. 564 (2019) 308-317. DOI:10.1016/j.ijpharm.2019.04.055 |
| [8] |
X. Zeng, M. Luo, G. Liu, et al., Adv. Sci. (Weinh) 5 (2018) 1800510. DOI:10.1002/advs.201800510 |
| [9] |
F. Zhang, G. Lu, X. Wen, et al., J. Control. Release 326 (2020) 131-139. DOI:10.1016/j.jconrel.2020.06.015 |
| [10] |
R.R. Castillo, D. Lozano, B. Gonzalez, et al., Expert. Opin. Drug Deliv. 16 (2019) 415-439. DOI:10.1080/17425247.2019.1598375 |
| [11] |
M.H. Chan, H.M. Lin, Biomaterials 46 (2015) 149-158. DOI:10.1016/j.biomaterials.2014.12.034 |
| [12] |
M. Vallet-Regi, M. Colilla, I. Izquierdo-Barba, M. Manzano, Molecules 23 (2018) 47. |
| [13] |
J. Yang, D. Dai, X. Lou, et al., Theranostics 10 (2020) 615-629. DOI:10.7150/thno.40066 |
| [14] |
J. Lu, M. Liong, Z. Li, et al., Small 6 (2010) 1794-1805. DOI:10.1002/smll.201000538 |
| [15] |
M. Vallet-Regi, F. Balas, D. Arcos, Angew. Chem. Int. Ed. 46 (2007) 7548-7558. DOI:10.1002/anie.200604488 |
| [16] |
R. Zhao, T. Li, G. Zheng, et al., Biomaterials 143 (2017) 1-16. DOI:10.1016/j.biomaterials.2017.07.030 |
| [17] |
X. Hong, X. Zhong, G. Du, et al., Sci. Adv. 6 (2020) eaaz4462. DOI:10.1126/sciadv.aaz4462 |
| [18] |
C. Chen, W. Yao, W. Sun, et al., Int. J. Biol. Macromol. 122 (2019) 1090-1099. DOI:10.1016/j.ijbiomac.2018.09.058 |
| [19] |
X. Ding, W. Yu, Y. Wan, et al., Carbohydr. Polym. 245 (2020) 116493. DOI:10.1016/j.carbpol.2020.116493 |
| [20] |
H. Li, X. Wu, B. Yang, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 94 (2019) 453-464. DOI:10.1016/j.msec.2018.09.053 |
| [21] |
S.P. Hadipour Moghaddam, R. Mohammadpour, H. Ghandehari, J. Control. Release 311-312 (2019) 1-15. DOI:10.1016/j.jconrel.2019.08.028 |
| [22] |
G. Yang, S.Z.F. Phua, A.K. Bindra, Y. Zhao, Adv. Mater. 31 (2019) e1805730. DOI:10.1002/adma.201805730 |
| [23] |
M. Gautam, R.K. Thapa, B.K. Poudel, et al., Acta Biomater. 88 (2019) 448-461. DOI:10.1016/j.actbio.2019.02.029 |
| [24] |
A. Noureddine, A. Maestas-Olguin, E.A. Saada, et al., Acta Biomater. 114 (2020) 358-368. DOI:10.1016/j.actbio.2020.07.027 |
| [25] |
C. Argyo, V. Weiss, C. Bräuchle, T. Bein, Chem. Mater. 26 (2013) 435-451. |
| [26] |
A. Baeza, M. Vallet-Regi, Pharmaceutics 12 (2020) 957. DOI:10.3390/pharmaceutics12100957 |
| [27] |
P. Mi, Theranostics 10 (2020) 4557-4588. DOI:10.7150/thno.38069 |
| [28] |
G. Helmlinger, F. Yuan, M. Dellian, R.K. Jain, Nat. Med. 3 (1997) 177-182. DOI:10.1038/nm0297-177 |
| [29] |
J. Jiang, N. Shen, T. Ci, et al., Adv. Mater. 31 (2019) e1904278. DOI:10.1002/adma.201904278 |
| [30] |
C.R. Thomas, D.P. Ferris, J.H. Lee, et al., J. Am. Chem. Soc. 132 (2010) 10623-10625. DOI:10.1021/ja1022267 |
| [31] |
Z. Zhang, L. Wang, J. Wang, et al., Adv. Mater. 24 (2012) 1418-1423. DOI:10.1002/adma.201104714 |
| [32] |
C. Qi, Y.J. Zhu, X.Y. Zhao, et al., Mater. Res. Bull. 48 (2013) 1536-1540. DOI:10.1016/j.materresbull.2012.12.052 |
| [33] |
Z. Shi, Q. Li, L. Mei, Chin. Chem. Lett. 31 (2020) 1345-1356. DOI:10.1016/j.cclet.2020.03.001 |
| [34] |
Y. Qiu, Y. Liu, L. Wang, et al., Biomaterials 31 (2010) 7606-7619. DOI:10.1016/j.biomaterials.2010.06.051 |
| [35] |
Y. Yang, Y. Lin, D. Di, et al., J. Colloid Interface Sci. 508 (2017) 323-331. DOI:10.1016/j.jcis.2017.08.050 |
| [36] |
J.L. Vivero-Escoto, I.I. Slowing, C.W. Wu, V.S. Lin, J. Am. Chem. Soc. 131 (2009) 3462-3463. DOI:10.1021/ja900025f |
| [37] |
M. Shourian, H. Ghourchian, M. Boutorabi, Anal. Chim. Acta 895 (2015) 1-11. DOI:10.1016/j.aca.2015.07.013 |
| [38] |
R. Cheng, F. Feng, F. Meng, et al., J. Control. Release 152 (2011) 2-12. DOI:10.1016/j.jconrel.2011.01.030 |
| [39] |
G. Saito, J.A. Swanson, K.D. Lee, Adv. Drug Deliv. Rev. 55 (2003) 199-215. DOI:10.1016/S0169-409X(02)00179-5 |
| [40] |
P. Eskandari, B. Bigdeli, M.Porgham Daryasari, et al., J. Drug Target. 27 (2019) 1084-1093. DOI:10.1080/1061186x.2019.1599379 |
| [41] |
L. Zhu, P. Kate, V.P. Torchilin, ACS Nano 6 (2012) 3491-3498. DOI:10.1021/nn300524f |
| [42] |
Y. Wang, S. Song, J. Liu, et al., Angew. Chem. Int. Ed. 54 (2015) 536-540. |
| [43] |
M. Liu, X. Sun, Z. Liao, et al., Drug Deliv. 26 (2019) 732-743. DOI:10.1080/10717544.2019.1642419 |
| [44] |
F. Muhammad, M. Guo, W. Qi, et al., J. Am. Chem. Soc. 133 (2011) 8778-8781. DOI:10.1021/ja200328s |
| [45] |
X. Fang, T. Zhai, U.K. Gautam, et al., Prog. Mater. Sci. 56 (2011) 175-287. DOI:10.1016/j.pmatsci.2010.10.001 |
| [46] |
Y. Salinas, C. Hoerhager, A. Garcia-Fernandez, et al., ACS Appl. Mater. Interfaces 10 (2018) 34029-34038. DOI:10.1021/acsami.8b13698 |
| [47] |
Y. Sun, L. Zheng, Y. Yang, et al., Nano Micro Lett. 12 (2020) 103. DOI:10.1007/978-981-15-7056-8_7 |
| [48] |
S. Zhang, X. Pei, H. Gao, et al., Chin. Chem. Lett. 31 (2020) 1060-1070. DOI:10.1016/j.cclet.2019.11.036 |
| [49] |
X. Qian, S. Deng, X. Chen, et al., Chin. Chem. Lett. 31 (2020) 2211-2214. DOI:10.1016/j.cclet.2019.09.024 |
| [50] |
S. Rojas, F.J. Carmona, C.R. Maldonado, et al., Inorg. Chem. 55 (2016) 2650-2663. DOI:10.1021/acs.inorgchem.6b00045 |
| [51] |
H. Zhang, W. Jiang, R. Liu, et al., ACS Appl. Mater. Interfaces 9 (2017) 19687-19697. DOI:10.1021/acsami.7b05142 |
| [52] |
C. Adhikari, A. Mishra, D. Nayak, A. Chakraborty, J. Drug Deliv. Sci. Technol. 47 (2018) 1-11. DOI:10.1016/j.jddst.2018.06.015 |
| [53] |
L. Guo, I. Panderi, D.D. Yan, et al., ACS Nano 7 (2013) 8780-8793. DOI:10.1021/nn403202w |
| [54] |
L. An, X. Wang, X. Rui, et al., Angew. Chem. Int. Ed. 57 (2018) 15782-15786. DOI:10.1002/anie.201810082 |
| [55] |
M. Zhang, X. Liu, Q. Luo, et al., Chem. Eng. J. 389 (2020) 124450. DOI:10.1016/j.cej.2020.124450 |
| [56] |
Q. Wei, Y. Chen, X. Ma, et al., Adv. Funct. Mater. 28 (2017) 1704634. |
| [57] |
X. Cheng, D. Li, A. Lin, et al., Int. J. Nanomed. 13 (2018) 3661-3677. DOI:10.2147/ijn.s167407 |
| [58] |
H. Liang, B. Zhou, D. Wu, et al., Adv. Colloid Interface Sci. 272 (2019) 102019. DOI:10.1016/j.cis.2019.102019 |
| [59] |
T.S. Sileika, D.G. Barrett, R. Zhang, et al., Angew. Chem. Int. Ed. 52 (2013) 10766-10770. DOI:10.1002/anie.201304922 |
| [60] |
H. Ejima, J.J. Richardson, K. Liang, et al., Science 341 (2013) 154-157. DOI:10.1126/science.1237265 |
| [61] |
C. Park, B.J. Yang, K.B. Jeong, et al., Angew. Chem. Int. Ed. 56 (2017) 5485-5489. DOI:10.1002/anie.201701152 |
| [62] |
B. Yang, S. Zhou, J. Zeng, et al., Nano Res. 13 (2020) 1013-1019. DOI:10.1007/s12274-020-2736-6 |
| [63] |
A.F. Moreira, V.M. Gaspar, E.C. Costa, et al., Eur. J. Pharm. Biopharm. 88 (2014) 1012-1025. DOI:10.1016/j.ejpb.2014.09.002 |
| [64] |
A.D. Trofimov, A.A. Ivanova, M.V. Zyuzin, A.S. Timin, Pharmaceutics 10 (2018) 167. DOI:10.3390/pharmaceutics10040167 |
| [65] |
P. Srivastava, S.K. Hira, D.N. Srivastava, et al., ACS Appl. Mater. Interfaces 10 (2018) 6917-6929. DOI:10.1021/acsami.7b18729 |
| [66] |
Y. He, B. Zeng, S. Liang, et al., ACS Appl. Mater. Interfaces 9 (2017) 44402-44409. DOI:10.1021/acsami.7b16787 |
| [67] |
J. Ma, H. Wu, Y. Li, et al., Pharm. Res. 35 (2018) 57. DOI:10.1134/s0424857018110063 |
| [68] |
G. Crini, Chem. Rev. 114 (2014) 10940-10975. DOI:10.1021/cr500081p |
| [69] |
Y.M. Zhang, Y.H. Liu, Y. Liu, Adv. Mater. 32 (2020) e1806158. DOI:10.1002/adma.201806158 |
| [70] |
B. Schmidt, C. Barner-Kowollik, Angew. Chem. Int. Ed. 56 (2017) 8350-8369. DOI:10.1002/anie.201612150 |
| [71] |
J.J. Hu, Q. Lei, M.Y. Peng, et al., Biomaterials 128 (2017) 136-146. DOI:10.1001/jamainternmed.2016.7068 |
| [72] |
Y. Wu, Z. Xu, W. Sun, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 103 (2019) 109831. DOI:10.1016/j.msec.2019.109831 |
| [73] |
A. Llopis-Lorente, A. Garcia-Fernandez, N. Murillo-Cremaes, et al., ACS Nano 13 (2019) 12171-12183. DOI:10.1021/acsnano.9b06706 |
| [74] |
M. Dash, F. Chiellini, R.M. Ottenbrite, E. Chiellini, Prog. Polym. Sci. 36 (2011) 981-1014. DOI:10.1016/j.progpolymsci.2011.02.001 |
| [75] |
R. Jayakumar, M. Prabaharan, S.V. Nair, H. Tamura, Biotechnol. Adv. 28 (2010) 142-150. DOI:10.1016/j.biotechadv.2009.11.001 |
| [76] |
H. Yi, L.Q. Wu, W.E. Bentley, et al., Biomacromolecules 6 (2005) 2881-2894. DOI:10.1021/bm050410l |
| [77] |
M.N. Kumar, R.A. Muzzarelli, C. Muzzarelli, et al., Chem. Rev. 104 (2004) 6017-6084. DOI:10.1021/cr030441b |
| [78] |
T. Yan, J. He, R. Liu, et al., Carbohydr. Polym. 231 (2020) 115706. DOI:10.1016/j.carbpol.2019.115706 |
| [79] |
B.P. Toole, Nat. Rev. Cancer 4 (2004) 528-539. DOI:10.1038/nrc1391 |
| [80] |
R. Stern, M.J. Jedrzejas, Chem. Rev. 106 (2006) 818-839. DOI:10.1021/cr050247k |
| [81] |
G. Tripodo, A. Trapani, M.L. Torre, et al., Eur. J. Pharm. Biopharm. 97 (2015) 400-416. DOI:10.1016/j.ejpb.2015.03.032 |
| [82] |
Q. Zhao, S. Wang, Y. Yang, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 78 (2017) 475-484. DOI:10.1016/j.msec.2017.04.059 |
| [83] |
W. Ma, Q. Chen, W. Xu, et al., Nano Res. 14 (2020) 846-857. DOI:10.1109/ei250167.2020.9347325 |
| [84] |
J. Meng, Z. Jin, P. Zhao, et al., Sci. Adv. 6 (2020) eaba1362. DOI:10.1126/sciadv.aba1362 |
| [85] |
A.B. Cook, S. Perrier, Adv. Funct. Mater. 30 (2019) 1901001. |
| [86] |
M. He, L. Yu, Y. Yang, et al., Chin. Chem. Lett. 31 (2020) 3178-3182. DOI:10.1016/j.cclet.2020.05.034 |
| [87] |
Z. Li, X. Shan, Z. Chen, et al., Adv. Sci. (Weinh) 8 (2020) 2002589. |
| [88] |
Y. Zhang, C.Y. Ang, M. Li, et al., ACS Appl. Mater. Interfaces 7 (2015) 18179-18187. DOI:10.1021/acsami.5b05893 |
| [89] |
H. Peng, R. Dong, S. Wang, et al., Int. J. Pharm. 446 (2013) 153-159. DOI:10.1016/j.ijpharm.2013.01.071 |
| [90] |
P.W. Chung, R. Kumar, M. Pruski, V.S.Y. Lin, Adv. Funct. Mater. 18 (2008) 1390-1398. DOI:10.1002/adfm.200701116 |
| [91] |
A. Kienzle, S. Kurch, J. Schloder, et al., Adv. Healthc. Mater. 6 (2017) 1700012. DOI:10.1002/adhm.201700012 |
| [92] |
L. Chen, T. Zhao, M. Zhao, et al., Chem. Sci. 11 (2020) 2819-2827. DOI:10.1039/c9sc06260b |
| [93] |
L. Li, W. Sun, L. Li, et al., Nanoscale 9 (2017) 314-325. DOI:10.1039/C6NR07004C |
| [94] |
S. Mu, Y. Liu, T. Wang, et al., Acta Biomater. 63 (2017) 150-162. DOI:10.1016/j.actbio.2017.08.050 |
| [95] |
D. Wu, Z.Q. Zhu, H.X. Tang, et al., Theranostics 10 (2020) 9808-9829. DOI:10.7150/thno.43631 |
| [96] |
Q. Yan, X. Guo, X. Huang, et al., ACS Appl. Mater. Interfaces 11 (2019) 24377-24385. DOI:10.1021/acsami.9b04142 |
| [97] |
W. Cheng, X. Zeng, H. Chen, et al., ACS Nano 13 (2019) 8537-8565. DOI:10.1021/acsnano.9b04436 |
| [98] |
W. Wang, Z. Tang, Y. Zhang, et al., Macromol. Biosci. 20 (2020) e2000222. DOI:10.1002/mabi.202000222 |
| [99] |
H. Lee, S.M. Dellatore, W.M. Miller, P.B. Messersmith, Science 318 (2007) 426-430. DOI:10.1126/science.1147241 |
| [100] |
W. Cheng, J. Nie, L. Xu, et al., ACS Appl. Mater. Interfaces 9 (2017) 18462-18473. DOI:10.1021/acsami.7b02457 |
| [101] |
D. Chang, Y. Gao, L. Wang, et al., J. Colloid Interface Sci. 463 (2016) 279-287. DOI:10.1016/j.jcis.2015.11.001 |
| [102] |
W. Cheng, C. Liang, L. Xu, et al., Small 13 (2017) 1700623. DOI:10.1002/smll.201700623 |
| [103] |
Y. Yang, W. Zeng, P. Huang, et al., View 2 (2020) 20200042. |
| [104] |
W. Cheng, J. Nie, N. Gao, et al., Adv. Funct. Mater. 27 (2017) 1704135. DOI:10.1002/adfm.201704135 |
| [105] |
K. Li, C. Lin, Y. He, et al., ACS Nano 14 (2020) 14164-14180. DOI:10.1021/acsnano.0c07071 |
| [106] |
H. Zhang, Q. Xia, D. Zhou, Colloids Surf. B: Biointerfaces 193 (2020) 111107. DOI:10.1016/j.colsurfb.2020.111107 |
| [107] |
X. Chen, H. Sun, J. Hu, et al., Colloids Surf. B: Biointerfaces 152 (2017) 77-84. DOI:10.1016/j.colsurfb.2017.01.010 |
| [108] |
R. Bhat, I. Garcia, E. Aznar, et al., Nanoscale 10 (2017) 239-249. DOI:10.31142/ijtsrd5940 |
| [109] |
B. Zhang, Z. Luo, J. Liu, et al., J. Control. Release 192 (2014) 192-201. DOI:10.1016/j.jconrel.2014.06.037 |
| [110] |
R.R. Castillo, A. Baeza, M. Vallet-Regi, Biomater. Sci. 5 (2017) 353-377. DOI:10.1039/C6BM00872K |
| [111] |
L. Pascual, C. Cerqueira-Coutinho, A. Garcia-Fernandez, et al., Nanomedicine 13 (2017) 2495-2505. DOI:10.1016/j.nano.2017.08.006 |
| [112] |
À. Ribes, S. Santiago-Felipe, A. Aviñó, et al., Sens. Actuators B: Chem. 277 (2018) 598-603. DOI:10.1016/j.snb.2018.09.026 |
| [113] |
X. Zeng, G. Liu, W. Tao, et al., Adv. Funct. Mater. 27 (2017) 1605985. DOI:10.1002/adfm.201605985 |
| [114] |
C. Liang, H. Wang, M. Zhang, et al., J. Colloid Interface Sci. 525 (2018) 1-10. DOI:10.1016/j.jcis.2018.04.058 |
| [115] |
W. Cheng, C. Liang, X. Wang, et al., Nanoscale 9 (2017) 17063-17073. DOI:10.1039/C7NR05450E |
| [116] |
Z. Wang, F. Zhang, D. Shao, et al., Adv. Sci. (Weinh) 6 (2019) 1901690. DOI:10.1002/advs.201901690 |
| [117] |
X. Liang, X. Ye, C. Wang, et al., J. Control. Release 296 (2019) 150-161. DOI:10.1016/j.jconrel.2019.01.027 |
| [118] |
J. Su, H. Sun, Q. Meng, et al., Theranostics 7 (2017) 523-537. DOI:10.7150/thno.17259 |
| [119] |
D. Zhang, Z. Lin, Y. Zheng, et al., ACS Nano 14 (2020) 8985-8999. DOI:10.1021/acsnano.0c03833 |
| [120] |
D. Nie, Z. Dai, J. Li, et al., Nano Lett. 20 (2020) 936-946. DOI:10.1021/acs.nanolett.9b03817 |
| [121] |
D. Shao, F. Zhang, F. Chen, et al., Adv. Mater. 32 (2020) e2004385. DOI:10.1002/adma.202004385 |
| [122] |
Z.A. Chen, S.H. Wu, P. Chen, et al., ACS Appl. Mater. Interfaces 11 (2019) 4790-4798. DOI:10.1021/acsami.8b18434 |
 2021, Vol. 32
2021, Vol. 32 

