Quinoline-2, 4-diones are an important class of biologically nitrogen containing fused heterocycles and have a wide application in pharmaceuticals and agrochemicals [1]. As a consequence, the research of efficient and novel methods for the preparation of quinoline-2, 4-diones attracts an ever-increasing attention in organic chemistry [2]. The intramolecular cyclization of N-acylanthranilates and the oxidation of 4-hydroxyquinolin-2-ones were the two classical synthetic methods. In recent years, the radical cyclization reaction for the synthesis of various heterocyclic compounds has achieved much progress in organic chemistry [3]. For example, the cyclization of N-acylanthranilates has received much attentions and great progress has been made. In 2019, Li group realized the cascade methylation/cyclization of ortho cyanoarylacrylamides with dicumyl peroxide, providing a simple and straightforward approach to methylated quinoline-2, 4-diones [4]. The nitro-containing quinoline-2, 4(1H, 3H)-diones was also realized by the same group in 2018 [5]. The difluoro-containing quinoline-2, 4-diones were reported by Shi group [6]. In addition, difluoromethylated [7], carbonyl substituted [8], sulfonated [9], trifluoromethylated [10], phosphonylated [11] quinoline-2, 4-diones were also prepared from ortho-cyanoarylacrylamides via radical cyclization reaction.
Reactions conducted under metal-free conditions are ecofriendly and play an important role in organic synthesis [12]. As a readily available, environmentally friendly, easily handled oxidant, persulfates have been greatly applied in organic synthesis [13]. Generally speaking, persulfate can decompose to give sulfate radical anions under heating, light irradiation or transition metal reduction conditions. This radical anion has strong single-electron oxidizing properties, and can oxidize various neutral molecules or anions to produce corresponding radical active intermediates. These newly generated reactive intermediates can undergo a series of chemical transformations to give useful compounds with diverse structures. As our continuous efforts on heterocyclic compounds [14], herein, we disclosed ammonium persulfate promoted cyclization of ortho-cyanoarylacrylamides with oxamic acids for the construction of carbamoyl quinoline-2, 4-diones under metal-free conditions (Scheme 1).

|
Download:
|
| Scheme 1. Synthesis of quinoline-2, 4-diones. | |
For the optimization of the reaction conditions, the cyclic reaction of N-(2-cyanophenyl)-N-methylmethacrylamide A1 with 2-oxo-2-(phenylamino)acetic acid B1 was chosen as a model reaction. With examination of a series of solvents, it revealed that acetone/H2O was suitable for this transformation and the corresponding product was isolated with 86% yield (Table 1, entry 1). When other solvents dioxane/H2O, CH3CN/H2O, EtOAc/H2O, DMF/H2O, THF/H2O, DMSO/H2O were used for this reaction, products with slight lower yields were obtained (Table 1, entries 2–7). To our disappointment, no desired product was generated when toluene/H2O, DCE/H2O or PhCl/H2O was used as solvent (Table 1, entries 8–10). We next turn our attention to investigate the effect of oxidants on the cyclic reaction. Moderate yields of the products were obtained when the reaction was performed in the presence of K2S2O8 or Na2S2O8 (Table 1, entries 11 and 12). PIDA, TBPB and DTBP were not effective for this reaction (Table 1, entries 13–15). Further optimization of the oxidants loading reveal that 2 equiv. of (NH4)2S2O8 provided the best yield of the product (Table 1, entries 16 and 17).
|
|
Table 1 Optimization of the reaction conditions.a |
After completion of the search for the optimized reaction conditions, the scope of the substrates was examined and the results were shown in Scheme 2. A series of substituted oxamic acids with different substituents on the phenyl subunit were investigated. To our delight, wide range of substituted oxamic acids can be used in the reaction to give the desired quinoline-2, 4-diones. Various substituents such as CH3, CH3O, F, Br and CF3 were well tolerated in this system (Scheme 2, C1-C11). The electronic properties and steric hindrance of the substituents did not show significant effect on the efficiency of the reaction. Additionally, when 2-(naphthalen-1-ylamino)-2-oxoacetic acid was subjected to this reaction, the corresponding product was isolated in 94% yield (C12). Besides aryl substituted oxamic acids, alkyl substituted oxamic acids also reacted well, providing the corresponding products in 74%–88% yields (C13–C15). The effect of substituents on the N-aryl moiety was also investigated. To our delight, a variety of N-(2-cyanoaryl)acrylamides we tested showed good performance (C16–C31). It was noted that N-benzyl substituted acrylamide also showed high reactivity, affording C32 in 88% yield. However, acrylamide with a free N–H bond was not suitable for this reaction (C33).
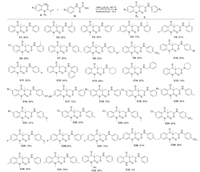
|
Download:
|
| Scheme 2. Reaction scope. A (0.2 mmol), B (0.4 mmol), (NH4)2S2O8 (2.0 equiv.), acetone/H2O (2 mL). 80 ℃, 12 h. | |
In order to shed some light on the reaction mechanism, some control experiments were conducted. It was found that the reaction was completely inhibited when the reaction was performed in the presence of TEMPO (2, 2, 6, 6-tetramethylpiperidine-1-oxyl). Additionally, when 1.1-diphenylethylene as another radical inhibitor was added under standard conditions, the carbamoyl radical captured intermediate D1 was detected by HRMS. These results indicated that a radical mechanism was involved it the cyclic reaction (Scheme 3).
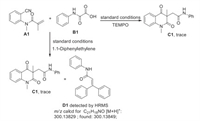
|
Download:
|
| Scheme 3. Control experiments. | |
On the basis of the above mentioned results and previous reports [15], a proposed reaction mechanism was described in Scheme 4. First, oxidation of 2-oxo-2-(phenylamino)acetic acid B1 by persulfate generated carbamoyl radical E, subsequently, alkyl radical F was formed via the addition reaction of carbamoyl radical E with N-(2-cyanophenyl)-N-methylmethacrylamide A1. Intramolecular addition of the alkyl radical to the nitrile provided imine radical G, which was converted to imine H via H-abstraction. Finally, the hydrolysis of imine in the presence of H2O could result in the desired product C.
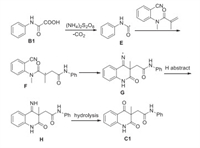
|
Download:
|
| Scheme 4. Tentative reaction mechanism. | |
In conclusion, we have developed an efficient and practical methods for the synthesis of carbamoyl quinoline-2, 4-diones via the reaction of ortho-cyanoarylacrylamides with oxamic acids. This cyclic reaction could be performed efficiently under metal free conditions. Various products with functional groups could be obtained with moderate to high yields. Radical mechanism was proposed for this reaction. This procedure is expected to complement the current methods for the synthesis of quinoline-2, 4-diones derivatives.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21772107), Shandong Province Key Research and Development Plan (No. 2019GSF108017). We also thank Cai-Zhen Ding, Yan-Li Wang and Hong-Di Yang for their useful help.
| [1] |
(a) S. Han, F.F. Zhang, H.Y. Qian, et al., J. Med. Chem. 58 (2015) 5751-5769; (b) Y.X. Liu, H.P. Zhao, Z.W. Wang, et al., Mol. Divers. 17 (2013) 701-710; (c) J.L. McCormick, T.C. McKee, J.H. Cardellina, M.R. Boyd, J. Nat. Prod. 59 (1996) 469-471; (d) Z. Liu, X. Zhang, H. Zhang, et al., Chin. J. Org. Chem. 40 (2020) 2755; (e) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241-3244; (f) Y.F. Si, K. Sun, X.L. Chen, et al., Org. Lett. 22 (2020) 6960-6965. |
| [2] |
(a) T. Yang, W.J. Xia, J.Q. Shang, et al., Org. Lett. 21 (2019) 444-447; (b) L.J. Wu, Y. Yang, R.J. Song, et al., Chem. Commun. 54 (2018) 1367-1370; (c) Y. Zhang, T. Zhang, H. Zhan, X. Li, Chin. J. Catal. 35 (2014) 1840-1845; (d) L. Tao, C. Li, Y. Ren, et al., Chin. J. Catal. 40 (2019) 1548-1556; (e) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (f) H.Y. Guo, Y. Yu, Chin. Chem. Lett. 21 (2010) 1435-1438; (g) Z. Wang, L. Chen, G. Mao, C. Wang, Chin. Chem. Lett. 31 (2020) 1890-1894. |
| [3] |
(a) L. Xiong, H. Hu, C.W. Wei, B. Yu, Eur. J. Org. Chem. 2020 (2020) 1588-1597; (b) J. Xuan, A. Studer, Chem. Soc. Rev. 46 (2017) 4329-4346; (c) F. Zhao, X. Jia, D. Wang, et al., Chin. J. Org. Chem. (2017) 37; (d) X.X. Meng, Q.Q. Kang, J.Y. Zhang, et al., Green Chem 22 (2020) 1388-1392; (e) Z. Wang, W.M. He, Chin. J. Org. Chem. 39 (2019) 3594-3595; (f) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258; (g) D. Dong, Y. Sun, G. Li, et al., Chin. J. Org. Chem. 40 (2020) 4071-4086; (h) Z.Y. Gan, G.Q. Li, X.B. Yang, et al., Sci. China Chem. 63 (2020) 1652-1658; (i) K. Sun, G. Li, Y. Li, et al., Adv. Synth. Catal. 362 (2020) 1947-1954; (j) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262; (k) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett 60 (2019) 1845-1848; (l) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368; (m) X. Ren, Z. Lu, Chin. J. Catal. 40 (2019) 1003-1019; (n) R. Yi, L. Qian, B. Wan, Chin. J. Catal. 40 (2019) 177-183; (o) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258; (p) Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021) 1907-1910; (q) W.H. Bao, Z. Wang, Z. Cao, et al., Adv. Synth. Catal. 363 (2021) 757-761; (r) D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575; (s) D.Q. Dong, Q.Q. Han, S.H. Yang, et al., ChemistrySelect 5 (2020) 13103-13134. |
| [4] |
T. Yang, W.J. Xia, B. Zhou, et al., Eur. J. Org. Chem. 2019 (2019) 5749-5755. DOI:10.1002/ejoc.201900918 |
| [5] |
Y.M. Li, T. Yang, J.L. Zhou, et al., Synthesis(Mass) 50 (2018) 3460-3466. DOI:10.1055/s-0037-1610070 |
| [6] |
F. Ding, Y. Fang, Y. Jiang, K. Lin, L. Shi, Chem. Asian J. 13 (2018) 636-640. DOI:10.1002/asia.201701780 |
| [7] |
H. Sun, Y. Jiang, Y.S. Yang, et al., Org. Biomol. Chem. 17 (2019) 6629-6638. DOI:10.1039/c9ob01213c |
| [8] |
(a) S.S. Wang, H. Fu, G. Wang, M. Sun, Y.M. Li, RSC Adv. 6 (2016) 52391-52394; (b) S.S. Wang, H. Fu, Y. Shen, M. Sun, Y.M. Li, J. Org. Chem. 81 (2016) 2920-2929. |
| [9] |
S. Wang, X. Huang, Q. Wang, et al., RSC Adv. 6 (2016) 11754-11757. DOI:10.1039/C5RA27878C |
| [10] |
H. Fu, S.S. Wang, Y.M. Li, Adv. Synth. Catal. 358 (2016) 3616-3626. DOI:10.1002/adsc.201600693 |
| [11] |
Y.M. Li, S.S. Wang, F. Yu, Y. Shen, K.J. Chang, Org. Biomol. Chem. 13 (2015) 5376-5380. DOI:10.1039/C5OB00617A |
| [12] |
(a) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308; (b) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173; (c) Y. Wu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 31 (2020) 2999-3000; (d) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, Chemistryselect 4 (2019) 2480-2483; (e) J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Chin. J. Catal. 42 (2021) 1445-1450; (f) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39 (2019) 3190-3198; (g) X.Y. Li, Y. Liu, X.L. Chen, et al., Green Chem. 22 (2020) 4445-4449; (h) K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500; (i) J. Jiang, F. Xiao, W.M. He, L. Wang, Chin. Chem. Lett. 32 (2021) 1637-1644. |
| [13] |
(a) J.F. Zhao, X.H. Duan, L.N. Guo, Chin. J. Org. Chem. 37 (2017) 2498-2511; (b) S. Mandal, T. Bera, G. Dubey, J. Saha, J.K. Laha, ACS Catal. 8 (2018) 5085-5144; (c) number>L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464; (d) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138; (e) W. Wu, S. Yi, W. Huang, D. Luo, H. Jiang, Org. Lett. 19 (2017) 2825-2828; (f) J. Liu, D. Yu, Y. Yang, et al., Org. Lett. 22 (2020) 4844-4847; (g) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. 61 (2020) 151704; (h) H.B. Qiu, P.C. Guo, L. Yuan, G.P. Sheng, Chin. Chem. Lett. 31 (2020) 2614-2618; (i) Y. Li, W. Xiang, T. Zhou, et al., Chin. Chem. Lett. 31 (2020) 2757-2761; (j) Z. Chen, Q. Huang, B. Huang, F. Zhang, C. Li, Chin. J. Catal. 40 (2019) 38-42. |
| [14] |
(a) D. Dong, G. Li, D. Chen, et al., Chin. J. Org. Chem. 40 (2020) 1766-1771; (b) D.M. Chen, Y.Y. Sun, Q.Q. Han, Z.L. Wang, Tetrahedron Lett. 61 (2020) 152482; (c) S.Q. Yan, D.Q. Dong, C.W. Xie, W.S. Wang, Z.L. Wang, Chin. J. Org. Chem. 39 (2019) 2560-2566; (d) G.H. Li, D.Q. Dong, Y. Yang, X.Y. Yu, Z.L. Wang, Adv. Synth. Catal. 361 (2019) 832-835. |
| [15] |
(a) J.W. Yuan, J.L. Zhu, H.L. Zhu, et al., Org. Chem. Front. 7 (2020) 273-285; (b) J.W. Yuan, Q. Chen, C. Li, et al., Org. Biomol. Chem. 18 (2020) 2747-2757; (c) M.T. Westwood, C.J.C. Lamb, D.R. Sutherland, A.L. Lee, Org. Lett. 21 (2019) 7119-7123; (d) Q.Q. Han, D.M. Chen, Z.L. Wang, et al., Chin. Chem. Lett. 32 (2021) 2559-2561; (e) D. Chen, Y. Sun, D. Dong, Q. Han, Z. Wang, Chin. J. Org. Chem. 40 (2020) 4267-4273; (f) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498; (g) Z. Gan, X. Zhu, Q. Yan, X. Song, D. Yang, Chin. Chem. Lett. 32 (2020) 1705-1708; (h) J.Y. Chen, Y.W. Lin, W.M. He, Chin. Chem. Lett. 31 (2020) 2989-2990; (i) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864; (j) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378; (k) M.M. Xu, L.Y. Kou, X.G. Bao, X.P. Xu, S.J. Ji, Chin. Chem. Lett. 32 (2021) 1915-1919. |
 2021, Vol. 32
2021, Vol. 32 


