b College of Pharmaceutical Science, Zhejiang University, Hangzhou 310058, China
Quinoxalin-2(1H)-one, as a significant heterocyclic unit, has been found important applications in synthetic chemistry, materials, natural products and pharmaceuticals because of their innate outstanding biological activities and excellent chemical characters [1], and their biological activities can be significantly influenced if the substituents is introduced into the N1- and C3-positions of the quinoxalin-2(1H)-one [2]. In particular, 3-substituted quinoxalin-2(1H)-ones have been developed into powerful drugs due to their strong pharmacological effects [3], such as ataquimast, antinicrobial, anticancer, Fxa coagulation inhibitors and glycogen phosphorylase inhibitor (Fig. 1) [4]. Therefore, a number of methods have been developed for their synthesis [5]. Generally, they are synthesized by cyclization of derivatives of aniline or 1, 2-diaminobenzene with suitable partners. However, the disadvantages including pre-functionalization of the partners and multi-step synthesis limit its application [6]. In recent years, direct C-H bond functionalization at the C3-position of quinoxalin-2(1H)-one has become a straightforward access to the 3-substituted quinoxalin-2(1H)-one derivatives, and various remarkable work has been achieved [7-12]. For instances, our group in 2019 reported a first example of oxidative C-H fluoroalkoxylation of quinoxalinones with fluoroalkyl alcohols under transition-metal and solvent-free conditions [8b]. This method can also be extended to the facile and efficient synthesis of histamine-4 receptor. The same year, Sun's group presented an efficient electrochemical approach for the C(sp2)–H phosphonation of quinoxalin-2(1H)-ones and C(sp3)–H phosphonation of xanthenes [9a]. More interestingly, the group of Pan disclosed a photocatalyst-free visible-light-promoted sulfenylation of quinoxalinones with thiols via cross-dehydrogenative coupling [10b]. Shortly after this discovery, He's group demonstrated a visible-light-promoted amidation of quinoxalin-2(1H)-ones [11b]. In a very recent contribution, a mild and eco-friendly visible-light-induced decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo carboxylic acids using ambient air as the sole oxidant at room temperature was also established by the same group [12a]. In sharp contrast, the alkenylation of quinoxalin-2(1H)-ones was rarely reported.

|
Download:
|
| Fig. 1. Examples of quinoxalin-2(1H)-one skeleton-based bioactive molecules. | |
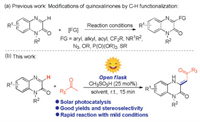
Photocatalysis has become a powerful strategy for organic synthesis due to the advantages of low energy consumption and environmental protection [13]. For example, MacMillan et al. in 2016 reported a photocatalyzed C-H arylation of aliphatic amines with aryl bromides, providing a complement to existing cross-coupling technologies [13a]. In 2021, He' group developed the first example of visible-light induced one-pot tandem reaction of arylacrylamides, CHF2CO2H and PhI(OAc)2, affording an eco-friendly and practical method to access various difluoromethylated oxindoles [13b]. The same year, Jin and coworkers developed photocatalyst-free radical tandem cyclization of quinazolinones containing an unactivated alkene moiety with difluoro bromides under illumination, giving a practical method for the synthesis of fluorine-containing ring-fused quinazolinones [13f]. In recent years, with increasing attention to renewable energy, considerable efforts have been switched to the development of photocatalytic reactions that excited by the sunlight, which is known as a renewable and simple accessible light source [14]. Our research interests focus on the development of novel and effective methodologies for the direct modification ofN-containing heterocycles [15], herein, we demonstrated a direct alkenylation reaction between quinoxalin-2(1H)-ones and methyl ketones. Compared with our previous work [15a], this transformation was achieved through a combination of Mannich-type reaction and solar photocatalysis, which could be completed within 15min, providing a green and efficient solution for the synthesis of potentially bioactive compounds that containing a 3, 4-dihydroquinoxalin-2(1H)-one structure (Scheme 1b).
|
Download:
|
| Scheme 1. Modification of quinoxalin-2(1H)-ones by C-H functionalization. | |
1-Methylquinoxalin-2(1H)-one (1a) and acetone (2a) were chosen as starting materials to screen the reaction conditions. The target product (3a) was obtained in 80% yield when the reaction was performed by using 25mol% of CH3SO3H as a catalyst under the irradiation of sunlight for 15min (Table 1, entry 1). Other acid catalyst, such as CF3COOH and HBF4 gave the relative lower yield under the same conditions (Table 1, entries 2 and 3). No product was obtained in the absence of any acid catalyst (Table 1, entry 4). When used MeCN or DMF as solvent and 2.0 equiv. of acetone as substrate, 76% or 52% yield was obtained respectively (Table 1, entries 5 and 6). Extended reaction time to 30min did not enhanced product yield (Table 1, entry 7). There was no desired product generated when the reaction was carried out under dark condition (Table 1, entry 8).
|
|
Table 1 Screening of reaction conditions.a |
With the optimum reaction conditions in hands, we then examined the substrate scope of the reaction by employing various quinoxalin-2(1H)-ones (1) with acetone (2a) (Scheme 2). Firstly, the N-substituted groups such as N-methyl, N-ethyl, N-cyclopropylmethyl, N-keto and N-ester were well compatible under the standard conditions, giving the desired products (3a-e) in 72%–80% yields. It is worth mentioning that quinoxalin-2(1H)-one with a sensitive allyl group, which could be further functionalized, also could give the product (3f) in 69% yield. A wide range of quinoxalin-2(1H)-ones with different benzyl groups, bearing both electron-donating and electron-withdrawing substituents at ortho-, meta-, or para-position could undergo the reaction smoothly, affording the corresponding products (3g–n) in 40%–72% yields. Importantly, the N-free quinoxalin-2(1H)-one could undergo the reaction smoothly, providing the product (3o) in 45% yield. Besides, plenty of quinoxalin-2(1H)-ones that bear the functional groups including methyl, halogen, tert-butyl, methoxy or trifluoromethyl at C5-, C6- or C7-position also gave the desired products in satisfactory yield (3p-3w). To expand the substrate scope of N-heterocycles, we also tested quinoline, isoquinoline, quinoxaline, benzimidazole and benzothiazole under standard conditions, however, no corresponding product was obtained (see Supporting information).

|
Download:
|
| Scheme 2. Substrate scope of quinoxalin-2(1H)-ones. Reaction conditions: 1 (0.2 mmol), 2a (1.0 mL), CH3SO3H (25 mol%), open flask, sunlight, room temperature, 15 min. Isolated yields. a Reaction was performed on a 1 mmol scale. | |
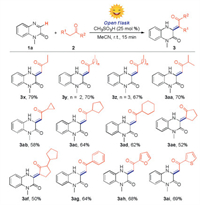
Subsequently, we evaluated the substrate scope of methyl ketones for the reaction (Scheme 3). To reduce the dosage of reactant, the reactions were performed with 2.0 equiv. of methyl ketones by using acetonitrile as solvent. To our delight, both long-chain and cycloalkyl methyl ketones could undergo the reaction smoothly, giving the corresponding products (3x–3ad) in 58%–79% yields. The molecular structure of 3y was confirmed by X-ray crystallographic analysis (CCDC: 2060383). It was found that the molecular structure was more stable in (Z)-configuration probably because the effect of hydrogen bond interaction between amine and carbonyl group. Then, we found that cyclopentanone skeleton could also react with quinoxalin-2(1H)-one smoothly to deliver the target products (3ae and 3af) in moderate yield. The subsequent exploration found that the aryl methyl ketones, such as acetophenone, 1-(furan-2-yl)ethan-1-one and 1-(thiophen-2-yl)ethan-1-one were also could be converted into target products (3ag-3ai) in acceptable yields. Unfortunately, the substrates like ethyl acetate, acetonitrile, nitromethane, ethyl acetoacetate, and acetylacetone were not compatible under standard conditions (Supporting information).
|
Download:
|
| Scheme 3. Substrate scope of methyl ketones. Reaction conditions: 1a (0.2 mmol), 2 (2.0 equiv.), CH3SO3H (25 mol%), MeCN (1.0 mL), open flask, sunlight, room temperature, 15min. Isolated yields. | |
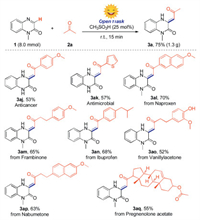
To show the synthetic utility of this protocol, a gram-scale synthesis experiment was performed to give the target product (3a) in 75% yield (Scheme 4). Interestingly, the anticancer compound (3aj) and antimicrobial compound (3ak) were obtained in moderate yields by using our strategy [16]. Moreover, since the molecules that bearing a 3, 4 dihydroquinoxalin-2(1H)-one framework are a promising class of biologically active compounds, in this regard, several bioactive molecules such as naproxen derivative, frambinone, ibuprofen derivative, vanillylacetone, nabumetone and pregnenolone acetate were selected to react with 1-methylquinoxalin-2(1H)-one directly, providing the potentially active molecules (3al-3aq) in 52%–70% yields.
|
Download:
|
| Scheme 4. Gram-scale synthesis and application. Reaction conditions: 1a (0.2mmol), 2 (2.0 equiv.), CH3SO3H (25mol%), MeCN (1.0mL), open flask, sunlight, room temperature, 15min. Isolated yields. | |
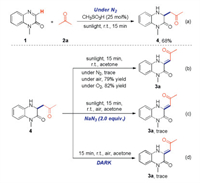
To study the reaction mechanism, a series of control experiments were carried out. Product 4 was generated instead of target product 3a when the reaction was performed under nitrogen atmosphere (Scheme 5). This result showed that oxygen in air was included in the subsequent oxidation process. To confirm the assumption, the oxidation process of compound 4 was studied. First, target product 3 was formed in 0%, 79% and 82% yields when the reaction performed under nitrogen, air or oxygen atmosphere respectively (Scheme 5). Second, the reaction was inhibited when singlet oxygen inhibitor (NaN3) was involved in the transformation (Scheme 5). Furthermore, compound 4 could not be converted into target product 3 when the reaction was performed in dark condition (Scheme 5). These experimental results strongly supported that the singlet oxygen 1O2, which was generated from triplet oxygen 3O2 through photocatalysis, serves as the real oxidant.
|
Download:
|
| Scheme 5. Control experiments. | |
On the basis of above results and previous reports [8-12], we proposed a possible mechanism for this reaction (Scheme 6). Firstly, substrate 1a was transformed into intermediate A through a protonation process. Meanwhile, acetone 2a was converted to the enol form B under acidic condition. Then, a Mannich-type reaction took place between intermediates A and B to give the intermediate C, which underwent a deprotonation process to provide the key compound 4. It was found that organic molecules that containing a quinoxalin-2(1H)-one skeleton could act as a photosensitizer to generate 1O2 from O2 under the irradiation of visible light [12p]. In this regard, compounds1a, 4 or 3a was excited by visible light to provide the excited-species 1a*, 4* or 3a*, which acted as a photosensitizer and underwent an energy transfer (ET) process with O2 to give 1O2, along with the regeneration of ground-state compounds 1a, 4 or 3a. Finally, compound 4 underwent the single-electron-transfer (SET) process with 1O2 to give the desired product with the generation of H2O2, which was detected by H2O2 test paper (Supporting information) [12a] [17]. We proposed that the electron-withdrawing effects of carbonyl group that exists in quinoxalin-2(1H)-one skeleton lower down the electron cloud density of the enamine moiety, making it difficult to be oxidized and can survive under this H2O2 oxidation conditions.

|
Download:
|
| Scheme 6. Plausible mechanism. | |
In conclusion, this study described a novel strategy for the olefination of quinoxalin-2(1H)-ones with methyl ketones. Various substrates were compatible under standard condition, providing the corresponding products in moderate to good yields. Control experiments revealed that a Mannich-type reaction and oxidative process were involved in the transformation.
Declaration of competing interestThe authors declare that they have no conflict of interest.
AcknowledgmentsWe thank the Natural Science Foundation of Zhejiang Province (No. LY21B060009) and the National Natural Science Foundation of China (No. 21871071) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.04.016.
| [1] |
(a) R.E. TenBrink, W.B. Im, V.H. Sethy, et al., J. Med. Chem. 37 (1994) 758-768; (b) A. Monge, F.J. Martinez-Crespo, A.L. Cerai, et al., J. Med. Chem. 38 (1995) 4488-4494; (c) M.M. Badran, K.A.M. Abouzid, M.H.M. Hussein, Arch. Pharmacal Res. 26 (2003) 107-113; (d) H.M. Refaat, A.A. Moneer, O.M. Khalil, Arch. Pharmacal Res. 27 (2004) 1093-1098; (e) A. Carta, S. Piras, G. Loriga, G. Paglietti, Mini-Rev. Med. Chem. 6 (2006) 1179-1200; (f) J.H. Fu, J.W. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390; (g) W. Wei, L.L. Wang, H.L. Yue, et al., ACS Sustain. Chem. Eng. 6 (2018) 17252-17257; (h) J.W. Yuan, J.H. Fu, S.N. Liu, et al., Org. Biomol. Chem. 16 (2018) 3203-3212. |
| [2] |
X.B. Zeng, C.L. Liu, X.Y. Wang, Org. Biomol. Chem. 15 (2017) 8929-8935. DOI:10.1039/C7OB02187A |
| [3] |
(a) J.A. Willardsen, D.A. Dudley, W.L. Cody, et al., J. Med. Chem. 47 (2004) 4089-4099; (b) S.Y. Zhang, F.M. Zhang, Y.Q. Tu, Chem. Soc. Rev. 40 (2011) 1937-1949; (c) J.R. Zbieg, E. Yamaguchi, E.L. Mclnturff, M.J. Krische, Science 336 (2012) 324-327; (d) T.Y. Chen, M.J. Krische, Org. Lett. 15 (2013) 2994-2997; (e) D. Liu, C. Liu, H. Li, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 4453-4456; (f) X.Q. Chu, H. Meng, Y. Zi, X.P. Xu, S.J. Ji, Chem. Commun. 50 (2014) 9718-9721; (g) X. Qin, X. Hao, H. Han, et al., J. Med. Chem. 58 (2015) 1254-1267; (h) J.K. Cheng, T.P. Loh, J. Am. Chem. Soc. 137 (2015) 42-45. |
| [4] |
(a) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533; (b) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792; (c) Q.M. Yang, Z.B. Yang, Y.S. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667. |
| [5] |
X. Li, K.H. Yang, W.L. Li, W.F. Xu, Drugs Fut. 31 (2006) 979. DOI:10.1358/dof.2006.031.11.1037128 |
| [6] |
J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182. DOI:10.1002/adsc.202000116 |
| [7] |
Q. Ke, G. Yan, J. Yu, X. Wu, Org. Biomol. Chem. 17 (2019) 5863-5881. DOI:10.1039/C9OB00782B |
| [8] |
(a) J.Z. Jin, J.Y. Tong, W.B. Yu, J. Qiao, C. Shen, Catal. Commun. 141 (2020) 106008; (b) J. Xu, H. Yang, H. Cai, et al., Org. Lett. 21 (2019) 4698-4702; (c) J. Zhou, P. Zhou, T. Zhao, Q. Ren, J. Li, Adv. Synth. Catal. 361 (2019) 5371-5382; (d) S. Peng, D. Hu, J.L. Hu, et al., Adv. Synth. Catal. 361 (2019) 5721-5726; (e) L. Zhao, L. Wang, Y. Gao, Z. Wang, P. Li, Adv. Synth. Catal. 361 (2019) 5363-5370; (f) Q. Yang, X. Han, J. Zhao, H.Y. Zhang, Y. Zhang, J. Org. Chem. 84 (2019) 11417-11424. |
| [9] |
(a) K.J. Li, Y.Y. Jiang, K. Xu, C.C. Zeng, B.G. Sun, Green Chem. 21 (2019) 4412-4421; (b) W.P. Mai, J.W. Yuan, J.L. Zhu, et al., ChemistrySelect 4 (2019) 11066-11070; (c) J. Wang, J. Li, Y. Wei, J. Yang, C. Huo, Org. Chem. Front. 5 (2018) 3534-3537; (d) Y. Kim, D.Y. Kim, Tetrahedron Lett. 59 (2018) 2443-2446; (e) M. Gao, Y. Li, L. Xie, R. Chauvin, X. Cui, Chem. Commun. 52 (2016) 2846-2849. |
| [10] |
(a) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955; (b) Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245. |
| [11] |
(a) J. Yuan, J. Zhu, J. Fu, et al., Org. Chem. Front. 6 (2019) 925-935; (b) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustain. Chem. Eng. 7 (2019) 19993-19999; (c) J.W. Yuan, J.L. Zhu, B. Li, et al., Org. Biomol. Chem. 17 (2019) 10178-10187; (d) Q. Yang, Z. Yang, Y. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667; (e) Q. Yang, Y. Zhang, Q. Sun, et al., Adv. Synth. Catal. 360 (2018) 4509-4514; (f) W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130; (g) T.T. Hoang, T.A. To, V.T.T. Cao, et al., Catal. Commun. 101 (2017) 20-25; (h) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792. |
| [12] |
(a) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725; (b) P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498; (c) J. Xu, H. Yang, L. He, et al., Org. Lett. 23 (2021) 195-201; (d) J. Wang, B. Sun, L. Zhang, et al., Org. Chem. Front. 7 (2020) 113-118; (e) J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042; (f) J. Shen, J. Xu, L. Huang, Q. Zhu, P. Zhang, Adv. Synth. Catal. 362 (2020)230-241; (g) H. Zhang, J. Xu, M. Zhou, et al., Org. Biomol. Chem. 17 (2019) 10201-10208; (h) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464; (i) L.X. Liu, N. Pan, W. Sheng, et al., Adv. Synth. Catal. 361 (2019) 4126-4132; (j) W. Zhang, Y.L. Pan, C. Yang, et al., J. Org. Chem. 84 (2019) 7786-7795; (k) L.Y. Xie, L.L. Jiang, J.X. Tan, etal., ACSSustain. Chem. Eng. 7 (2019)14153-14160; (l) G. Hong, J. Yuan, J. Fu, et al., Org. Chem. Front. 6 (2019) 1173-1182; (m) L. Wang, H. Liu, F. Li, et al., Adv. Synth. Catal. 361 (2019) 2354-2359; (n) C. Jin, X. Zhuang, B. Sun, D. Li, R. Zhu, AsianJ. Org. Chem. 8 (2019) 1490-1494; (o) W. Xue, Y. Su, K.H. Wang, et al., Asian J. Org. Chem. 8 (2019) 887-892; (p) J. Wang, B. Sun, L. Zhang, et al., Asian J. Org. Chem. 8 (2019) 1942-1946; (q) W. Wei, L. Wang, H. Yue, etal., ACSSustain. Chem. Eng. 6 (2018)17252-17257; (r) S. Liu, Y. Huang, F.L. Qing, X.H. Xu, Org. Lett. 20 (2018) 5497-5501; (s) L. Hu, J. Yuan, J. Fu, et al., Eur. J. Org. Chem. 2018 (2018) 4113-4120; (t) J. Fu, J. Yuan, Y. Zhang, Org. Chem. Front. 5 (2018) 3382-3390; (u) J. Yuan, J. Fu, J. Yin, et al., Org. Chem. Front. 5 (2018) 2820-2828; (v) K. Yin, R. Zhang, Synlett 29 (2018) 597-602; (w) B. Ramesh, C.R. Reddy, G.R. Kumar, B.V.S. Reddy, Tetrahedron Lett. 59 (2018) 628-631; (x) L. Wang, Y. Zhang, F. Li, Adv. Synth. Catal. 360 (2018) 3969-3977; (y) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533; (z) J. Yuan, S. Liu, L. Qu, Adv. Synth. Catal. 359 (2017) 4197-4207. |
| [13] |
(a) M.H. Shaw, V.W. Shurtleff, J.A. Terrett, J.D. Cuthbertson, D.W.C. MacMillan, Science 352 (2016) 1304-1308; (b) Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021)1907-1910; (c) B. Sun, P. Huang, Z. Yan, et al., Org. Lett. 23 (2021) 1026-1031; (d) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378; (e) K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500; (f) J. Yang, B. Sun, H. Ding, et al., Green Chem. 23 (2021) 575-581; (g) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258; (h) W. Ou, R. Zou, M. Han, L. Yu, C. Su, Chin. Chem. Lett. 31 (2020) 1899-1902; (i) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867; (j) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70; (k) L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21 (2019) 3362-3369; (l) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298; (m) J. Shen, J. Xu, L. He, Y. Ouyang, et al., Org. Lett. 23 (2021) 1204-1208; (n) J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 40 (2011) 102-113. |
| [14] |
(a) P. Esser, B. Pohlmann, H.D. Scharf, Angew. Chem. Int. Ed. 33 (1994) 2009-2023; (b) M. Okada, T. Fukuyama, K. Yamada, et al., Chem. Sci. 5 (2014) 2893-2898; (c) S. Park, W.H. Jeon, W.S. Yong, P.H. Yong, Org. Lett. 17 (2015) 5060-5063; (d) S.Y. Ni, J. Cao, H.B. Mei, et al., Green Chem. 18 (2016) 3935-3939. |
| [15] |
(a) J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 2123-2129; (b) C. Shen, A. Wang, J. Xu, et al., Chem 5 (2019) 1059-1107; (c) J. Xu, K. Du, J. Shen, et al., ChemCatChem 10 (2018) 3675-3679; (d) J. Xu, K. Cheng, C. Shen, et al., ChemCatChem 10 (2018) 965-970; (e) C. Shen, M. Yang, J. Xu, et al., RSC Adv. 7 (2017) 49436-49439; (f) J. Xu, C. Shen, X. Zhu, et al., Chem. Asian J. 11 (2016) 882-892; (g) J. Xu, X. Zhu, G. Zhou, et al., Org. Biomol. Chem. 14 (2016) 3016-3021. |
| [16] |
E.E. Stepanova, D.N. Lukmanova, S.O. Kasatkina, M.V. Dmitriev, A.N. Maslivets, ChemistrySelect 4 (2019) 12774-12778. DOI:10.1002/slct.201902900 |
| [17] |
(a) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173; (b) D. Rawat, R. Kumar, A. Subbarayappa, Green Chem. 22 (2020) 6170-6175. |
 2021, Vol. 32
2021, Vol. 32 


