b University of the Chinese Academy of Sciences, Beijing 100049, China;
c College of Chemistry, Fuzhou University, Fuzhou 350108, China;
d Chemical Refining Laboratory, Refining Department, Egyptian Petroleum Research Institute, Nasr City, Cairo 11727, Egypt
As one of the primary nuclear fuels, deuterium (D2) plays an irreplaceable role in controlled nuclear fusion and is also widely used in non-radioactive isotope tracing, chemical reaction mechanism tracing, as well as medicine and life sciences [1-4]. However, the separation and purification of D2 from H2 isotopic mixture is a challenging task due to their similar sizes and chemical properties. Conventional D2/H2 separation methods on industrial plant scale, such as cryogenic distillation process and Girdler sulfide process, are highly energy- and time-intensive. Furthermore, these technologies can only provide very low separation factors, making it difficult for extensive application [5-10]. Therefore, it is still an urgent and daunting task to explore new alternative methods for D2/H2 separation.
Recently, the strategy of separating hydrogen isotopes based on the kinetic quantum sieving (KQS) and chemical affinity quantum sieve (CAQS) effects of porous materials has attracted considerable attention. Based on KQS effect, which means that different diffusion rates can separate hydrogen isotopes in restricted pores [11], many reports have studied hydrogen isotope separation behavior of porous materials such as porous carbons [12-15], zeolites [16-18], metal-organic frameworks (MOFs) [19-23], covalent organic frameworks (COFs) [24] and porous organic cages [25]. However, the KQS effect is only obvious at extremely low temperatures (as low as 20 K), increasing separation costs dramatically. Unlike the KQS effect, CAQS effect can effectively separate hydrogen isotopes in porous materials under higher temperatures (≥ 77 K) because hydrogen isotopes demonstrate different adsorption capacity and enthalpies under low pressure [26]. Based on CAQS effect, heavier D2 preferentially adsorbed on strong active sites, achieving high D2/H2 selectivity. Compared to porous carbons, zeolites and COFs, MOFs exhibit more advantages for D2/H2 separation because their pore sizes and open metal sites (OMSs) are controllable and further optimize gas uptake and adsorption enthalpy, which are critical factors for D2/H2 separation based on CAQS effect. In recent years, MOFs have been widely used in gas separation [27-30]. However, very few MOFs, such as Cu(Ⅰ)-MFU-4L [31, 32], Co-MOF-74 [33] and FJI-Y11 [34], are applied to D2/H2 separation based on obvious CAQS effect. Low-temperature thermal desorption spectroscopy indicated that Cu(Ⅰ)-MFU-4L only showed high H2/D2 selectivity at very low temperature (20 K). Furthermore, it is well known that Cu(Ⅰ)-MOFs are always unstable in air and can be easily oxidized by oxygen, limiting its practical application. Therefore, we devote to screening stable materials with strong binding sites and high D2/H2 uptakes for D2/H2 separation under more mild conditions.
Compared to low-temperature thermal desorption spectroscopy, the breakthrough experiment used for D2/H2 separation is closer to simulated industrial separation processes. Recently, our group carried out breakthrough experiments to explore D2/H2 separation performance of the famous MOF-74 series frameworks with a high density of OMSs, especially Co-MOF-74 that exhibited a satisfying D2 retention time of 300 min/g (H2/D2/Ne: 1/1/98) at 77 K [35]. As structural isomers of MOF-74, M2(m-dobdc) (M = Co, Ni, Mg, Mn) frameworks possess a narrower pore size of 9.8 Å than that of MOF-74 (11 Å). The smaller pore size may be more conducive to mass transfer process of hydrogen isotope gas molecules in the channels. Based on the above considerations, we evaluate the D2/H2 separation ability of M2(m-dobdc) frameworks by considering the competitive and synergistic effect between adsorption enthalpy and capacity during the breakthrough process.
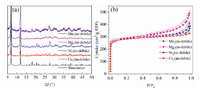
M2(m-dobdc) (M = Co, Ni, Mg, Mn) were synthesized by solvothermal method (Fig. S1 in Supporting information) according to previous reports [36]. M2(m-dobdc) frameworks feature a three-dimensional honeycomb structure constructed by one-dimensional [M(μ−COO)(μ−OH)]n chains. The calculated pore size of M2(m-dobdc) is 9.8 Å, smaller than that of MOF-74 (11 Å). This facilitates mass transfer process of hydrogen isotope molecules in the channels. Since the hydroxyl oxygen atom and the carboxyl oxygen atom in m-dobdc ligands are involved in coordination, this series of materials can maintain their original frameworks under high vacuum at 180 ℃. The removal of coordinated solvent molecules leaves large number of OMSs, with a calculated density of 4.6 OMSs/nm−3 in M2(m-dobdc), much higher than those of HKUST-1 (2.6 OMSs/nm−3) [37] and PCN-16 (1.2 OMSs/nm−3) [37]. The purity of prepared frameworks was confirmed by their PXRD patterns (Fig. 1a), and typical type-Ⅰ N2 sorption isotherms (Fig. 1b) at 77 K indicate their microporous characteristics.
|
Download:
|
| Fig. 1. (a) PXRD patterns of M2(m-dobdc) (M = Co, Ni, Mg, Mn). (b) N2 adsorption isotherms of M2(m-dobdc) at 77 K (filled, adsorption; empty, desorption). | |
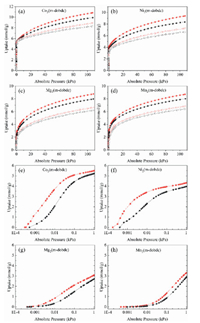
To better understand the adsorption behavior of hydrogen isotope for M2(m-dobdc), sorption isotherms of H2 and D2 at 77 K and 87 K were obtained, respectively. Among these four materials, Co2(m-dobdc) shows the highest adsorption capacity as 9.62 mmol/g for H2 and 10.7 mmol/g for D2 at 800 mmHg and 77 K, higher than Ni2(m-dobdc) (H2: 8.24 mmol/g, D2: 9.24 mmol/g), Mg2(m-dobdc) (H2: 7.97 mmol/g, D2: 8.75 mmol/g) and Mn2(m-dobdc) (H2: 7.88 mmol/g, D2: 8.56 mmol/g) (Fig. 2). As for the low-pressure part (1 kPa, a partial pressure of H2 and D2 during the breakthrough process) of the adsorption curve, the D2(H2) adsorption capacity of Co2(m-dobdc) and Ni2(m-dobdc) sharply increase to 5.49(5.22) and 4.32(3.96) mmol/g, respectively, which are contrast to the near-flat growth of adsorption curve for Mg2(m-dobdc) (3.07(2.70) mmol/g) and Mn2(m-dobdc) (3.29(2.90) mmol/g), which can be attributed to the strong binding force between OMSs and hydrogen isotope molecules in the framework. The results further indicate their great potential for D2/H2 separation. The results demonstrate that metal centers significantly influence host-guest interactions, resulting in different performance in the selective adsorption of H2 and D2.
|
Download:
|
| Fig. 2. (a-d) D2 (red) and H2 (black) sorption isotherms for M2(m-dobdc) at 77 K (filled) and 87 K (empty). (e-h) Adsorption isotherms of D2 (red) and H2 (black) for pressure in the range of 0–1 kPa (77 K). | |
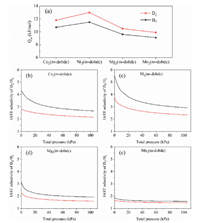
Adsorption enthalpy contributes towards understanding the interaction strength between gas molecules and adsorbates. Herein, the adsorption enthalpies of H2 and D2 are calculated through the Clausius-Clapeyron equation. As shown in Fig. 3a, among these four materials, Ni2(m-dobdc) shows the highest adsorption enthalpy of H2 and D2 at zero coverage (H2: 11.5 kJ/mol, D2: 13.0 kJ/mol), slightly higher than Co2(m-dobdc) (H2: 10.7 kJ/mol, D2: 11.8 kJ/mol). Such high adsorption enthalpy implies that Co2(m-dobdc) and Ni2(m-dobdc) may be good candidates for D2/H2 separation.
|
Download:
|
| Fig. 3. (a) H2 and D2 adsorption enthalpies of M2(m-dobdc) at zero coverage. (b-e) H2/D2(50/50) IAST selectivity of M2(m-dobdc) at 77 K (black) and 87 K (red). | |
To predict the D2/H2 separation ability of M2(m-dobdc), the separation performance for D2/H2(50/50) mixtures was evaluated through ideal adsorbed solution theory (IAST) [38] (Figs. 3b-e). At 77 K, the D2/H2(50/50) selectivity of Co2(m-dobdc) and Ni2(m-dobdc) can reach 4.3 and 5.5 at zero coverage, respectively, higher than some famous reported porous materials such as CuBOTf (1.3) [39], Fe-MOF-74 (2.5) [26] and Co-MOF-74 (3.2) [26]. When the pressure rises to 1 kPa, these two values still maintain at 4.22 and 5.39, respectively. According to previous reports, materials with such high IAST selectivity at 77 K were rarely reported, meaning the potential ability of Co2(m-dobdc) and Ni2(m-dobdc) for separating hydrogen isotopes.
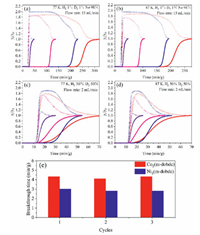
Breakthrough experiments were performed to check the actual separation performance of M2(m-dobdc). Herein, we simulated two hydrogen isotope mixtures with different compositions (H2/D2/Ne: 1/1/98) and (H2/D2: 50/50) and evaluated the actual separation ability of M2(m-dobdc) adsorbents through breakthrough experiments at 77 K and 87 K, respectively. For these four M2(m-dobdc) adsorbents, when the gas mixture (H2/D2/Ne: 1/1/98) flowed over the packed column with a flow rate of 15 mL/min at 77 K, H2 always flowed out first due to its lower adsorption capacity and weaker binding force with adsorbent than D2. After the hydrogen broke up, D2 can still be retained in the packed column filled with Co2(m-dobdc) for 180 min/g, higher than those of Ni2(m-dobdc) (150 min/g), Mg2(m-dobdc) (80 min/g) and Mn2(m-dobdc) (3 min/g) (Fig. 4). When breakthrough experiments were conducted with H2/D2(50:50), effective separation of D2/H2 can still be achieved with a breakthrough time of 4.5 min/g for Co2(m-dobdc) and 3 min/g for Ni2(m-dobdc), while Mg2(m-dobdc) and Mn2(m-dobdc) showed no obvious separation ability. Surprisingly, although Ni2(m-dobdc) has the highest D2 and H2 adsorption enthalpy, Co2(m-dobdc) showed the longest D2 retention time. As for Mg2(m-dobdc) and Mn2(m-dobdc), they have similar adsorption capacity, but Mn2(m-dobdc) has inferior separation effect due to its low adsorption enthalpy. The similar rule can also be found in our previous D2/H2 separation research on MOF-74. On the other hand, although M2(m-dobdc) (M = Co, Ni, Mg) exhibit similar hydrogen isotope adsorption enthalpies with M2(dobdc) (M = Co, Ni, Mg), M2(m-dobdc) always showed shorter breakthrough time due to their narrower pore sizes and lower adsorption capacities of D2 and H2. Based on the above comparison, although adsorption enthalpy and adsorption capacity are both important to breakthrough experiment performance, adsorption capacity seems to play a more decisive role in D2/H2 separation because D2/H2 separation conducted by breakthrough experiments requires MOFs to display high D2/H2 uptakes as well as significant difference of D2/H2 uptakes to extend D2 retention time. The regeneration of adsorbents is also an important evaluation criterion in industrial applications, and cyclic experiments revealed that during three cycles, the breakthrough times of D2 in Co2(m-dobdc) and Ni2(m-dobdc) remained (Fig. 4e), indicating their excellent cycling stability for D2/H2 separation.
|
Download:
|
| Fig. 4. (a–d) Breakthrough curves for D2/H2 separation on Co2(m-dobdc) (red), Ni2(m-dobdc) (blue), Mg2(m-dobdc) (pink), Mn2(m-dobdc) (violet). The hollow circle represents H2, and the solid circle represents D2. (e) Breakthrough time of D2 in cycling tests on Co2(m-dobdc) and Ni2(m-dobdc) for H2/D2 (50:50) mixture. | |
In conclusion, D2/H2 separation performance of M2(m-dobdc) is explored by breakthrough experiments, in which two critical factors, including gas uptake and adsorption enthalpy, are considered to investigate the competitive and synergistic effect between adsorption enthalpy and capacity in D2/H2 separation ability of M2(m-dobdc) frameworks. The results showed that for MOF materials with approximate adsorption enthalpies, MOF with higher gas uptake exhibits greater D2/H2 separation performance. Therefore, among these materials, Co2(m-dobdc) exhibits the second-highest hydrogen isotope adsorption enthalpy and the highest gases uptake (5.22 mmol/g for H2 and 5.49 mmol/g for D2) under 1 kPa, making it the best material for D2/H2 separation with D2 retention time of 180 min/g for (H2/D2/Ne: 1/1/98) at 77 K. Compared to Co-MOF-74 (Table S1 in Supporting information), Co2(m-dobdc) has lower adsorption enthalpy and capacity, resulting in shorter retention time of D2. The results demonstrate that the adsorption capacity shows a more significant impact than adsorption enthalpy on D2/H2 separation performance. This work demonstrates a noteworthy design consideration to develop materials with excellent hydrogen isotope separation ability.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB20000000), the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (No. QYZDB-SSW-SLH019) and the National Natural Science Foundation of China (Nos. 21771177, 51603206 and 21203117).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.02.063.
| [1] |
H. Oh, M. Hirscher, Eur. J. Inorg. Chem. (2016) 4278-4289. DOI:10.1002/ejic.201600253 |
| [2] |
S. Niimura, T. Fujimori, D. Minami, et al., J. Am. Chem. Soc. 134 (2012) 18483-18486. DOI:10.1021/ja305809u |
| [3] |
J. Cai, Y. Xing, X. Zhao, RSC Adv. 2 (2012) 8579-8586. DOI:10.1039/c2ra01284g |
| [4] |
J.Y. Kim, H. Oh, H.R. Moon, Adv. Mater. 31 (2019) 1805293. DOI:10.1002/adma.201805293dene.2016.01.106 |
| [5] |
R. Bhattacharyya, K. Bhanja, S. Mohan, Int. J. Hydrog. Energy 41 (2016) 5003-5018. DOI:10.1016/j.ijhydene.2016.01.106 |
| [6] |
A. Lazar, S. Brad, M. Vijulie, A. Oubraham, Fusion Eng. Des. 146 (2019) 1998-2001. DOI:10.1016/j.fusengdes.2019.03.085 |
| [7] |
F. Huang, Int. J. Hydrog. Energy 43 (2018) 1718-1724. DOI:10.1016/j.ijhydene.2017.11.147 |
| [8] |
H.K. Rae, Selecting Heavy Water Processes, ACS Symposium Series, Washington D.C., 1978.
|
| [9] |
X. Deng, D. Luo, C. Qin, et al., Int. J. Hydrog. Energy 44 (2019) 16675-16683. DOI:10.1016/j.ijhydene.2019.04.282 |
| [10] |
M.V. Ananyev, A.S. Farlenkov, E.K. Kurumchin, Int. J. Hydrog. Energy 43 (2018) 13373-13382. DOI:10.1016/j.ijhydene.2018.05.150 |
| [11] |
J.J.M. Beenakker, V.D. Borman, S.Y. Krylov, Chem. Phys. Lett. 232 (1995) 379-382. DOI:10.1016/0009-2614(94)01372-3 |
| [12] |
H. Tanaka, H. Kanoh, M. Yudasaka, S. Iijima, K. Kaneko, J. Am. Chem. Soc. 127 (2005) 7511-7516. DOI:10.1021/ja0502573 |
| [13] |
X.Z. Chu, Z.P. Cheng, Y.J. Zhao, et al., Sep. Purif. Technol. 146 (2015) 168-175. DOI:10.1016/j.seppur.2015.03.036 |
| [14] |
X. Zhao, S. Villar-Rodil, A.J. Fletcher, K.M. Thomas, J. Phys. Chem. B 110 (2006) 9947-9955. DOI:10.1021/jp060748p |
| [15] |
Y. Xing, J. Cai, L. Li, M. Yang, X. Zhao, Phys. Chem. Chem. Phys. 16 (2014) 15800-15805. DOI:10.1039/C4CP00709C |
| [16] |
J. Perez-Carbajo, J.B. Parra, C.O. Ania, P.J. Merkling, S. Calero, ACS Appl. Mater. Interfaces 11 (2019) 18833-18840. DOI:10.1021/acsami.9b02736 |
| [17] |
J.M. Salazar, S. Lectez, C. Gauvin, et al., Int. J. Hydrog. Energy 42 (2017) 13099-13110. DOI:10.1016/j.ijhydene.2017.03.222 |
| [18] |
R. Xiong, R.B. Xicohtencatl, L. Zhang, et al., Microporous Mesoporous Mater. 264 (2018) 22-27. DOI:10.1016/j.micromeso.2017.12.035 |
| [19] |
J.Y. Kim, L. Zhang, R. Balderas-Xicohtencatl, et al., J. Am. Chem. Soc. 139 (2017) 17743-17746. DOI:10.1021/jacs.7b10323 |
| [20] |
L. Zhang, S. Jee, J. Park, et al., J. Am. Chem. Soc. 141 (2019) 19850-19858. DOI:10.1021/jacs.9b10268 |
| [21] |
J. Teufel, H. Oh, M. Hirscher, et al., Adv. Mater. 25 (2013) 635-639. DOI:10.1002/adma.201203383 |
| [22] |
D. Cao, H. Huang, Y. Lan, et al., J. Mater. Chem. A 6 (2018) 19954-19959. DOI:10.1039/C8TA05707A |
| [23] |
D. Cao, J. Ren, Y. Gong, et al., J. Mater. Chem. A 8 (2020) 6319-6327. DOI:10.1039/C9TA14254A |
| [24] |
H. Oh, S.B. Kalidindi, Y. Um, et al., Angew. Chem. Int. Ed. 52 (2013) 13219-13222. DOI:10.1002/anie.201307443 |
| [25] |
M. Liu, L. Zhang, M.A. Little, et al., Science 366 (2019) 613-620. DOI:10.1126/science.aax7427 |
| [26] |
S.A. FitzGerald, C.J. Pierce, J.L.C. Rowsell, E.D. Bloch, J.A. Mason, J. Am. Chem. Soc. 135 (2013) 9458-9464. DOI:10.1021/ja402103u |
| [27] |
Q. Li, J. Duan, W. Jin, Chin. Chem. Lett. 29 (2018) 854-856. DOI:10.1016/j.cclet.2017.11.008 |
| [28] |
W. Fan, Y. Wang, Z. Xiao, et al., Chin. Chem. Lett. 29 (2018) 865-868. DOI:10.1016/j.cclet.2017.11.020 |
| [29] |
Y.P. Xia, C.X. Wang, M.H. Yu, X.H. Bu, Chin. Chem. Lett. 32 (2021) 1153-1156. DOI:10.1016/j.cclet.2020.09.014 |
| [30] |
X. Wang, Y. Wang, K. Lu, W. Jiang, F. Dai, Chin. Chem. Lett. 32 (2021) 1169-1172. DOI:10.1016/j.cclet.2020.09.036 |
| [31] |
I. Weinrauch, I. Savchenko, D. Denysenko, et al., Nat. Commun. 8 (2017) 14496. DOI:10.1038/ncomms14496 |
| [32] |
S.A. Fitzgerald, D. Mukasa, K.H. Rigdon, N. Zhang, B.R. Barnett, J. Phys, Chem. C 123 (2019) 30427-30433. DOI:10.1021/acs.jpcc.9b09332 |
| [33] |
H. Oh, I. Savchenko, A. Mavrandonakis, T. Heine, M. Hirscher, ACS Nano 8 (2014) 761-770. DOI:10.1021/nn405420te=11 |
| [34] |
Y. Si, X. He, J. Jiang, et al., Nano Res. 14 (2021) 518-525. DOI:10.1007/s12274-019-2571-9 |
| [35] |
Y. Si, W. Wang, E.S.M. El-Sayed, D. Yuan, Sci. China Chem. 63 (2020) 881-889. DOI:10.1007/s11426-020-9722-2 |
| [36] |
M.T. Kapelewski, S.J. Geier, M.R. Hudson, et al., J. Am. Chem. Soc. 136 (2014) 12119-12129. DOI:10.1021/ja506230r |
| [37] |
H.-M. Wen, H. Wang, B. Li, et al., Inorg. Chem. 55 (2016) 7214-7218. DOI:10.1021/acs.inorgchem.6b00748 |
| [38] |
A.L. Myers, J.M. Prausnitz, AIChE J. 11 (1965) 121-127. DOI:10.1002/aic.690110125 |
| [39] |
D. Noguchi, H. Tanaka, A. Kondo, et al., J. Am. Chem. Soc. 130 (2008) 6367-6372. DOI:10.1021/ja077469f |
 2021, Vol. 32
2021, Vol. 32 

