b General Education Division, School of Humanities & Social Science, The Chinese University of Hong Kong, Shenzhen, Shenzhen 518172, China;
c School of Civil and Environmental Engineering, Harbin Institute of Technology, Shenzhen, Shenzhen 518055, China
Abundant biogas produced from anaerobic treatment process of municipal wastewater and digestion of solid organic wastes can be employed as a renewable energy source. Conventional direct combustion of biogas produces thermoelectricity, but suffers from low efficiencies and emission of a variety of green-house gas pollutants such as NOx, SOx, CH4 and COx into the air [1]. Solid oxide fuel cells (SOFCs) provide an alternative solution of direct employment with the biogas fuel and efficiently generate electricity using low-cost electrocatalysts [2]. Recently, there is increasing research on this type of directly biogas fueled SOFCs from single cells to complete stacks [3]. However, one of the significant issues limiting the further development of biogas fueled SOFCs is the carbon deposition on the anode, which results in the rapid performance degradation of the SOFCs cells [4]. Therefore, extensive work has been focused on the development of SOFCs cells with coking-resistant capability [5]. There are several strategies addressing this problem, one of which is adding the oxygen content in the fuel (H2O, CO2 or O2) from thermodynamic point of view [6, 7]. The O2 or O2− can easily oxidize the carbon species into CO and CO2 under cell operating temperature. Whereas, another strategy is design and modification of anode materials to inhibit carbon formation or remove carbon by water-gas reaction via enhanced H2O adsorption on the anode [8-10]. Furthermore, adding an inter-reforming layer on top of anode which can convert CH4 into H2 has also been demonstrated effective at reducing carbon deposition [11].
Semiconductor photocatalysis has been widely studied in degradation of various types of pollutants in aqueous phase and gas phase, since it can in situ generate oxidative species including hydroxyl radicals, superoxide radicals etc. on surface of photocatalysts under excitation of proper energetic photons [12-17]. In addition, solid phase mineralization of carbon soot by migrating hydroxyl radicals on TiO2 powder under UV light irradiation was also reported [18]. In order to utilize the full spectrum of solar light, a series of photocatalysts (e.g., defective oxides) with broad light absorption capacity up to 2500 nm have been developed [19], demonstrating that the photocatalytic redox process can be achieved under longer wavelength (IR range) of solar photons. The heat driven near IR or IR light photoactivity referred as thermo-photocatalytic activity has been demonstrated at elevated temperature together with a UV–vis lamp for a variety of applications. For example, thermo-photocatalytic CO2 reduction or hydrogenation on Ru/TiO2 [20], methane reforming on Pt/black TiO2 [21], propane oxidation on Pt/TiO2/WO3 [22], near IR driven water splitting on WO2-NaxWO3 [23] and organic pollutants degradation on BiO2-xBi2O2.75 [24]etc. have been reported recently. Many defective oxides present the thermo-photocatalytic behavior, since the defects on the surface or in the bulk solid oxide semiconductor can yield intermediate energy states between the band−gap and significantly change its optical absorption property and charge carrier mobility. However, highly concentrated defects in an oxide semiconductor can cause the intrinsic change of its conductive property from a semiconductor to a metallic one, and thus the photogenerated charge transition from occupied bands to unoccupied bands cannot be explained by the conventional band-gap theory. For example, a red metallic Sr1-xNbO3 photocatalyst has been demonstrated as an effective photocatalyst for the oxidation of methylene blue and reduction of water, and the authors proposed that the electron transition occurs from the conduction band to a higher level unoccupied band [25]. Recently, we also demonstrated that highly reduced transitional metal oxides (e.g., TiO2-x) can stabilize electrons in the bulk and release them into water upon thermal or near infrared light excitation, corresponding to defects formation upon reduction and removal upon oxidation [26, 27], can be repeatedly proceeded.
So far, most of the thermo-photocatalytic processes for liquid/solid phase reaction were carried out under moderately elevated temperature (T≤100 ℃) and gas/solid phase reaction were performed at relatively higher temperature (T≤500 ℃), whereas the thermo-photocatalytic process in a gas/solid/solid triple phase under higher temperature (> 500 ℃) is still not explored yet. Under condition of such high temperature, intense IR light would be irradiated to a heated solid catalyst, which may induce the charge carriers' transition and then initiate the photocatalytic redox process. Upon this hypothesis, oxidative hydroxyl radicals could be produced on the surface of a semiconducting or metallic solid by oxidizing water or adsorbed hydroxyl group under high temperature.
In this work, we aim to explore the IR-light driven thermo-photocatalytic reactions occurring on a gas/solid/solid triple phase boundary for mineralization of carbon species under high temperature utilizing the in situ generated hydroxyl radical as the oxidant, so as to solve the carbon deposition problem on the anode of biogas fueled SOFCs. Firstly, commercial TiO2 nanopowder was physically mixed into NiO-YSZ based anode, then metallic TiOx particles can be produced in the composite anode during fabrication of single cell upon the high temperature H2 reduction process. The introduction of TiOx particles into anode shows a positive effect on the electrocatalytic activity on the methane oxidation and coking resistance of single cell in simulated biogas fuel. The detailed information on cell fabrication and testing approach were described in supporting information.
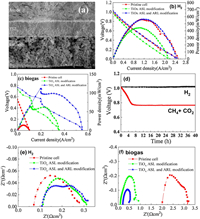
From the cross-sectional view of a single cell as shown in Fig. 1a, the optimized single cell was composed by LSM+YSZ/LSM/LSM+LSCF based cathode layer (~100μm), a dense YSZ electrolyte layer (~50μm), and TiOx modified Ni/YSZ anode support layer (ASL, ~700μm) and TiOx/Ni/YSZ anode reforming layer (ARL, ~20μm). In the ARL and ASL, a certain amount of TiO2 particles were physically mixed with anode powder and converted to TiOx after H2 reduction at 850 ℃ for 5h, which is expected to play dual roles during cell operation. Firstly, it has been demonstrated that Pt/TiO2-x catalyst can markedly improve the thermo-kinetics of reforming CH4 to H2 with a quantum efficiency of 60% at 500 ℃ under visible light irradiation [21]. More importantly, hydroxyl radical could be generated by thermo-photocatalytic process on TiOx particles, which can be utilized for deposited carbon oxidation and removal. Through systematic optimization of ratio of doped TiO2 and ARL components and thickness, we found that the cell composed by 1 wt% TiO2/Ni-YSZ composite and 3 wt% TiO2/Ni-YSZ as the ARL exhibited the best electrochemical performance, compared to other cells with different components.
|
Download:
|
| Fig. 1. Structure and performance of original and modified SOFCs. (a) A scanning electron micrograph image of a single cell composed by different layers. I-V and I-P curves of a single cell operated at 850 ℃ using (b) H2 and (c) biogas. (d) Open circuit potential of single cells operated in H2 or biogas. Impedance analysis of single cells under (e) H2 and (f) biogas. | |
We evaluated the electrochemical performance of the unmodified cell and TiOx modified cells using H2 (as reference) and simulated biogas (70% CH4 + 30% CO2) mixed with ~ 3% H2O vapor as the fuel and ambient air was used at 850 ℃. Representative I-V and I-P curves of a single cell are shown in Figs. 1b and c. Under H2 operating condition, the peak power densities of unmodified cell, 1 wt% TiOx ASL modified cell and 1 wt% TiOx ASL and ARL modified cell were 0.559, 0.442, 0.560 W/cm2 at 850 ℃ (Tables S1–S4 in Supporting information). The TiOx ASL modification slightly deteriorated the electrochemical performance, probably because the gas transfer and electrocatalytic activity was inhibited. Whereas the TiOx ASL and ARL modified cell exhibited similar electrochemical performance with unmodified one. It is commonly considered that the ARL is beneficial for improving electrochemical activity by increasing triple phase boundary length, which can compensate the negative effect introduced by TiOx introduction. When these cells run in simulated biogas, the peak powder densities of unmodified cell were significantly reduced to ~0.014 W/cm2 at 850 ℃, whereas average peak powder densities of 0.06 and 0.10 W/cm2 for those of TiOx ASL modified and TiOx ASL and ARL modified cells (Tables S1–S4). The distinct differences imply the significant roles of TiOx and ARL modification. The sharp spike peaks of the power density maybe attributed to the redox reactions during running biogas fuel. The stable open circuit potentials (1.0 V for H2 and 0.78 V for biogas) for 40 h (Fig. 1d) suggests that the composite anode is robust in direct utilization of methane. The impedance spectra imply that both electrolyte and electrodes contribute small ohmic resistances to the total cell resistance under H2 (Fig. 1e). The ohmic resistance at 850 ℃ was only about 0.07~0.10 Ωcm2 while the polarization resistance was ~0.20 Ωcm2, indicating that polarization resistances contribute more to the total cell resistance. When fueled with biogas, the pristine cell exhibited much higher ohmic and polarization resistances which was about 2.0 and 1.2 Ωcm2 (Fig. 1f), indicating that Ni-YSZ anode has lower catalytic activity for CH4 oxidation while it has higher activity towards H2 oxidation. For the TiOx ASL and TiOx ASL and ARL modified cells, both of the ohmic and polarization resistances were markedly reduced when fueled by biogas. The results imply that the modified cells have better electrochemical performances towards CH4 fuel.
In addition, the coking resistance of the composite anode can be clearly presented as shown from the SEM and EDS images of these cells after reaction. For the pristine cell, many carbon particles appeared on the surface of anode after reaction (Fig. 2a and Fig. S1 in Supporting information), whereas the composite cells with TiOx ASL or TiOx ASL and ARL modification show less carbon particles deposition on the surface of anode after reaction (Figs. 2b and c). The deposited carbon was further confirmed by EDS mapping images (Figs. 2d–f) showing that O and Ni elements are composed as the backbone components, whereas carbon species homogeneously existed on the anode surface. Some carbon species may come from the sample contamination in the air. Furthermore, the porosity of anode for the unmodified cell after reaction was also reduced due to the carbon deposition, resulting in the decay of its performance.

|
Download:
|
| Fig. 2. SEM images of different anodes after reaction: (a) unmodified Ni-YSZ anode. (b) TiOx modified Ni-YSZ anode. (c) TiOx ASL and ARL modified anode.EDS images of anode for TiOx modified anode as shown in panel b: (d) C element; (e) Ni element; (f) O element. | |
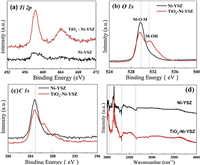
To gain insights into the enhanced performance of the modified anode, we analyzed the commercial NiO/YSZ powder and 1 wt% TiOx-Ni/YSZ composite powder, which were pressed and sintered at 1100 ℃ in air for 2 h and reduced in pure H2 atmosphere at 850 ℃ for 5h. The XRD patterns (Fig. S2 in Supporting information) of the reduced Ni/YSZ and TiOx-Ni/YSZ composite samples indicate that anatase/rutile phase of TiO2 at 2θ of 25.7° and 27.6° was disappeared after high temperature reduction, and there are new phases formed at 2θ of 37.2°, 39.6°, 43.3°, 46.7° in the modified anode materials, which can be indexed to titanium oxides (TiOx) such as TiO (PDF#89-3360), TiO0.975 (PDF#75-0312), TiO1.2 (PDF#85-1381) and TiO1.69 (PDF#89-3076). For further proving the reduction behavior, we carried out the powder reduction experiment under the same reduction condition. White TiO2 powder can be converted to the nonstoichiometric black TiOx, which has been reported with reduced band-gap and metallic property [26, 27]. XPS analysis of Ni-YSZ anode and TiOx modified anode clearly shows the difference between the two samples. As shown in Fig. 3a, the weak Ti 2p peaks at binding energies of ~458 eV and ~464 eV originate from the doped TiOx particles in reference with the Ni-YSZ sample. The O 1s peaks are slightly different for the two samples (Fig. 3b), the peak at binding energy of 530 eV can be assigned for M-O-M bonds, whereas the new peak at binding energy of 532 eV for the modified sample can be assigned for M-OH bond. The H2 reduction creates the oxygen defects and thus the Ti−OH bond may exist at the defect sites. On the other hand, the carbon elements shown for both samples may come from the carbon deposition during cell operation with CH4 fuel and sample contamination in the air (Fig. 3c). Thus, it is not convincible to quantify the amounts of carbon species on the anode samples by XPS analysis. The FTIR spectra shows little difference between the two samples (Fig. 3d), there was no water absorption peaks appeared at 3450−3550 cm−1. In literature [10], it was found that increasing the absorption of water and surface hydroxyl group on the anode was the key for improving coking resistance performance in direct CH4 fueled SOFCs with alkaline metal or rare earth metal modified anodes. Therefore, the reduced carbon deposition performance of the TiOx modified anode should be explained by other reasons.
|
Download:
|
| Fig. 3. XPS analysis (a) Ti 2p, (b) O 1s, (c) C 1s and (d) FTIR analysis of Ni-YSZ anode and modified TiOx-Ni-YSZ anode. | |
For demonstrating our hypothesized mechanism accounting for reduced carbon deposition, we carried out the thermo-photocatalytic powder experiments by direct mixing TiO2 powder with graphite powder together, which was firstly reduced by H2 and then filled with N2 and water vapor maintained at 850 ℃ for 30 h. The mass change of powder and generated gas products were monitored for multicycle tests. As shown in Fig. S3a (Supporting information), the powder mass change was negligible without running the temperature ramping program for the repeated batch 1–3 experiments. However, the total mass of powder was significantly reduced after 30 h reaction at 850 ℃ for the batch 4–6 experiments in Fig. S3b (Supporting information). The reduced amount of powder with an average value of 27.2 mg far exceed the amount of reduced mass assuming that TiO2 was completely converted into TiO, indicating that the graphite powder was oxidized to gaseous species (e.g., CO, CO2, CxHy) and removed during the high temperature reaction. The generation of CO2 was demonstrated from the color change and precipitation occurred in the two gas indexing bottles filled with bromothymol blue and limewater (Fig. S4 in Supporting information), implying that CO2 was continuously produced during the reaction.
The utilization of CH4 and CO2 as the SOFCs fuels and the carbon formation process during the high temperature reaction can be expressed by reactions (1) to (5). Furthermore, the addition of reforming layer can partially convert CH4 into H2 and improve the cell performance according reaction (6), both the Ni particles and TiOx particles play the reforming roles at high temperature. The carbon deposition on the anode would cause the decay of electrochemical performance of fuel cells as shown from the unmodified cell. According to the experiments observation, we propose the IR-light driven photocatalytic removal of carbon under high temperature in the direct CH4 fueled SOFCs through the reactions of (7)–(11).
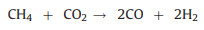
|
(1) |

|
(2) |

|
(3) |
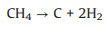
|
(4) |
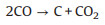
|
(5) |

|
(6) |

|
(7) |

|
(8) |
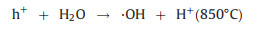
|
(9) |
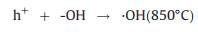
|
(10) |

|
(11) |
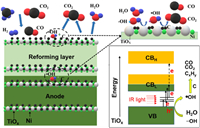
As shown in Scheme 1, TiO2 was converted to defective metallic TiOx, and could be excited by strong IR light irradiation to generate electron-hole pairs by carrier transition from occupied valence band (VB) to lower unoccupied conduction band (CBL) via the mediation of intermediate defects states or from the lower unoccupied conduction band CBL to a higher unoccupied band (CBH). The remained holes could oxidation water vapor or surface hydroxyl group to generated oxidative hydroxyl radical, which can be diffused into the vicinity of deposited carbon species and further oxidize them into CO, CO2 or CxHy. The oxidation process took place on the water vapor/carbon/TiOx triple phase boundary. The generated CO2 was indirectly demonstrated, however more in situ characterization and analysis techniques are required to directly demonstrate the proposed mechanism.
|
Download:
|
| Scheme 1. Proposed mechanism for thermo-photocatalytic removal of carbon deposited on the anode. | |
In summary, by integrating TiOx powder into the Ni-YSZ anode and ARL layer, the SOFCs cell performance was significantly improved when simulated biogas was used as the fuel and ambient air was used as the oxidant. The peak power density was enhanced about seven times for the optimized anodes. More importantly, the coking resistance of the composite anode is exceptional in contrast to the state-of-the-art Ni-YSZ anode. The remarkable performance and stability of the developed fuel cell may result from the IR light driven photocatalytic removal of deposited carbon on the anode, in which in situ generated hydroxyl radical is the key oxidant for carbon removal. This work provides a novel strategy for solving the carbon deposition problems in direct methane fueled SOFCs.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by Shenzhen Science and Technology Innovation Commission (No. JCYJ20190813171403664), Basic research program of Guangdong Province (No. 2018A030313851), Longgang District Technology Supporting Project (No. LGKCKJPT2019074), the Fundamental Research Funds for the Central Universities (No. HIT. NSRIF. 2020074). We also thanks professor Zheng Zhong for providing collaboration research at Institute of Hydrogen and Fuel Cell.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.01.050.
| [1] |
W. Wang, R. Ran, Z. Shao, J. Power Sources 196 (2011) 90-97. DOI:10.1016/j.jpowsour.2010.07.033 |
| [2] |
W. Wang, R. Ran, Z. Shao, Int. Hydrogen Energy 36 (2011) 755-764. DOI:10.1016/j.ijhydene.2010.09.048 |
| [3] |
Z. Tao, G. Hou, N. Xu, Q. Zhang, H. Ding, Electrochim. Acta 150 (2014) 55-61. DOI:10.1016/j.electacta.2014.10.126 |
| [4] |
X. Yao, P. Li, B. Yu, et al., Int. Hydrogen Energy 42 (2017) 22192-22200. DOI:10.1016/j.ijhydene.2017.02.008 |
| [5] |
N. Hou, T. Yao, P. Li, et al., ACS Appl. Mater. Interfaces 11 (2019) 6995-7005. DOI:10.1021/acsami.8b19928 |
| [6] |
J. Ma, C. Jiang, P.A. Connor, M. Cassidy, J.T.S. Irvine, J. Mater. Chem. A 3 (2015) 19068-19076. DOI:10.1039/C5TA06421J |
| [7] |
B.J.M. Sarruf, J.E. Hong, R. Steinberger-Wilckens, P.E.V. de Miranda, Int. Hydrogen Energy 43 (2018) 6340-6351. DOI:10.1016/j.ijhydene.2018.01.192 |
| [8] |
J. Qu, W. Wang, Y. Chen, X. Deng, Z. Shao, Appl. Energy 164 (2016) 563-571. DOI:10.1016/j.apenergy.2015.12.014 |
| [9] |
D. Yoon, A. Manthiram, Energy Environ. Sci. 7 (2014) 3069-3076. DOI:10.1039/C4EE01455C |
| [10] |
J. Qu, W. Wang, Y. Chen, et al., ChemSusChem 11 (2018) 3112-3119. DOI:10.1002/cssc.201801539 |
| [11] |
Y. Chen, B. deGlee, Y. Tang, et al., Nat. Energy 3 (2018) 1042-1050. DOI:10.1038/s41560-018-0262-5 |
| [12] |
M. Zhang, J. He, Y. Chen, P.-Y. Liao, M. Zhu, Chin. Chem. Lett. 31 (2020) 2721-2724. DOI:10.1016/j.cclet.2020.05.001 |
| [13] |
G. Liu, J. Zhou, W. Zhao, Z. Ao, T. An, Chin. Chem. Lett. 31 (2020) 1966-1969. DOI:10.1016/j.cclet.2019.12.023 |
| [14] |
B. Jing, Z. Ao, W. Zhao, et al., J. Mater. Chem. A 8 (2020) 20363-20372. DOI:10.1039/d0ta06060g |
| [15] |
H. Xu, J. Shi, S. Lyu, X. Lang, Chin. J. Catal. 41 (2020) 1468-1473. DOI:10.1016/S1872-2067(20)63640-3 |
| [16] |
X. Li, X. Lang, J. Chem. Phys. 152 (2020) 044705. DOI:10.1063/1.5142512 |
| [17] |
S. Hu, Y. Yu, Y. Guan, et al., Chin.Chem. Lett. 31 (2020) 2839-2842. DOI:10.1016/j.cclet.2020.08.021 |
| [18] |
M.C. lee, W. Choi, J. Phys. Chem. B 106 (2002) 11818-11822. DOI:10.1021/jp026617f |
| [19] |
M.Q. Yang, L. Shen, Y. Lu, et al., Angew. Chem. Int. Ed. 58 (2019) 3077-3081. DOI:10.1002/anie.201810694 |
| [20] |
C. Wang, S. Fang, S. Xie, Y. Zheng, Y.H. Hu, Chemistry 8 (2020) 7390-7394. DOI:10.1039/c9ta13275a |
| [21] |
B. Han, W. Wei, M. Li, K. Sun, Y.H. Hu, Chem. Commun. 55 (2019) 7816-7819. DOI:10.1039/c9cc04193a |
| [22] |
L. Wang, X.Y. Liu, A. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 12909-12916. DOI:10.1002/anie.202001701 |
| [23] |
G. Cui, W. Wang, M. Ma, et al., Nano Lett. 15 (2015) 7199-7203. DOI:10.1021/acs.nanolett.5b01581 |
| [24] |
M. Wang, G. Tan, D. Zhang, et al., Appl. Catal. B: Environ. 254 (2019) 98-112. DOI:10.3390/plants8040098 |
| [25] |
X. Xu, C. Randorn, P. Efstathiou, J.T.S. Irvine, Nature Mater. 11 (2012) 595-598. DOI:10.1038/nmat3312 |
| [26] |
X. Zhang, G. Zhang, J. Zou, New J. Chem. 42 (2018) 6084-6090. DOI:10.1039/C8NJ00560E |
| [27] |
G. Zhang, J. Zou, X. Xu, Adv. Sustain. Systems 2 (2018) 1700139. DOI:10.1002/adsu.201700139 |
 2021, Vol. 32
2021, Vol. 32 

