b Green Catalysis Center, College of Chemistry, Zhengzhou University, Zhengzhou 450001, China;
c National Rubber Additive Engineering Technology Center, Liaocheng 252059, China
The direct functionalization of C-H bonds for the synthesis of heterocyclic compounds has emerged as an increasingly valuable tool for step-economical carbon-heteroatom bond-forming organic reactions [1-3]. Traditionally, transition metal complexes were generally used as efficient catalysts for the functionalization of C-H bonds, and a handful of eminent works have been disclosed [4, 5]. Although these methods are very efficient, it often suffers from some drawbacks, such as the generation of waste, the use of expensive hazardous transition metals and ligands. With the development of green organic chemistry and enhancement of environmental protection consciousness of chemists [6, 7], the application of a microwave-assisted strategy for the functionalization of C-H bonds to synthesize heterocyclic compounds under metal-free conditions is highly attractive.
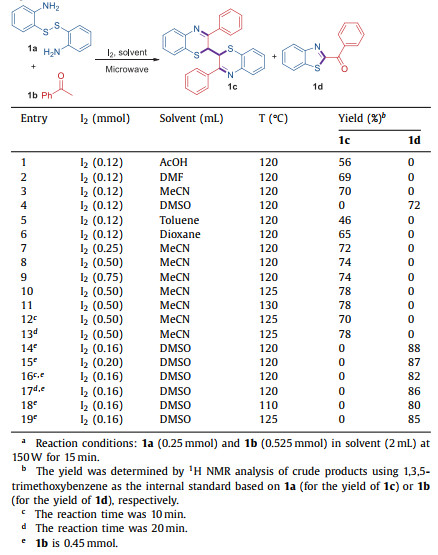
2-Acylbenzothiazoles and bibenzo[b][1, 4]thiazines are two classes of heterocyclic compounds containing both nitrogen and sulfur in the five- and six-membered ring, respectively, which could be used as multifunctional building units and valuable structural units in pharmaceutical chemistry, pesticides, industrial dyes and functional materials [8, 9]. For example, benzothiazole is an important type of heterocyclic skeleton, which can be used for anticancer agents and enzyme inhibitors, and so on [10]. In recent years, some practical methodologies have been reported to synthesize benzothiazole compounds [11]. For instance, in 2012, Wu and co-workers reported an I2-promoted oxidative cyclization reaction for the one-pot synthesis of 2-acylbenzothiazoles from methyl o-aminobenzenethiols and ketones [12]. In 2016, Wan et al. also developed a tunable procedure for the synthesis of benzothiazole-based vicinal diketones or 2-aroylbenzothiazoles from o-aminothiophenols [13]. For the preparation of thiazines, several synthesis protocols have been achieved. For example, the oxidative coupling of 3-phenyl-2H-1, 4-benzothiazine with highly explosive picric acid [14]. Later, our group and Nguyen et al. found that bibenzo[b][1, 4]thiazines can be constructed through a one-step reaction of readily available 2, 2′-dithiodianiline or 2-aminothiophenol in the presence AIBN or TFA, respectively [15, 16]. Some other methods require halogenated reagent, harsh reaction conditions, expensive catalysts, and additional ligands to proceed with the reactions, which accordingly would generate stoichiometric amounts of waste after the reactions[17]. However, in the above-mentioned advances, most of the reactions need a long reaction time (up to 24 h) to get good yields. Thus, there is an incentive to develop efficient and green catalytic processes based on microwave technology to minimize the reaction time in terms of sustainability. We herein report an efficient condition-controlled reaction between aryl methyl ketones and disulfanediyldianilines under microwave-assisted conditions, which would generate bibenzo[b][1, 4]thiazines or 2-acylbenzothiazoles as the main products in only 15 min, respectively (Scheme 1).
|
Download:
|
| Scheme 1. Condition-controlled selective synthesis of 2-acylbenzothiazoles and bibenzo[b][1, 4]thiazines. | |
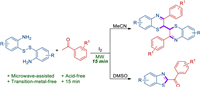
Initially, 2, 2′-disulfanediyldianiline 1a and acetophenone 1b were selected to optimize the microwave-assisted reaction parameters (Table 1). We conducted the reaction with the conditions of I2 (0.12 mmol), 1a (0.25 mmol) and 1b (0.525 mmol) in AcOH (2 mL) at 150 W/120 ℃ for 15 min, only 3, 3′-diphenyl-2H, 2′H-2, 2′-bibenzo[b][1, 4]thiazine1c was isolated in 56% yield (entry 1). Some other solvents, including DMF, MeCN, DMSO, toluene and dioxane were tested (entries 2–6), which disclosed that MeCN displayed the best efficiency for the synthesis of 1c (entry 3). While the reaction performed in DMSO gave the sole product benzo[d]thiazol-2-yl(phenyl)methanone1d in 72% yield (entry 4). To further optimize the reaction conditions of product 1c, the amount of I2 was surveyed. It turns out that 2 equiv. of I2 is the best loading for generating of 1c (entries 7–9). Next, we checked the influence of the reaction temperature. The reaction yield did not increase when the reaction temperature was higher than 125 ℃ (entries 10 and 11). Moreover, changing the reaction time did not increase the yield of 1c (entries 12 and 13). On the other hand, we also evaluated the reaction parameters including I2 loading, reaction temperature, and reaction time for the synthesis of product 1d (entries 14–19). From the above experimental results, it could be found that the application of DMSO as a solvent is critical for the formation of 1d, which is similar with that of the reported case [18].
|
|
Table 1 Optimization of the reaction conditions.a. |
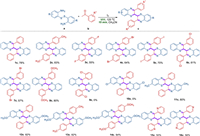
With the optimized reaction conditions, the scope of the substrates for the synthesis of bibenzo[b][1, 4]thiazines was firstly investigated (Scheme 2). Initially, when aryl methyl ketones with electron-donating groups and electron- withdrawing groups in the meta- and para-position were used as reactants, and the corresponding products 1c-8c could be obtained in moderate to good yields. Unfortunately, the ortho-position substituted aryl methyl ketone could not proceed in the reaction (9c) probably owing to the stereo-hindrance effect. The yields of the ketones bearing electron-donating groups (–CH3, –OMe) (2c and 8c) were better than those of substrates bearing electron-withdrawing groups (–F, –Cl, –Br, –I) (3c-7c). Next, the substituted 2, 2′-disulfanediyldianilines were used to react with acetophenone (10c-12c). No product was detected when 2, 2′-disulfanediylbis(4-chloroaniline) was used as the substrate (10c). The scope of the reaction was further explored by substituted dithiodianilines with substituted acetophenones bearing electron-donating groups, the products were isolated in good yields (13c and 14c). From the above results, we found that the reaction was significantly influenced by both the electronic effect and stereo hindrance effect.
|
Download:
|
| Scheme 2. Scope of synthesis of bibenzo[b][1, 4]thiazines. Reaction conditions: I2 (0.5 mmol), a (0.25 mmol) and b (0.525 mmol) in MeCN (2 mL) at 150 W/125 ℃ for 15 min. Isolated yield after flash chromatography (Al2O3) based on a. | |
Next, we evaluated the substrate scope for the synthesis of 2-acylbenzothiazoles d after establishing the optimized reaction conditions. The procedure was extended to a variety of aromatic ketones for coupling with substituted dithiodianilines (Scheme 3, 1d-16d). The reaction of aromatic ketones with both electron-donating and electron-withdrawing groups at ortho-, meta- and para-position of the phenyl group were suitable for obtaining the corresponding products in moderate to excellent yields (1d-12d). The reaction yields of the ketones bearing electron-donating groups at para-position (–CH3, –OMe) (2d and 3d) were slightly better than those of substrates bearing electron-withdrawing groups (–NO2, –CN, –F, –Cl, –Br, –I) (4d-9d). The reaction yields were mainly influenced by the steric hindrance effect of group R1. Relatively low yields were observed when ortho- and meta-substituted substrates were used (10d-12d). Notably, substituted 2, 2′-disulfanediyldianilines reacted smoothly with 2a to afford the desired corresponding products in good yields (13d-16d). Notably, the moderate yield could also be achieved when 2-acetylfuran was presented in the reaction (17d).

|
Download:
|
| Scheme 3. Scope of the synthesis of 2-acylbenzothiazoles. Reaction conditions: a (0.25 mmol), b (0.45 mmol) and I2 (0.16 mmol) in DMSO (2 mL) at 150 W/120 ℃ for 15 min. Isolated yields after flash chromatography based on b. | |
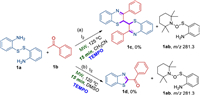
To clarify the mechanism of the two reactions, a series of control experiments were designed and conducted. The reaction intermediates were examined and monitored by 1H NMR and GC–MS (Scheme 4). When the radical scavenger 2, 2, 6, 6-tetramethylpiperidin-1-oxyradical (TEMPO, 1.0 mmol) was added to probe the radical nature of the two reactions, no corresponding products 1c and 1d were detected. While, the adduct 1ab was observed by GC–MS in both reactions (Scheme 4 and Fig. S29 in Supporting information), suggesting that the thiol radical was involved in the reaction.
|
Download:
|
| Scheme 4. Control experiments. | |
On the basis of the above results and previous reports [12, 15, 19], the plausible reaction pathways are proposed for the synthesis of bibenzo[b][1, 4]thiazines c and 2-acylbenzothiazoles d, respectively (Scheme 5). First, the thiyl radical 1aa was generated from 1a under the irradiation of microwave. When DMSO was applied as the solvent, 1b was transformed into α-iodoacetophenone 1ba in presence of I2, which was further oxidized by DMSO to form 1bb [20]. Then the condensation of 1aa and 1bb afforded the intermediate 1bc. Subsequently, 1bc underwent an intramolecular cyclization and hydrogen atom abstraction to afford the product 1d On the other hand, when the reaction was conducted in CH3CN the direct condensation of 1aa and 1b gave the intermediate 1ab, which was converted into the corresponding isomer 1ac. Then the intermediate 1ac suffered from an intramolecular cyclization and hydrogen atom abstraction to generate the intermediate 1ae, which was further dimerized into the desired product 1c in the presence of I2 (Scheme 5). On the other hand, I2 could be regenerated from the reaction of HI and DMSO in the pathway of the generation of product d [12]. The reason of high I2 loading was probably due to the low efficiency of the regeneration process.
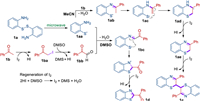
|
Download:
|
| Scheme 5. Possible mechanisms. | |
In summary, we have developed an efficient microwave-assisted condition-controlled C(sp3)-H activation strategy for the selective synthesis of 2-acylbenzothiazoles and bibenzo[b][1, 4]thiazines from aryl methyl ketones and disulfanediyldianilines. In these reactions, a wide array of aryl methyl ketones were well compatible. The reaction was switchable by simply changing the reaction conditions (I2/MeCN or I2/DMSO) without the involvement of explosive oxidants and additional activating reagents.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe work was financially supported by the National Natural Science Foundation of China (No. 21871125); the Natural Science Foundation of Shandong Province, China (Nos. ZR2019MB043 and ZR2019QB022) and the Construction Project of Quality Curriculum for Postgraduate Education of Shandong Province (No. SDYKC19057).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.069.
| [1] |
(a)J.F. Yu, J.J. Li, P. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 18141–18145; (b)J.S. Wright, P.J.H. Scott, P.G. Steel, et al., Angew. Chem. Int. Ed. 59 (2020) 2–28; (c)Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361–1368. |
| [2] |
(a) Y. Wang, J. Zhu, R. Guo, et al., Chem. Sci. 11 (2020) 12316–12322; (b)X. Zhang, A. McNally, et al., ACS Catal. 9 (2019) 4862–4866. |
| [3] |
(a) U.H.F. Bunz, J. Freudenberg, et al., Acc. Chem. Res. 52 (2019) 1575–1587; (b)X.M. Xu, D.M. Chen, Z.L. Wang, et al., Chin. Chem. Lett. 31 (2020) 49–57. |
| [4] |
(a) U. Dhawa, C. Tian, W. Li, et al., ACS Catal. 10 (2020) 6457–6462; (b)M. Liu, L.J. Li, J. Zhang, et al., Chin. Chem. Lett. 31 (2020) 1301–1304. |
| [5] |
(a) J.R. Hummel, J.A. Boerth, J.A. Ellman, et al., Chem. Rev. 117 (2017) 9163–9227; (b)X. Lu, S.J. He, W.M. Cheng, et al., Chin. Chem. Lett. 29 (2018) 1001–1008. |
| [6] |
(a) X.Y. Li, Y. Liu, X.L. Chen, et al., Green Chem. 22 (2020) 4445–4449; (b)Q. Zhang, Z. Mao, K. Wang, et al., Green Chem. 22 (2020) 3239–3247. |
| [7] |
(a) W.P. Teh, D.C. Obenschain, B.M. Black, et al., J. Am. Chem. Soc. 142 (2020) 16716–16722; (b)J.L. Peltier, E. Tomas-Mendivil, D.R. Tolentino, et al., J. Am. Chem. Soc. 142 (2020) 18336–18340. |
| [8] |
(a) X. Meng, X. Bi, C. Yu, et al., Green Chem. 20 (2018) 4638–4644; (b)K. Bahrami, M.M. Khodaei, F. Naali, et al., J. Org. Chem. 73 (2008) 6835–6837. (c)L. Leone, O. Crescenzi, R. Amorati, et al., Org. Lett. 15 (2013) 4944–4947. |
| [9] |
D.A. Horton, G.T. Bourne, M.L. Smythe, et al., Chem. Rev. 103 (2003) 893-930. |
| [10] |
(a) E. Meltzer-Mats, G. Babai-Shani, L. Pasternak, et al., J. Med. Chem. 56 (2013) 5335–5350; (b)A. Spadaro, M. Frotscher, R.W. Hartmann, et al., J. Med. Chem. 55 (2012) 2469–2473. |
| [11] |
(a) X. Meng, X. Bi, C. Yu, et al., Green Chem. 20 (2018) 4638–4644; (b)B. Wang, Q. Zhang, Z. Guo, et al., Synthesis 52 (2020) 3058–3064; |
| [12] |
Y.P. Zhu, M. Lian, F.C. Jia, et al., Chem. Commun. 48 (2012) 9086-9088. DOI:10.1039/c2cc34561g |
| [13] |
J.P. Wan, Y. Zhou, Y. Liu, et al., Green Chem 18 (2016) 402-405. DOI:10.1039/C5GC01821H |
| [14] |
F. Giordano, L. Mazzarella, G. Prota, et al., J. Chem. Soc. C. (1971) 2610-2616. |
| [15] |
X. Huang, N. Rong, P. Li, et al., Org. Lett. 20 (2018) 3332-3336. DOI:10.1021/acs.orglett.8b01238 |
| [16] |
T.B. Nguyen, P. Retailleau, et al., Adv. Synth. Catal. 23 (2020) 5380-5384. DOI:10.1002/adsc.202000964 |
| [17] |
(a) O. Prakash, N. Sharma, K. Pannu, et al., Synth. Commun. 37 (2007) 1995–1999; (b)D.C. Loukrakpam, P. Phukan, et al., Chem. Select. 4 (2019) 3180–3184; (c)Y.P. Zhu, F.C. Jia, M.C. Liu, et al., Org. Lett. 14 (2012) 4414–4417; (d)X. Fan, Y. He, X. Zhang, et al., Tetrahedron 67 (2011) 6369–6374; (e)K. Yang, C. Zhang, P. Wang, et al., Chem. Eur. J. 20 (2014) 7241–7244; (f)Q. Feng, Q. Song, Adv. Synth. Catal. 356 (2014) 2445–2452. |
| [18] |
(a) W. Fan, Z. Yang, B. Jiang, et al., Org. Chem. Front. 4 (2017) 1091–1102; (b)Z. Yao, X. Lin, R. Chauvin, et al., Chin. Chem. Lett. 31 (2020) 3250–3254. |
| [19] |
(a) K. Farshadfar, A. Gouranourimi, A. Ariafard, et al., Org. Biomol. Chem. 18 (2020) 8103–8108; (b)R. Fan, W. Li, D. Pu, et al., Org. Lett. 11 (2009) 1425–1428. |
| [20] |
R. Ma, Y. Ding, R. Chen, et al., J. Org. Chem. 86 (2021) 310-321. DOI:10.1021/acs.joc.0c02095 |
 2021, Vol. 32
2021, Vol. 32