b School of Pharmaceutical and Materials Engineering, Taizhou University, Taizhou 318000, China;
c State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China;
d School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
The introduction of sulfonyl motifs into organic molecules has attracted continuous interest in medicinal chemistry, due to the dramatic improvement in biologically active compounds and pharmaceutical agents [1]. Therefore, enormous efforts have been made for the preparation of diverse sulfonyl compounds [2]. Among them, diarylmethyl sulfones have attracted significant focus recently, and are often used as building blocks in synthetic chemistry [3]. They are valuable synthetic intermediates for the construction of triarylmethane derivatives, which are ubiquitous in the area of medicinal chemistry and materials science [4]. However, only several classical strategies for the preparation of diarylmethyl sulfones have been developed, including nucleophilic addition of aromatic aldehydes [5], nucleophilic substitution of alcohols [6], transition metal-catalyzed arylation of methyl sulfones [7] and nucleophilic 1, 6-addition of para-quinone methides (p-QMs) [8]. We noticed that previous works described above were initiated by sulfinyl anion starting from sodium arylsulfinates or arylsulfonyl hydrazines. Meanwhile, p-QMs as versatile Michael acceptors are structurally characterized by the unique assembly of carbonyl and olefinic moieties, which have been applied in the synthesis of natural products and bioactive agents [9]. Compared to the reports on nucleophilic 1, 6-addition of p-QMs [10], radical 1, 6-addition of p-QMs is much less, which also provides an important and alternative route for the generation of valuable entities [11]. Despite these significant advances, development of more straightforward methods as well as environmentally friendly conditions to construct diverse diarylmethyl sulfones is still highly desirable.
Using sulfur dioxide surrogates of DABCO·(SO2)2 or potassium/sodium metabisulfite as the source of sulfur dioxide has become a facile pathway for the preparation of sulfonyl compounds [12], [13]. Recently, we accomplished the generation of sulfonyl compounds through radical insertion of sulfur dioxide. Several radical precursors including aryldiazonium tetrafluoroborates [14], potassium alkyltrifluoroborates [15], Katritzky salts [16] and 4-substituted Hantzsch esters [17] have been utilized in the sufonylation process. During the reaction process, sulfonyl radicals formed in situ would be the key intermediates. So far, 1, 2-addition of sulfonyl radicals with the difunctionalization of olefins has been realized [18]. Prompted by the recent advance of 1, 6-addition of p-QMs and the radical chemistry of sulfur dioxide, we envisioned that radical 1, 6-addition of p-QMs with the insertion of sulfur dioxide would be feasible as well, which would give rise to diarylmethyl sulfones. Herein, we report a photoinduced reaction of potassium alkyltrifluoroborates, sulfur dioxide, and p-QMs under visible light irradiation at room temperature, leading to diarylmethyl alkylsulfones in moderate to good yields. This reaction works well under photocatalysis with a broad substrate scope by using DABCO·(SO2)2 as the source of sulfur dioxide. Various functional groups are compatible. Mechanistic study shows that this transformation is initiated by an alkyl radical generated in situ from potassium alkyltrifluoroborate in the presence of photocatalyst. The subsequent insertion of sulfur dioxide and radical 1, 6-addition of p-QMs with alkylsulfonyl radical intermediate afford the corresponding diarylmethyl alkylsulfone.
Guided by the hypothesis as mentioned above, we therefore started to investigate the feasibility of this proposed route. Initial studies were performed for the reaction of 4-benzylidene-2, 6-di-tert-butylcyclohexa-2, 5-dienone 1a, potassium cyclopentyltrifluoroborate 2a and DABCO·(SO2)2. At the beginning, the reaction was carried out in 1, 2-dichloroethane (DCE) in the presence of 30 W blue LEDs (465–475 nm) at room temperature. Although we discovered that the starting materials were consumed and the phenol-type diarylmethyl sulfone was produced, the product could not be purified and isolated since the elimination of sulfinic acid might be reversible [19]. Thus, a late-modification by indium(III) triflate catalyzed acylation was introduced [20], and the optimization of reaction conditions for the radical 1, 6-addition was provided in Table 1. A brief screening of photocatalysts revealed that 9-mesityl-10-methyl acridinium perchlorate [Mes-Acr]ClO4 was the best one, and the corresponding diarylmethyl alkylsulfone 3aa was obtained as expected in 52% yield (Table 1, entry 1). Inferiors results were observed when other photocatalysts were used in the reaction (Table 1, entries 2–6). We further explored the reaction in different solvents, and DCE was found to be the best choice (Table 1, entries 7–13). Next, evaluation of additives was carried out, showing that the presence of 0.5 equiv. of KH2PO4 gave rise to compound 3aa in 86% yield (Table 1, entry 14. For details, see Tables S1 in Supplementary information). Considering the easy availability, sodium metabisulfite or potassium metabisulfite was used as the source of sulfur dioxide. However, no product was detected (Table 1, entry 15). Additionally, no reaction occurred when the reaction was performed in dark (Table 1, entry 16).
|
|
Table 1 Initial studies for the reaction of 4-benzylidene-2, 6-di-tert-butylcyclohexa-2, 5-dienone 1a, potassium cyclopentyltrifluoroborate 2a and DABCO·(SO2)2.a |
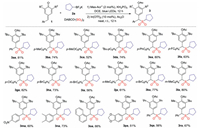
After obtaining the optimized conditions, we then evaluated the substrate scope of this radical 1, 6-addition of p-QMs. As illustrated in Scheme 1, a variety of substituted para-quinone methides 1 could react smoothly with potassium cyclopentyltrifluoroborate 2a and DABCO·(SO2)2 under the standard conditions, providing the desired diarylmethyl alkylsulfones in moderate to excellent yields.
|
Download:
|
| Scheme 1. Reaction of substituted para-quinone methides 1, potassium cyclopentyltrifluoroborate 2a and DABCO·(SO2)2. Isolated yield based on para-quinone methides 1. | |
From Scheme 1, it was found that para-quinone methides bearing electron-donating or electron-withdrawing groups located at the para-position of aromatic ring exhibited good reaction efficiency and diverse functional groups including methyl, methoxyl, tert-butyl, phenyl, fluoro, chloro, bromo and nitro were well tolerated under the optimal conditions, leading to the desired products 3ba-3 ha and 3ma in moderate to good yields (52%−82% yields). Additionally, the efficiency of this protocol was not impeded by the ortho- or meta-substituents on the aromatic ring, which gave rise to the corresponding products 3ia-3la in 56%−80% yields. Interestingly, muti-substituted para-quinone methides 1n-1p could also take part in this transformation. Notably, the para-quinone methides 1q and 1r could react with compound 2a smoothly, affording the desired products 3qa and 3ra in 56% and 67% yield, respectively.
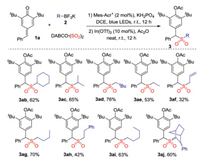
In order to further verify the applicability of the strategy, a range of potassium alkyltrifluoroborates 2 were examined in the reaction of 4-benzylidene-2, 6-di-tert-butylcyclohexa-2, 5-dienone 1a and DABCO·(SO2)2 (Scheme 2). As expected, all reactions worked well, affording the corresponding diarylmethyl alkylsulfones in moderate to good yields. The alkyl groups including cyclohexyl, isopropyl, neopentyl, n-pentyl, n-butyl and ethyl groups were compatible under the conditions. Compound 3aj with diastereoisomers was furnished in 60% yield. In this transformation, the substrate scope was limited since only para-quinone methides as 1, 6-acceptor with 2, 6-dialkyl groups could be employed. It was reported that the presence of 2, 6-dialkyl groups could not be avoided for the successful conversion, as they provided stability to para-quinone methides [21]. Additionally, the bulky tert-butyl or isopropyl groups could block the C-4 position of para-quinone methides, thus directing the para-quinone methides to undergo 1, 6-conjugate addition over 1, 4-conjugate addition in a highly regioselective manner.
|
Download:
|
| Scheme 2. Reaction of para-quinone methide 1a, potassium alkyltrifluoroborate 2 and DABCO·(SO2)2. Isolated yield based on para-quinone methide 1a. | |
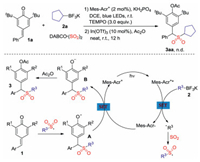
Since a radical 1, 6-addition was hypothesized, a control experiment was subsequently performed with the addition of 2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO) as a radical scavenge under the standard conditions (Scheme 3). As expected, the reaction was completely hampered, and no desired product 3aa was detected. Thus, combined with our previous work and related reports [12], a plausible mechanism was proposed (Scheme 3). We reasoned that under visible light irradiation, the photocatalyst Mes-Acr+ would convert to the excited state of Mes-Acr+*, which would promote the transformation from potassium alkyltrifluoroborate 2 to alkyl radical intermediate and the reduced species Mes-Acr·. Subsequently, alkyl radical would be trapped by sulfur dioxide, leading to the formation of alkylsulfonyl radical. Then, radical 1, 6-addition process would occur between alkylsulfonyl radical and para-quinone methides 1 giving rise to radical intermediate A. Radical intermediate A would be reduced by Mes-Acr· to provide anionic intermediate B along with the regeneration of photocatalyst Mes-Acr+. Further acylation would produce the corresponding diarylmethyl alkylsulfone 3.
|
Download:
|
| Scheme 3. Control experiment and plausible mechanism. | |
In conclusion, we have described a photoinduced reaction of potassium alkyltrifluoroborates, sulfur dioxide, and para-quinone methides under visible light irradiation at room temperature. Diverse diarylmethyl alkylsulfones are produced in moderate to good yields. This reaction works well under photocatalysis with a broad substrate scope by using DABCO·(SO2)2 as the source of sulfur dioxide. Mechanistic study shows that this transformation is initiated by alkyl radicals generated in situ from potassium alkyltrifluoroborates in the presence of photocatalyst. The subsequent insertion of sulfur dioxide and radical 1, 6-addition of para-quinone methides with alkylsulfonyl radical intermediates afford the corresponding diarylmethyl alkylsulfones.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (Nos. 22007017 and 21871053), the Talents' Start-up Fund of Gannan Medical University (No. QD201831), the Education Bureau of Jiangxi Province (No. GJJ190799), the Research Fund of Gannan Medical University (No. ZD201905), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (No. 2020ZD04) is gratefully acknowledged. We also thank Prof. Pornchai Rojsitthisak in Chulalongkorn University for English review.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.007.
| [1] |
(a) M.R. Prinsep, J.W. Blunt, M.H.G. Munro, J. Nat. Prod. 54 (1991) 1068-1076; (b) M. Teall, P. Oakley, T. Harrison, et al., Bioorg. Med. Chem. Lett. 15 (2005) 2685-2688; (c) L. Legros, J.R. Dehli, C. Bolm, Adv. Synth. Catal. 347 (2005) 19-31; (d) I. Churcher, D. Beher, J.D. Best, et al., Bioorg. Med. Chem. Lett. 16 (2006) 280-284; (e) Y. Harrak, G. Casula, J. Basset, et al., J. Med. Chem. 53 (2010) 6560-6571. |
| [2] |
N.S. Simpkins, Sulfones in Organic Synthesis. Oxford: Pergamon Press, 1993.
|
| [3] |
(a) J. Zhou, M.L. Wang, X. Gao, G.F. Jiang, Y.G. Zhou, Chem. Commun. 53 (2017) 3531-3534; (b) M. Nambo, Z.T. Ariki, D. Canseco-Gonzalez, D.D. Beattie, C.M. Crudden, Org. Lett. 18 (2016) 2339-2342; (c) M. Nambo, C.M. Crudden, Angew. Chem. Int. Ed. 53 (2014) 742-746. |
| [4] |
(a) D.F. Duxbury, Chem. Rev. 93 (1993) 381-433; (b) M.S. Shchepinov, V.A. Korshun, Chem. Soc. Rev. 32 (2003) 170-180; (c) V. Nair, S. Thomas, S.C. Mathew, K.G. Abhilash, Tetrahedron 62 (2006) 6731-6747. |
| [5] |
(a) P. Thirupathi, S.S. Kim, Eur. J. Org. Chem. 2010 (2010) 1798-1808; (b) J.L. Zhao, S.H. Guo, J. Qiu, et al., Tetrahedron Lett 57 (2016) 2375-2378; (c) R.R. Kuchukulla, F. Li, Z. He, L. Zhou, Q. Zeng, Green Chem 21 (2019) 5808-5812. |
| [6] |
(a) M.A. Reddy, P.S. Reddy, B. Sreedhar, Adv. Synth. Catal. 352 (2010) 1861-1869; (b) K. Xu, L. Li, W. Yan, et al., Green Chem 19 (2017) 4494-4497; (c) H. Yamamoto, K. Nakata, Eur. J. Org. Chem. 2019 (2019) 4906-4910. |
| [7] |
(a) B. Zheng, T. Jia, P.J. Walsh, Org. Lett. 15 (2013) 1690-1693; (b) M. Nambo, C.M. Crudden, Angew. Chem. Int. Ed. 53 (2014) 742-746. |
| [8] |
(a) T. Liu, J. Liu, S. Xia, et al., ACS Omega 3 (2018) 1409-1415; (b) X.Y. Guan, L.D. Zhang, P.S. You, S.S. Liu, Z.Q. Liu, Tetrahedron Lett 60 (2019) 244-247; (c) Z.Q. Liu, P.S. You, L.D. Zhang, et al., Molecules 25 (2020) 539-553. |
| [9] |
(a) M.G. Peter, 1989 Angew. Chem. Int. Ed., 28555-570; (b) H.U. Wagner, R. Gompper, Quinone Methides, in S. Patai, Z. Rappport (Eds. ), The Chemistry of Quinonoid Compounds, Wiley, New York, 1974, pp. 1145-1178; (c) A.B.Q. Turner, 1964 Q. Rev. Chem. Soc., 18347-360; (d) D.J. Hart, P.A. Cain, D.A. Evans, 1978 J. Am. Chem. Soc., 1001548-1557. |
| [10] |
(a) V. Reddy, R. Vijaya Anand, Org. Lett. 17 (2015) 3390-3393; (b) F.S. He, J.H. Jin, Z.T. Yang, et al., ACS Catal 6 (2016) 652-656; (c) K. Zhao, Y. Zhi, A. Wang, D. Enders, ACS Catal 6 (2016) 657-660; (d) N. Dong, Z.P. Zhang, X.S. Xue, X. Li, J.P. Cheng, Angew. Chem. Int. Ed. 55 (2016) 1460-1464; (e) Y.H. Deng, X.Z. Zhang, K.Y. Yu, et al., Chem. Commun. 52 (2016) 4183-4186; (f) Z.P. Zhang, N. Dong, X. Li, Chem. Commun. 53 (2017) 1301-1304; (g) B.M. Sharma, D.R. Shinde, R. Jain, et al., Org. Lett. 20 (2018) 2787-2791; (h) Z. Liu, H. Xu, T. Yao, J. Zhang, L. Liu, Org. Lett. 21 (2019) 7539-7543; (i) G.M. Nan, X. Li, T.Y. Yao, et al., Org. Biomol. Chem. 18 (2020) 1780-1784; (j) J.Y. Wang, W.J. Hao, S.J. Tu, B. Jiang, Org. Chem. Front. 7 (2020) 1743-1778. |
| [11] |
(a) M. Ke, Q. Song, Adv. Synth. Catal. 359 (2017) 384-389; (b) Q.Y. Wu, Q.Q. Min, G.Z. Ao, F. Liu, Org. Biomol. Chem. 16 (2018) 6391-6394; (c) Q.Y. Wu, G.Z. Ao, F. Liu, Org. Chem. Front. 5 (2018) 2061-2064; (d) W. Zhang, C. Yang, Z.P. Zhang, X. Li, J.P. Cheng, Org. Lett. 21 (2019) 4137-4142; (e) H.D. Zuo, W.J. Hao, C.F. Zhu, et al., Org. Lett. 22 (2020) 4471-4477. |
| [12] |
(a) P. Bisseret, N. Blanchard, 2013 Org. Biomol. Chem., 115393-5398; (b) A.S. Deeming, E.J. Emmett, C.S. Richards-Taylor, M.C. Willis, 2014 Synthesis (Mass), 462701-2710; (c) G. Liu, C. Fan, J. Wu, 2015 Org. Biomol. Chem., 131592-1599; (d) E.J. Emmett, M.C. Willis, 2015 Asian J. Org. Chem., 4602-611; (e) D. Zheng, J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017; (f) G. Qiu, K. Zhou, L. Gao, J. Wu, 2018 Org. Chem. Front., 5691-705; (g) K. Hofman, N.W. Liu, G. Manolikakes, 2018 Chem. Eur. J., 2411852-11863; (h) G. Qiu, L. Lai, J. Cheng, J. Wu, 2018 Chem. Commun., 5410405-10414; (i) G. Qiu, K. Zhou, J. Wu, 2018 Chem. Commun., 5412561-12569; (j) S. Ye, G. Qiu, J. Wu, 2019 Chem. Commun., 551013-1019; (k) S. Ye, M. Yang, J. Wu, 2020 Chem. Commun., 564145-4155; (l) S. Ye, X. Li, W. Xie, J. Wu, 2020 Eur. J. Org. Chem. 1274-1287. |
| [13] |
(a) X. Wang, L. Xue, Z. Wang, Org. Lett. 16 (2014) 4056-4058; (b) N.W. Liu, S. Liang, G. Manolikakes, Adv. Synth. Catal. 359 (2017) 1308-1319; (c) H. Wang, S. Sun, J. Cheng, Org. Lett. 19 (2017) 5844-5847; (d) N. von Wolff, J. Char, X. Frogneux, T. Cantat, Angew. Chem. Int. Ed. 56 (2017) 5616-5619; (e) J. Sheng, Y. Li, G. Qiu, Org. Chem. Front. 4 (2017) 95-100; (f) Y. Wang, L. Deng, J. Zhou, et al., Adv. Synth. Catal. 360 (2018) 1060-1065; (g) A.L. Tribby, I. Rodríguez, S. Shariffudin, N.D. Ball, J. Org. Chem. 82 (2017) 2294-2299. |
| [14] |
(a) D. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55 (2016) 11925-11929; (b) J. Zhang, Y. An, J. Wu, Chem. Eur. J. 23 (2017) 9477-9480; (c) T. Liu, D. Zheng, Z. Li, J. Wu, Adv. Synth. Catal. 359 (2017) 2653-2659; (d) Y. An, J. Wu, Org. Lett. 19 (2017) 6028-6031; (e) T. Liu, D. Zheng, Y. Ding, X. Fan, J. Wu, Chem. Asian J. 12 (2017) 465-469; (f) Y. An, J. Zhang, H. Xia, J. Wu, Org. Chem. Front. 4 (2017) 1318-1321; (g) X. Wang, T. Liu, Q. Zhong, J. Wu, Org. Chem. Front. 4 (2017) 2455-2458; (h) T. Liu, D. Zheng, J. Wu, Org. Chem. Front. 4 (2017) 1079-1083; (i) J. Zhang, K. Zhou, J. Wu, Org. Chem. Front. 5 (2018) 813-816; (j) T. Liu, D. Zheng, Z. Li, J. Wu, Adv. Synth. Catal. 360 (2018) 865-869; (k) J. Zhang, F. Zhang, L. Lai, et al., Chem. Commun. 54 (2018) 3891-3894; (l) Zhou K., J. Zhang, G. Qiu, J. Wu, Org. Lett. 21 (2019) 275-278; (m) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867; (n) X. Wang, Y. Lin, J.B. Liu, et al., Chin. J. Chem. 38 (2020) 1098-1102; (o) F.S. He, Y. Yao, W. Xie, J. Wu, Chem. Commun. 56 (2020) 9469-9472; (p) T. Zhu, J. Wu, Org. Lett. 22(18) (2020) 7094-7097; (q) J. Huang, F. Ding, Z. Chen, G. Yang, J. Wu, Org. Chem. Front. 8 (2021) 1461-1465; (r) Y. Yao, Z. Yin, F.S. He, et al., Chem. Commun. 57 (2021) 2883-2886. |
| [15] |
(a) K. Zhou, J. Huang, J. Wu, G. Qiu, Chin. Chem. Lett. 32 (2021) 37-39; (b) T. Liu, Y. Ding, X. Fan, J. Wu, Org. Chem. Front. 5 (2018) 3153-3157; (c) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8 (2019) 893-898; (d) X. Gong, M. Yang, J.B. Liu, F.S. He, J. Wu, Org. Chem. Front. 7 (2020) 938-943. |
| [16] |
(a) X. Wang, Y. Kuang, S. Ye, J. Wu, Chem. Commun. 55 (2019) 14962-14964; (b) T. Zhu, J. Shen, Y. Sun, J. Wu, Chem. Commun. 57 (2021) 915-918. |
| [17] |
(a) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013; (b) X. Wang, H. Li, G. Qiu, J. Wu, Chem. Commun. 55 (2019) 2062-2065; (c) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013; (d) X. Gong, M. Yang, J.B. Liu, et al., Green Chem 22 (2020) 1906-1910. |
| [18] |
(a) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938; (b) F.S. He, M. Yang, S. Ye, J. Wu, Chin. Chem. Lett. 32 (2021) 461-464; (c) K. Zhou, J. Chen, J. Wu, Chin. Chem. Lett. 31 (2020) 2996-2998; (d) J. Chen, L. Wu, J. Wu, Chin. Chem. Lett. 31 (2020) 2993-2995. |
| [19] |
(a) D. Richter, N. Hampel, T. Singer, A.R. Ofial, H. Mayr, Eur. J. Org. Chem. (2009) 3203-3211; (b) Y.J. Fan, L. Zhou, S. Li, Org. Chem. Front. 5 (2018) 1820-1824. |
| [20] |
K.K. Chauhan, C.G. Frost, I. Love, D. Waite, Synlett 10 (1999) 1743-1744. |
| [21] |
P. Goswami, S. Sharma, G. Singh, R.V. Anand, J. Org. Chem. 83 (2018) 4213-4220. |
 2021, Vol. 32
2021, Vol. 32