b Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
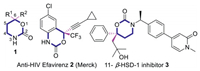
Nitrogen-containing heterocycles are abundant structural motifs in a large number of alkaloids [1], drug molecules [2] and biologically active substances [3]. Although significant accomplishments have been achieved towards the synthesis of various nitrogen heterocyclic compounds in the past few decades [4], modern organic synthesis still demands more efficient and divergent methodologies to access privileged motifs of biological active compounds [5]. As a prime instance, 1, 3-oxazinan-2-ones are not only a core scaffold within natural products [6] and pharmacologically interesting molecules [7], but also widely utilized as key intermediates [8] in the synthesis of drugs [8c, 8f] and bioactive natural products [7b, 8g]. Numerous synthetic approaches were reported to access various substituted 1, 3-oxazinan-2-ones, including halonium-mediated [6c, 9] or metal-catalyzed cyclization [10], intramolecular Michael addition of functionalized homoallylamines/homoallylic alcohols [11], allylic C-H amination [12], and tethered aminohydroxylation of olefins [13]. However, the effective methods to access 6, 6-disubstituted-1, 3-oxazinan-2-one 1, exemplified with the core structural unit of biologically active compounds such as anti-HIV Efavirenz 2 (Merck) [7e] and 11-β-HSD-1 inhibitor 3 [7c], are rare[14] (Fig. 1).
|
Download:
|
| Fig. 1. 6, 6-Disubstituted-1, 3-oxazinan-2-one scaffold and active compounds. | |
N-Acyliminium ions, acting as important organic synthetic intermediates, are widely used in the formation of C-C and C-heteroatom bonds [15], mostly through intermolecular addition [16] and intramolecular cyclization [17] with various nucleophilic reagents. For examples, the reactions of N-acyliminium ions with olefins could undergo Lewis acid-catalyzed intramolecular addition-cyclization to construct a series of heterocyclic skeletons (Fig. 2, Eq. 1) [18]. Intermolecular reactions of N-acyliminium ions with olefins were also reported [19]. Kobayashi achieved the ring-opening allylation of semicyclic N, O-acetals with allylic silanes (Fig. 2, Eq. 2a) [19a]. Later, Zhang developed the intermolecular coupling reaction of N-acyliminium ions with styrene (Fig. 2, Eq. 2b) [19b]. Notably, N-acyliminium ions could serve as part of electron-deficient dienes, undergoing [4 + 2] cycloaddition with various dienophiles (alkenes or alkynes) [20, 21]. For example, Yoshida established a cycloaddition process of N-acyliminium ions connecting with an alkoxycarbonyl group with alkenes to afford substituted 1, 3-oxazinan-2-one framework, but the formation of corresponding N-acyliminium dienes required anodic oxidation of α-silyl carbamate substrates (Fig. 2, Eq. 3) [20d]. On the basis of our continuous efforts in exploring chemical transformations of semicyclic N, O-acetals [22], we envisioned that such [4 + 2] cycloaddition could lead to various important units. Herein we present an efficient synthetic approach to 4, 6, 6-trisubstituted-1, 3-oxazinan-2-ones6/7/9/10 through TMSOTf-mediated [4 + 2] cycloaddition of semicyclicN, O-acetals 4 with 1, 1-disubstituted ethylenes 5/8 (Fig. 2, Eq. 4).

|
Download:
|
| Fig. 2. The intra- or intermolecular reactions of N-acyliminium ions with olefins. | |
Our investigation started with the reaction of semicyclic N, O-acetal 4b with 1, 1-diphenylethene 5a. The reaction could not take place in the absence of Lewis acid (Table 1, entry 1). Several types of iron Lewis acids could lead to only faint products (Table 1, entries 2-6). When Ni(OTf)2, Cu(OTf)2 and Sc(OTf)3 were examined, no product was observed (Table 1, entries 7-9). SnCl4 could afford the desired product 7ba in 34% yield (Table 1, entry 10). Slight improvements in yields were achieved when TiCl4 and BF3˙Et2O were applied, and the desired product 7ba could be obtained in moderate yield (48% and 66% respectively, Table 1, entries 11 and 12). Delightfully, TMSOTf could significantly increase the yield of 7ba to 81% (Table 1, entry 13). It was worth noting that the reaction was conducted at -78 ℃. Either increasing or decreasing the loading of TMSOTf resulted in slight drop of the reaction yield (Table 1, entries 14 and 15). The reaction could also afford the desired product using THF and PhMe as solvents, but the corresponding yields were lower compared with that in dichloromethane (Table 1, entries 16 and 17).
|
|
Table 1 Optimization of reaction conditions.a |
With the above identified optimized reaction conditions, the olefin substrates 5b-5j with different electronic properties were examined and the results were summarized in Scheme 1. First, neither hex-1-ene 5b nor cyclohexene 5c could afford any desired product in the presence of TMSOTf. The dienophiles with electron-withdrawing groups, methyl acrylate 5d and (E)-4-phenylbut-3-en-2-one 5e, led to complicated reaction mixtures. Delightfully, styrene 5f and its derivatives (5g and 5h) could generate the desired products 6af, 7bf-7bh in moderate yields and diastereoselectivities. 2-Vinylnaphthalene could also react with 4a and 4b to give the corresponding products 6ai and 7bi in moderate yields and diastereoselectivities. It was worth mentioning that a complex result was obtained when (E)-1, 2-diphenylethene 5j was investigated, probably due to the steric effect during the intermolecular [4 + 2] cycloaddition process.

|
Download:
|
| Scheme 1. The reactions of semicyclic N, O-acetals 4a/4b with olefins 5b-5j. The reactions were performed with N, O-acetals 4 (0.5 mmol), olefins 5b-5j (1.0 mmol), and TMSOTf (1.0 mmol) in dry DCM (2 mL) at -78 ℃ for 5-10 h, isolated yield. dr was determined by HPLC or NMR of crude products. a 7bf (2.80 g, 51% yield) was obtained with 4b (22.0 mmol), 5f (44.0 mmol), and TMSOTf (44.0 mmol) in dry DCM (50 mL) at −78 ℃ for 8 h. | |
Next, we turned to investigate the scope and limitation of such addition-cyclization of semicyclic N, O-acetal (4a or 4b) with 1, 1-disubstituted ethylenes 5a, 5k-5v (Scheme 2). When prop-1-en-2-ylbenzene 5k was explored, desired products 6ak, 7bk were obtained in moderate yields and excellent diastereoselectivities. 4-Chloro substitution at phenyl ring (5l) led to slight decrease in yields of 6al, 7bl, but with excellent diastereoselectivities (dr up to 99:1). Replacement of the methyl group of 5k with other alkyl substitutions (5q: n-butyl and 5v: isopropyl) was tolerated, and the desired products 6aq, 7bq, 7bv were afforded in moderate yields under the optimized conditions. Although the n-butyl substituted products 6aq, 7bq showed moderate diastereoselectivities, the isopropyl substituted product 7bv was obtained with excellent diastereoselectivities. In addition, a series of diaryl substituted alkenes were surveyed under the optimized conditions. In general, all these substituted alkenes (5a, 5l-5o) could react with semicyclic N, O-acetals 4a and 4b, affording the desired products 6aa, 6am-6ao, 7ba, 7bm, 7bp in moderate yields. Several benzyl and phenyl olefins 5r-5t were also screened, most of them could give the desired products 6ar-6at, 7br-7bt in excellent yields and diastereoselectivities, except for the p-methoxyphenyl substituted olefin 5r. Substituted olefin 5u containing phenyl and phenethyl could also afford the desired products 6au and 7bu in excellent yields with moderate diastereoselectivities. The methyl and butyl substituted ethylene 5w could also react with N, O-acetal 4b to afford the desired product 7bw in 40% yield, but the diastereoselectivities was lower than those of aryl olefins. The chemical structures of 6aa, 6ak-6au, 7ba, 7bk-7bw were unambiguously confirmed based on the X-ray crystallographic analysis of compound 7bt (see Supporting information for detail).

|
Download:
|
| Scheme 2. The reactions of semicyclic N, O-acetals 4a/4b with substituted olefins 5a, 5k-5w. The reactions were performed with N, O-acetal 4 (0.5 mmol), olefins (0.75 mmol), and TMSOTf (1.0 mmol) in dry DCM (2 mL) at -78 ℃ for 5-10 h. Isolated yield. dr was determined by HPLC or NMR of crude products. | |
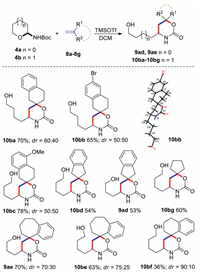
Next, we turned our attention to investigate the reaction of semicyclic N, O-acetal 4a or 4b with exocyclic olefins 8a-8g, aiming for the formation of 1, 3-oxazinan-2-ones containing a spiro quaternary carbon (Scheme 3). The reaction of 2-methylene-1, 2, 3, 4-tetrahydronaphthalene 8a with semicyclic N, O-acetal 4b afforded the desired product 10ba in 70% yield. The 6-bromo substituted olefin 8b led to 10bb in slightly lower yield of 65%, while the 5-methoxy substituted olefin 8c could generate 10bc in slightly higher yield of 78%. However, the diastereoselectivities of 10ba-10bc were low. The symmetric olefin, 2-methylene-2, 3-dihydro-1H-indene 8d, also worked well with semicyclic N, O-acetals 4a and 4b, affording the desired products 9ad and 10bd in moderate yields. Notably, a simple exocyclic olefin methylenecyclopentane 8g also worked well, and the corresponding product 10bg was obtained in 60% yield. Regarding olefin substrates with the exo-double bond adjacent to the phenyl ring, 5-methylene-6, 7, 8, 9-tetrahydro-5H-benzo[7]annulene 8e bearing a fused seven-membered ring could lead to the corresponding products in higher yields than that of 1-methylene-1, 2, 3, 4-tetrahydronaphthalene 8f bearing a fused six-membered ring. In detail, the desired products 9ae and 10be were obtained in 70% and 63% yields, while the yield of 10bf was only 36%. The diastereoselectivities of 9ae, 10be and 10bf were increased slightly, maybe due to the steric hindrance. The structures of 9ad, 9ae, 10ba-10bg were unambiguously confirmed based on the X-ray crystallographic analysis of compound10bb (see Supporting information for detail).
|
Download:
|
| Scheme 3. The reactions of semicyclic N, O-acetals 4a/4b with substituted olefins 8a-8g. The reactions were performed with N, O-acetal 4 (0.5 mmol), olefins (0.75 mmol), and TMSOTf (1.0 mmol) in dry DCM (2 mL) at -78 ℃ for 5-10 h. Isolated yield. dr was determined by HPLC or NMR of crude products. | |
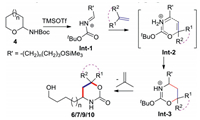
A possible mechanism for this TMSOTf-mediated [4 + 2] cycloaddition process is presented in Fig. 3 [19a, 20d, 20f]. When semicyclic N, O-acetals 4 reacted with alkenes 5, diene type of N-acyliminium ions Int-1 was first generated under Lewis acid conditions. The subsequent reaction with alkenes 5 gave a six-membered intermediate Int-2, which would define the stereochemical outcome and give Int-3. Upon the cleavage of t-butyl group, the corresponding cycloadducts 6/7/9/10 were produced, along with the release of 2-methylprop-1-ene.
|
Download:
|
| Fig. 3. Possible mechanism for the TMSOTf-mediated [4 + 2] cycloaddition process. | |
Finally, we focused on the utility of this intermolecular [4 + 2] process of N-acyliminium ions with alkenes in the synthesis of biologically active molecules. Scheme 4 showed a facile synthesis of norallosedamine 12. As a natural product, norallosedamine 12 was isolated from both the Sedum and Lobelia inflata plant family, and have attracted great interest in synthetic chemistry [23]. Starting from the cycloadduct 7bf, Dess-Martin oxidation (DMP) and subsequent reductive amination (Et3SiH/TMSOTf) could produce bicyclic pyrido [1,2-c][1,3]oxazin-1-one 11 in 75% overall yield. Then the ring opening (KOH) of 11 resulted in (±) norallosedamine 12 in 86% yield (dr = 94:6). The spectroscopic and physical data of the synthetic (±)-norallosedamine 12 were identical to the reported data [23a]. Norallosedamine 12 could be potentially converted to other alkaloids of its family by known process [23b, 23d].

|
Download:
|
| Scheme 4. Synthesis of (±)-Norallosedamine 12. Reagents and conditions: (a) i, DMP, DCM, r.t., 3 h; ii, Et3SiH/TMSOTf, MeCN, 0 ℃ to r.t., 40 min, 75% (2 steps); (b) t-BuOH/toluene (v/v = 1/1), KOH, 85 ℃, 30 min, 86%. | |
In summary, we established a novel and efficient approach for the synthesis of 4, 6-disubstituted- and 4, 6, 6-trisubstituted-1, 3-oxazinan-2-ones 6aa, 6af-6au, 7ba, 7bf-7bw and 6, 6-spiro containing 1, 3-oxazinan-2-ones 9ad, 9ae, 10ba-10bg. The Lewis acid TMSOTf could activate semicyclic N, O-acetals (4a and 4b), and the resulting N-alkoxycarbonyliminium ions readily underwent a [4 + 2] cycloaddition process with 1, 1-disubstituted ethylenes5a, 5k-5w and 8a-8g. The corresponding products were obtained in moderate to excellent yields and diastereoselectivities. In addition, the utility of this methodology was demonstrated by the facile synthesis of natural product (±)-norallosedamine12 from the cycloadduct 7bf.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the National Natural Science Foundation of China (No. 21772027 to B.-G. Wei and 21702032 to C.-M. Si) for financial support. The authors also thank Dr. Han-Qing Dong (Arvinas, Inc.) for helpful suggestions.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.003.
| [1] |
(a) C. Bian, J. Wang, X. Zhou, W. Wu, R. Guo, Chem. Biodiversity 17 (2020). e2000186; (b) T.P. Cushnie, B. Cushnie, A.J. Lamb, Int. J. Antimicrob. Agents 44 (2014) 377-386; (c) T.B. Hua, C. Xiao, Q.Q. Yang, J.R. Chen, Chin. Chem. Lett. 31 (2020) 311-323; (d) X. Liang, X.Z. Yang, L. Chen, et al., Med. Chem. Res. 30 (2021) 1-14; (e) X.D. Wu, X.N. Li, L.Y. Peng, Q.S. Zhao, J. Org. Chem. 85 (2020) 6803-6807; (f) D.D. Zhang, J.B. Xu, Y.Y. Fan, et al., J. Org. Chem. 85 (2020) 3742-3747. |
| [2] |
(a) N. Kerru, L. Gummidi, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Molecules 25 (2020) 1909; (b) A.M. Malebari, D. Fayne, S.M. Nathwani, et al., Eur. J. Med. Chem. 189 (2020) 112050; (c) M. Baiula, P. Galletti, G. Martelli, et al., J. Med. Chem. 59 (2016) 9721-9742. |
| [3] |
(a) A.A. Aly, A.A. Hassan, M.M. Makhlouf, S. Braese, Molecules 25 (2020) 3036; (b) A.M.S. El-Sharief, Y.A. Ammar, A. Belal, et al., Bioorg. Chem. 85 (2019) 399-412; (c) A.A. Ivashchenko, Y.A. Ivanenkov, A.G. Koryakova, et al., Eur. J. Med. Chem. 189 (2020) 112064; (d) S. Zhao, C. Pi, Y. Ye, L. Zhao, Y. Wei, Eur. J. Med. Chem. 180 (2019) 524-535. |
| [4] |
(a) P. Jain, P. Verma, G. Xia, J.Q. Yu, Nat. Chem. 9 (2017) 140-144; (b) P. Majumdar, A. Pati, M. Patra, R.K. Behera, A.K. Behera, Chem. Rev. 114 (2014) 2942-2977; (c) P. Singh, R.K. Varshnaya, R. Dey, P. Banerjee, Adv. Synth. Catal. 362 (2020) 1447-1484; (d) H. Matsuzaki, N. Takeda, M. Yasui, et al., Org. Lett. 22 (2020) 9249-9252; (e) Y. He, J. Yang, Q. Liu, X. Zhang, X. Fan, J. Org. Chem. 85 (2020) 15600-15609. |
| [5] |
(a) L. Li, Z. Chen, X. Zhang, Y. Jia, Chem. Rev. 118 (2018) 3752-3832; (b) S. Xu, H.M. Holst, S.B. McGuire, N.J. Race, J. Am. Chem. Soc. 142 (2020) 8090-8096. |
| [6] |
(a) G.M. Larson, B.T. Schaneberg, A.T. Sneden, J. Nat. Prod. 62 (1999) 361-363; (b) F. Taft, K. Harmrolfs, I. Nickeleit, et al., Chem. Eur. J. 18 (2012) 880-886; (c) R.B. Woodward, B.W. Au-Yeung, P. Balaram, et al., J. Am. Chem. Soc. 103 (1981) 3213-3215; (d) J.M. Cassady, K.K. Chan, H.G. Floss, E. Leistner, Chem. Pharm. Bull. 52 (2004) 1-26. |
| [7] |
(a) W. Yang, Y. Wang, A. Lai, et al., J. Med. Chem. 63 (2020) 7226-7242; (b) Z. Xu, C.M. Tice, W. Zhao, et al., J. Med. Chem. 54 (2011) 6050-6062; (c) Y. Zhang, J.P. Wu, G. Li, et al., J. Org. Chem. 81 (2016) 2665-2669; (d) L. Zhuang, C.M. Tice, Z. Xu, et al., Bioorg. Med. Chem. 25 (2017) 3649-3657; (e) S.D. Young, S.F. Britcher, L.O. Tran, et al., Antimicrob. Agents Chemother. 39 (1995) 2602-2605. |
| [8] |
(a) S.G. Davies, A.C. Garner, P.M. Roberts, et al., Org. Biomol. Chem. 4 (2006) 2753-2768; (b) J.R. Ella-Menye, V. Sharma, G. Wang, J. Org. Chem. 70 (2005) 463-469; (c) J.W. Hilborn, Z.H. Lu, A.R. Jurgens, et al., Tetrahedron Lett 42 (2001) 8919-8921; (d) G.S. Poindexter, K.M. Strauss, Synth. Commun. 23 (1993) 1329-1338; (e) S.C. Schmid, I.A. Guzei, J.M. Schomaker, Angew. Chem. Int. Ed. 56 (2017) 12229-12233; (f) M. Schulze, Synth. Commun. 40 (2010) 3415-3422; (g) Y.F. Wang, T. Izawa, S. Kobayashi, M. Ohno, J. Am. Chem. Soc. 104 (1982) 6465-6466. |
| [9] |
(a) H. Pan, H. Huang, W. Liu, H. Tian, Y. Shi, Org. Lett. 18 (2016) 896-899; (b) S. Mangelinckx, Y. Nural, H.A. Dondas, et al., Tetrahedron 66 (2010) 4115-4124. |
| [10] |
(a) U. Jakob, W. Bannwarth, Tetrahedron Lett 56 (2015) 6340-6344; (b) R. Robles-Machín, J. Adrio, J.C. Carretero, J. Org. Chem. 71 (2006) 5023-5026. |
| [11] |
(a) K. Narasaka, Y. Ukaji, S. Yamazaki, Bull. Chem. Soc. Jpn. 59 (1986) 525-533; (b) M. Hirama, T. Shigemoto, Y. Yamazaki, S. Ito, J. Am. Chem. Soc. 107 (1985) 1797-1798; (c) A.J. Borah, P. Phukan, J. Chem. Sci. 125 (2013) 1503-1510. |
| [12] |
S.A. Reed, A.R. Mazzotti, M.C. White, J. Am. Chem. Soc. 131 (2009) 11701-11706. DOI:10.1021/ja903939k |
| [13] |
T.J. Donohoe, C.J.R. Bataille, W. Gattrell, J. Kloesges, E. Rossignol, Org. Lett. 9 (2007) 1725-1728. DOI:10.1021/ol070430v |
| [14] |
(a) A.J. Boddy, C.J. Cordier, K. Goldberg, et al., Org. Lett. 21 (2019) 1818-1822; (b) T. Buyck, Q. Wang, J. Zhu, J. Am. Chem. Soc. 136 (2014) 11524-11528; (c) N. Uddin, J.S. Ulicki, F.H. Foersterling, M.M. Hossain, Tetrahedron Lett 52 (2011) 4353-4356; (d) R. Yousefi, T.J. Struble, J.L. Payne, et al., J. Am. Chem. Soc. 141 (2019) 618-625. |
| [15] |
(a) A.M. Jones, C.E. Banks, Beilstein J. Org. Chem. 10 (2014) 3056-3072; (b) U. Martínez-Estibalez, A. Gómez-SanJuan, O. García-Calvo, et al., Eur. J. Org. Chem. 2011 (2011) 3610-3633; (c) J. Royer, M. Bonin, L. Micouin, Chem. Rev. 104 (2004) 2311-2352; (d) M.G. Vinogradov, O.V. Turova, S.G. Zlotin, Russ. Chem. Rev. 86 (2017) 1-17; (e) A.K. Sahu, R. Unnava, S. Shit, A.K. Saikia, J. Org. Chem. 85 (2020) 1961-1971. |
| [16] |
(a) H.E. Zaugg, Synthesis (1970) 49-73; (b) H.E. Zaugg, Synthesis (1984) 85-110; (c) S. Pyne, A. Yazici, Synthesis 3 (2009) 339-368; (d) A. Yazici, S.G. Pyne, Synthesis 4 (2009) 513-541; (e) G.M. Ryder, U. Wille, A.C. Willis, S.G. Pyne, Org. Biomol. Chem. 17 (2019) 7025-7035; (f) T. Thaima, A. Yazici, C. Auranwiwat, et al., Org. Biomol. Chem. 19 (2021) 259-272. |
| [17] |
P. Wu, T.E. Nielsen, Chem. Rev. 117 (2017) 7811-7856. DOI:10.1021/acs.chemrev.6b00806 |
| [18] |
(a) M. Das, A.K. Saikia, J. Org. Chem. 83 (2018) 6178-6185; (b) K. Indukuri, R. Unnava, M.J. Deka, A.K. Saikia, J. Org. Chem. 78 (2013) 10629-10641; (c) Y. Krishna, K. Shilpa, F. Tanaka, Org. Lett. 21 (2019) 8444-8448; (d) Y. Zheng, L. Andna, O. Bistri, L. Miesch, Org. Lett. 22 (2020) 6771-6775; (e) S. Hanessian, M. Tremblay, Org. Lett. 6 (2004) 4683-4686; (f) S. Hanessian, M. Tremblay, J.F.W. Petersen, J. Am. Chem. Soc. 126 (2004) 6064-6071. |
| [19] |
(a) M. Sugiura, H. Hagio, R. Hirabayashi, S. Kobayashi, Synlett 2001 (2001) 1225-1228; (b) N. Lu, L. Wang, Z. Li, W. Zhang, Beilstein J. Org. Chem. 8 (2012) 192-200; (c) H. Richter, R. Frohlich, C.G. Daniliuc, O. Garcia Mancheno, Angew. Chem., Int. Ed. 51 (2012) 8656-8660; (d) A. Yazici, U. Wille, S.G. Pyne, J. Org. Chem. 81 (2016) 1434-1449. |
| [20] |
(a) S.M. Weinreb, P.M. Scola, Chem. Rev. 89 (1989) 1525-1534; (b) T. Shimizu, K. Tanino, I. Kuwajima, Tetrahedron Lett 41 (2000) 5715-5718; (c) D.M. Tomazela, L.A.B. Moraes, R.A. Pilli, M.N. Eberlin, M.G.M. D'Oca, J. Org. Chem. 67 (2002) 4652-4658; (d) S. Suga, Y. Tsutsui, A. Nagaki, J.I. Yoshida, Bull. Chem. Soc. Jpn. 78 (2005) 1206-1217; (e) S. Suga, D. Yamada, J. -i. Yoshida, Chem. Lett. 39 (2010) 404-406; (f) S. Suga, A. Nagaki, Y. Tsutsui, J.I. Yoshida, Org. Lett. 5 (2003) 945-947. |
| [21] |
(a) P.M. Esch, H. Hiemstra, W.N. Speckamp, Tetrahedron Lett 29 (1988) 367-370; (b) P.M. Esch, H. Hiemstra, W. Nico Speckamp, Tetrahedron 48 (1992) 3445-3462. |
| [22] |
(a) X.M. Wang, Y.W. Liu, R.J. Ma, C.M. Si, B.G. Wei, J. Org. Chem. 84 (2019) 11261-11267; (b) C. Wang, Z.Y. Mao, Y.W. Liu, et al., Adv. Synth. Catal. 362 (2020) 822-831; (c) R.C. Liu, W. Huang, J.Y. Ma, B.G. Wei, G.Q. Lin, Tetrahedron Lett 50 (2009) 4046-4049; (d) Y.W. Liu, R.J. Ma, J.H. Yan, Z. Zhou, B.G. Wei, Org. Biomol. Chem. 16 (2018) 771-779; (e) P. Han, Z.Y. Mao, C.M. Si, et al., J. Org. Chem. 84 (2019) 914-923; (f) Z.D. Chen, Z. Chen, Q.E. Wang, C.M. Si, B.G. Wei, Tetrahedron Lett 61 (2020) 152051; (g) X.L. Han, X.D. Nie, Z.D. Chen, et al., J. Org. Chem. 85 (2020) 13567-13578; (h) Y.X. Zhang, L.Y. Chen, J.T. Sun, C.M. Si, B.G. Wei, J. Org. Chem. 85 (2020) 12603-12613. |
| [23] |
(a) B.A.D. Neto, A.A.M. Lapis, A.B. Bernd, D. Russowsky, Tetrahedron 65 (2009) 2484-2496; (b) R.A. Pilli, L.C. Dias, Synth. Commun. 21 (1991) 2213-2229; (c) B.V. Subba Reddy, S. Ghanty, N.S.S. Reddy, Y.J. Reddy, J.S. Yadav, Synth. Commun. 44 (2014) 1658-1663; (d) C.Y. Yu, O. Meth-Cohn, Tetrahedron Lett 40 (1999) 6665-6668; (e) J.S. Yadav, Y. Jayasudhan Reddy, P. Adi Narayana Reddy, B.V. Subba Reddy, Org. Lett. 15 (2013) 546-549. |
 2021, Vol. 32
2021, Vol. 32 


