During the past decades, transition-metal catalyzed cascade C-H activation/annulation reaction has clearly served as an efficient method in synthetic chemistry [1-6] to construct diverse heterocycles such as isoquinolines [7], quinazolines [8], naphthalenone [9], naphthols [10] and others [11-24]. And this method has been well explored by numerous chemists because of its simple operation, fewer synthetic steps, high atom economy and high regioselectivity.
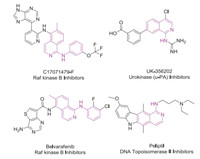
On the other hand, 1-aminoisoquinolines are frequently found in many drug molecules (Fig. 1) and exhibit a wide range of biological and pharmaceutical activities [25-31]. Therefore, straightforward strategy to access 1-aminoisoquinolines has intensively intrigued the scientists. According literatures, many methods have been reported including the coupling reaction of 1-aminoisoquinoline with phenylboronic acid [32], the transformation of 1-chloroisoquinoline with amine [33-35], and the amination between isoquinoline-N-oxide with amines [36-42], phenyl isothiocyanate [43]. Besides, benzamidines are efficient directing groups [44-53], which have been widely employed for the construction of 1-aminoisoquinolines derivatives via C-H activation/annulation adopting different C2 synthons, such as α-OMs/OTs acetophenone [54], diazo compounds [55-57], allyl carbonates [58], alkynes [59, 60] and sulfoxonium ylides (Scheme 1a) [61, 62]. However, all of these transformations only provided the aminoisquinolines with R2 or R3 (phenyl or alkyl), and the construction of nonsubstituted aminoisquinoline skeletons (R2, R3 = H) were seldom disclosed through C-H activation.
|
Download:
|
| Fig. 1. Selected examples of bioactive aminoisoquinolines. | |

|
Download:
|
| Scheme 1. Synthesis of aminoisoquinolines and other heterocycles via C-H activation/annulation. | |
Recently, vinylene carbonate, featuring higher operational security and commercial availability as acetylene surrogate, has been involved in the transition-metal-catalyzed C(sp2)-H activation reactions [63-66] by extruding a carbonate anion (Scheme 1b). In these cases, heterocyclic structures such as isoquinolinones and isocoumarins, have been successfully and efficiently synthesized utilizing vinylene carbonate as a new C2 synthon under Co(Ⅲ) or Rh(Ⅲ) catalysis with N-methoxybenzamide [67] or benzoic acid [68]. In this regard, we herein demonstrate a facile method to generate nonsubstituted aminoisoquinolines derivatives by a Rh(Ⅲ)-catalyzed cascade C-H activation/annulation reaction using benzimidamides with vinylene carbonate (Scheme 1c).
We commenced the reaction of Rh(Ⅲ)-catalyzed [4 + 2] annulation reaction with N-(tert-butyl)benzimidamide (1a) and vinylene carbonate (2a) as model substrates. To our delight, we obtained the expected aminoisoquinolines products in 20% yields by employing [Cp*RhCl2]2 (5 mol%) and AgSbF6 (20 mol%) as catalytic system in 1, 2-dichloroethane at 100 ℃ for 24 h under argon atmosphere (Table 1, entry 1). Initially, the yields of aminoisoquinolines did not increase when replacing the Rh catalyst with other metal catalysts (entries 2 and 3). Then several sliver salts were investigated, among which AgOTf gave the superior result compared with AgSbF6, AgBF4 and AgOTs (entries 4–6). In order to further increase the yield, different bases were screened including NaOAc, KPF6, K2CO3, KOPiv, and the results revealed that KOPiv was the best base for this reaction (entries 7–10). Finally, the use of toluene significantly increased the yield to 79%, which revealed solvents have a significant impact on the conversion (entries 11–13). In conclusion, the optimal conditions are shown as follows: the reaction proceeded in 79% yield under [Cp*RhCl2]2 (5 mol%) and AgOTf (20 mol%), using KOPiv (20 mol%) as additives in Tol (2.0 mL) at 100 ℃ for 24 h under Ar.
|
|
Table 1 Optimization of reaction conditions.a |
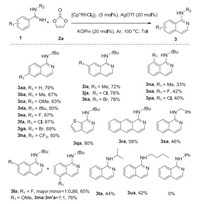
With the optimized conditions in hand, we started to investigate the scope and generality of benzamidines derivatives for the [4 + 2] annulation reactions. Generally, benzamidines bearing electron-donating (Me, OMe, t-Bu) and electron-withdrawing groups (F, Cl, Br) on the benzene ring could generate aminoisoquinolines derivatives (Scheme 2, 3aa-3ha) smoothly by reacting with vinylene carbonate in moderate to good yields. As shown in Scheme 2, it was obvious that the yields of the transformation decreased dramatically to 33%–42% for ortho-substituent substrates (3na-3pa), which indicated that the transformation was tremendously influenced by steric hindrance effect. Besides, it was interesting to note that the substrates with diverse groups at the meta position demonstrated higher efficiencies than that at the para position on the whole. In addition, meta-methyl-, meta-cholro- and meta-bromo-substituted benzamidines preferred the site with less hindrance (3ia-3ka), while meta-methoxyl- and meta-fluoro-substituted reactants gave the two regioselective products (3la-3ma). Delightfully, the product was isolated in 90% yields when reactant with a thiophene ring was used. Moreover, substituent with naphthalene was also suitable for the reaction, albeit in a 58% yield. Furthermore, when tert-butyl is replaced by other alkyl groups such as benzyl, isopropyl and n-butyl, this cascade procedure could also convert to generate corresponding products in 42%–46% yields (3sa-3ua). However, the reaction failed to work when N-phenylbenzimidamide was added.
|
Download:
|
| Scheme 2. Substrate scope of benzamidines. Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (5 mol%), AgOTf (20 mol%), KOPiv (20 mol%), Toluene (1.5 mL), Ar, 100 ℃, 24 h. | |
|
Download:
|
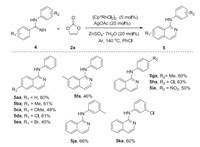
| Scheme 3. Substrate scope of N-phenylbenzimidamides. | |
Due to the inert reactivity of N-phenylbenzimidamide, a series of reaction conditions were examined for realization of the conversion by employing vinylene carbonate (see Supporting information for details). Fortunately, we obtained the expected N-phenylisoquinolin-1-amine successfully and confirmed the optimal conditions that the reaction proceeded in 60% yield with [Cp*RhCl2]2 (5 mol%), AgOAc (20 mol%) and ZnSO4·7H2O (20 mol%) as catalytic system in chlorobenzene (2.0 mL) at 140 ℃ for 24 h under Ar (Scheme 3). The electron-donating and electron-withdrawing groups were all compatible in 45%–66% yields without obvious electrical effect. Unfortunately, when the benzene ring was replaced by thiophene ring or naphthalene, we did not observe obvious products.
In order to explore whether the strategy could be applied for large scale, we enlarged the amount of 1a (2 mmol) and 2a (4 mmol) under the optimized conditions (Scheme 4). The result disclosed the transformation was less efficient in the yield of 55%.

|
Download:
|
| Scheme 4. Ten-fold-scale synthesis of aminoisoquinolines. | |
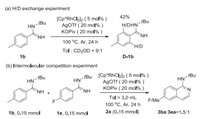
To further explore the experimental mechanism, we completed some mechanistic experiments. The H/D exchange experiment was carried out by using 1b with CD3OD at 100 ℃ for 2 h, which showed that 42% deuterium incorporation was estimated at ortho-position by 1H NMR indicating C-H bond cleavage of the transformation was reversible (Scheme 5a). When equimolar amounts of 1b and 1e were added in intermolecular competition experiment, the 3ba and 3ea were obtained in a ratio of 1.5:1, which indicated that electron-donating group was more effective (Scheme 5b).
|
Download:
|
| Scheme 5. Mechanistic studies. | |
Based on the above experiments and related literature, a plausible mechanism is presented in the Scheme 6. First, benzamidine transforms into a rhodacyclic intermediate A by C-H activation. Subsequently, the insertion of 2a with intermediate A results in the formation of seven-member intermediate B. Then, intermediate B undergoes reductive elimination to generate intermediate C releasing the Cp*Rh (I). The rhodium complexe D is obtained from oxidative addition of intermediate C and Cp*Rh (I). Finally, the rhodium complexe D generates aminoisoquinoline with the formation of the carbonate anion through β-oxygen elimination.

|
Download:
|
| Scheme 6. Plausible reaction mechanism. | |
In conclusion, we developed a novel method to construct aminoisoquinolines derivatives via Rh(Ⅲ)-catalyzed C-H activation/annulation by coupling benzamidines with vinylene carbonate as an acetylene surrogate. In this work, we realized the transformations of phenyl and alkyl-substituted benzamidines in good to moderate yields under different conditions, which was applied for larger scale.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe are grateful for the support from the National Natural Science Foundation of China (NSFC, Nos. 81602954, 81573286, 81373259 and 81773577).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.058.
| [1] |
A. Baccalini, G. Faita, G. Zanoni, D. Maiti, Chem. Eur. J. 26 (2020) 9749-9783. DOI:10.1002/chem.202001832 |
| [2] |
L. Song, E.V. Van der Eycken, Chem. Eur. J. 26 (2020) 1-25. DOI:10.1111/cwe.12349 |
| [3] |
M.R. Sk, S.S. Bera, S. Basuli, A. Metya, M.S. Maji, Asian J. Org. Chem. 9 (2020) 1701-1717. DOI:10.1002/ajoc.202000367 |
| [4] |
X. Huang, S. Liao, L. Xin, F. Zhang, Y. Yu, Synthesis 53 (2021) 238-254. DOI:10.1055/s-0040-1707268 |
| [5] |
Z. Wang, P. Xie, Y. Xia, Chin. Chem. Lett. 29 (2018) 47-53. DOI:10.11648/j.ajmsp.20180303.12 |
| [6] |
X. Han, P. Lin, Q. Li, Chin. Chem. Lett. 30 (2019) 1495-1502. DOI:10.1016/j.cclet.2019.04.027 |
| [7] |
Y.F. Wang, K.K. Toh, J.Y. Lee, S. Chiba, Angew. Chem. Int. Ed. 50 (2011) 5927-5931. DOI:10.1002/anie.201101009 |
| [8] |
R. Lai, X. Wu, S. Lv, et al., Chem. Commun. 55 (2019) 4039-4042. DOI:10.1039/c9cc01146c |
| [9] |
X. Chen, M. Wang, X. Zhang, X. Fan, Org. Lett. 21 (2019) 2541-2545. DOI:10.1021/acs.orglett.9b00340 |
| [10] |
Y. Xu, X. Yang, X. Zhou, L. Kong, X. Li, Org. Lett. 19 (2017) 4307-4310. DOI:10.1021/acs.orglett.7b01974 |
| [11] |
B. Ramesh, M. Tamizmani, M.J. Jeganmohan, J. Org. Chem. 84 (2019) 4058-4071. DOI:10.1021/acs.joc.9b00051 |
| [12] |
S.T. Mei, H.W. Liang, B. Teng, et al., Org. Lett. 18 (2016) 1088-1091. DOI:10.1021/acs.orglett.6b00197 |
| [13] |
L. Shi, B. Wang, Org. Lett. 18 (2016) 2820-2823. DOI:10.1021/acs.orglett.6b01234 |
| [14] |
R. Chen, S. Cui, Org. Lett. 19 (2017) 4002-4005. DOI:10.1021/acs.orglett.7b01728 |
| [15] |
X. Wu, H. Xiong, S. Sun, J. Cheng, Org. Lett. 20 (2018) 1396-1399. DOI:10.1021/acs.orglett.8b00119 |
| [16] |
C. Zhou, J. Zhao, W. Guo, J. Jiang, J. Wang, Org. Lett. 21 (2019) 9315-9319. DOI:10.1021/acs.orglett.9b03357 |
| [17] |
W. Chen, F.X. Liu, W. Gong, et al., Adv. Synth. Catal. 360 (2018) 2470-2475. DOI:10.1002/adsc.201800322 |
| [18] |
D. Li, N. Xu, Y. Zhang, L. Wang, Chem. Commun. 50 (2014) 14862-14865. DOI:10.1039/C4CC06457G |
| [19] |
Y. Wang, H. Zhou, K. Xu, M. Shen, H. Xu, Chin. Chem. Lett. 28 (2017) 92-96. DOI:10.1117/12.2285263 |
| [20] |
L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29 (2018) 907-910. DOI:10.1016/j.cclet.2018.05.007 |
| [21] |
H. Li, Y. Han, Z. Yang, Z. Yao, L. Wang, X. Cui, Chin. Chem. Lett. 32 (2021) 1263-1266. DOI:10.1016/j.cclet.2020.09.020 |
| [22] |
Y. Yu, Z. Xia, Q. Wu, D. Liu, L. Yu, Y. Xiao, Z. Tan, W. Deng, G. Zhu, Chin. Chem. Lett. 32 (2021) 1263-1266. DOI:10.1016/j.cclet.2020.09.020 |
| [23] |
J. Zhang, J.S. Sun, Y.Q. Xia, L. Dong, Adv. Synth. Catal. 361 (2019) 2037-2041. DOI:10.1002/adsc.201801606 |
| [24] |
K. Muralirajan, R. Kuppusamy, S. Prakash, C.H. Cheng, Adv. Synth. Catal. 358 (2016) 774-783. DOI:10.1002/adsc.201501056 |
| [25] |
C.E. Gutteridge, M.M. Hoffman, A.K. Bhattacharjee, W.K. Milhous, L. Gerena, Bioorg. Med. Chem. Lett. 21 (2011) 786-789. DOI:10.1016/j.bmcl.2010.11.099 |
| [26] |
R. Saari, J.C. Torma, T. Nevalainen, Bioorg. Med. Chem. 19 (2011) 939-950. DOI:10.1016/j.bmc.2010.11.059 |
| [27] |
A.L. Smith, F.F. DeMorin, N.A. Paras, et al., J. Med. Chem. 52 (2009) 6189-6192. DOI:10.1021/jm901081g |
| [28] |
N. Shelach, N. Lowenton-spier, Patent WO 2020165755 A1, 2020.
|
| [29] |
C. Rivalle, F. Wendling, P. Tambourin, et al., J. Med. Chem. 26 (1983) 181-185. DOI:10.1021/jm00356a012 |
| [30] |
C.G. Barber, R.P. Dickinson, P.V. Fish, Bioorg. Med. Chem. Lett. 14 (2004) 3227-3230. DOI:10.1016/j.bmcl.2004.03.094 |
| [31] |
I.H. Bae, J.B. Son, S.M. Han, et al., Patent WO 2013100632, 2013.
|
| [32] |
D.N. Rao, S. Rasheed, S. Aravinda, R.A. Vishwakarma, P. Das, RSC Adv. 3 (2013) 11472-11475. DOI:10.1039/c3ra40735g |
| [33] |
B.K. Lee, M.R. Biscoe, S.L. Buchwald, Tetrahedron Lett 50 (2009) 3672-3674. DOI:10.1016/j.tetlet.2009.03.137 |
| [34] |
C. Yang, F. Zhang, G.J. Deng, H. Gong, J. Org. Chem. 84 (2019) 181-190. DOI:10.1021/acs.joc.8b02588 |
| [35] |
X. Xie, T.Y. Zhang, Z. Zhang, J. Org. Chem. 71 (2006) 6522-6529. DOI:10.1021/jo060945k |
| [36] |
D. Kim, P. Ghosh, N.Y. Kwon, et al., J. Org. Chem. 85 (2020) 2476-2485. DOI:10.1021/acs.joc.9b03173 |
| [37] |
X. Chen, X. Li, Z. Qu, et al., Adv. Synth. Catal. 356 (2014) 1979-1985. DOI:10.1002/adsc.201301065 |
| [38] |
W.Z. Bi, K. Sun, C. Qu, et al., Org. Chem. Front. 4 (2017) 1595-1600. DOI:10.1039/C7QO00311K |
| [39] |
Y. Nanaji, S. Kirar, S.V. Pawar, A.K. Yadav, RSC Adv. 10 (2020) 7628-7634. DOI:10.1039/c9ra10397j |
| [40] |
M. Couturier, L. Caron, S. Tumidajski, K. Jones, T.D. White, Org. Lett. 8 (2006) 1929-1932. DOI:10.1021/ol060473w |
| [41] |
A.T. Londregan, S. Jennings, W. Liuqing, Org. Lett. 12 (2010) 5254-5257. DOI:10.1021/ol102301u |
| [42] |
P.J. Manley, M.T. Bilodeau, Org. Lett. 4 (2002) 3127-3129. DOI:10.1021/ol0264556 |
| [43] |
L.Y. Xie, S. Peng, L.L. Jiang, et al., Org. Chem. Front. 6 (2019) 167-171. DOI:10.1039/c8qo01128a |
| [44] |
S. Huang, H. Li, X. Sun, et al., Org. Lett. 21 (2019) 5570-5574. DOI:10.1021/acs.orglett.9b01902 |
| [45] |
Y. Li, C. Jia, H. Li, et al., Org. Lett. 20 (2018) 4930-4933. DOI:10.1021/acs.orglett.8b02057 |
| [46] |
Y. Li, Z. Qi, H. Wang, X. Yang, X. Li, Angew. Chem. Int. Ed. 55 (2016) 11877-11881. DOI:10.1002/anie.201606316 |
| [47] |
Z. Qi, S. Yu, X. Li, Org. Lett. 18 (2016) 700-703. DOI:10.1021/acs.orglett.5b03669 |
| [48] |
X. Song, Q. Zhou, J. Zhao, et al., Org. Lett. 22 (2020) 9506-9512. DOI:10.1021/acs.orglett.0c03515 |
| [49] |
F. Xu, Y. Song, W. Zhu, et al., Chem. Commun. 56 (2020) 11227-11230. DOI:10.1039/d0cc04885b |
| [50] |
L. Xu, L. Wang, Y. Feng, et al., Org. Lett. 19 (2017) 4343-4346. DOI:10.1021/acs.orglett.7b02028 |
| [51] |
J. Zhou, J. Li, Y. Li, et al., Org. Lett. 20 (2018) 7645-7649. DOI:10.1021/acs.orglett.8b03383 |
| [52] |
X. Zhou, Z. Qi, S. Yu, et al., Adv. Synth. Catal. 359 (2017) 1620-1625. DOI:10.1002/adsc.201601278 |
| [53] |
J. Ren, Y. Huang, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 32 (2021) 2592-2596. DOI:10.1016/j.cclet.2021.02.061 |
| [54] |
J. Li, Z. Zhang, M. Tang, X. Zhang, J. Jin, Org. Lett. 18 (2016) 3898-3901. DOI:10.1021/acs.orglett.6b01916 |
| [55] |
Y. Zuo, X. He, Y. Ning, Y. Wu, Y. Shang, J. Org. Chem. 83 (2018) 13463-13472. DOI:10.1021/acs.joc.8b02286 |
| [56] |
J. Li, M. Tang, L. Zang, et al., Org. Lett. 18 (2016) 2742-2745. DOI:10.1021/acs.orglett.6b01199 |
| [57] |
Q. Yang, C. Wu, J. Zhou, et al., Org. Chem. Front. 6 (2019) 393-398. DOI:10.1039/c8qo01148f |
| [58] |
C. Li, H.B. Xu, J. Zhang, M. Liu, L. Dong, Org. Biomol. Chem. 18 (2020) 1412-1416. DOI:10.1039/c9ob02553g |
| [59] |
J. Li, M. John, L. Ackermann, Chem. Eur. J. 20 (2014) 5403-5408. DOI:10.1002/chem.201304944 |
| [60] |
X. Wei, M. Zhao, Z. Du, X. Li, Org. Lett. 13 (2011) 4636-4639. DOI:10.1021/ol2018505 |
| [61] |
G. Zheng, M. Tian, Y. Xu, X. Chen, X. Li, Org. Chem. Front. 5 (2018) 998-1002. DOI:10.1039/C7QO01033H |
| [62] |
C. Wu, J. Zhou, G. He, et al., Org. Chem. Front. 6 (2019) 1183-1188. DOI:10.1039/c9qo00048h |
| [63] |
K. Ghosh, Y. Nishii, M. Miura, ACS Catal. 9 (2019) 11455-11460. DOI:10.1021/acscatal.9b04254 |
| [64] |
K. Ghosh, Y. Nishii, M. Miura, Org. Lett. 22 (2020) 3547-3550. DOI:10.1021/acs.orglett.0c00975 |
| [65] |
Y. Nishii, M. Miura, ACS Catal. 10 (2020) 9747-9757. DOI:10.1021/acscatal.0c02972 |
| [66] |
Z.H. Wang, H. Wang, H. Wang, L. Li, M.D. Zhou, Org. Lett. 23 (2021) 995-999. DOI:10.1021/acs.orglett.0c04200 |
| [67] |
X. Li, T. Huang, Y. Song, et al., Org. Lett. 22 (2020) 5925-5930. DOI:10.1021/acs.orglett.0c02016 |
| [68] |
G. Mihara, K. Ghosh, Y. Nishii, M. Miura, Org. Lett. 22 (2020) 5706-5711. DOI:10.1021/acs.orglett.0c02112 |
 2021, Vol. 32
2021, Vol. 32 


