The sodium trifluoromethanesulfinate (CF3SO2Na), also known as Langlois reagent, has been used as important source of trifluoromethyl in the synthesis of numerous CF3-functionalized molecules [1-3]. Generally, this cheap and stable reagent has found application in the trifluoromethylation reaction on a vast variety of chemical bonds, such as aryl C-H bond [4-6], alkenyl/formyl C-H bond [7-10], C-halogen bond [11], single C-C bond [12, 13], multiple carbon-carbon bond [14-17], heteroatom-heteroatom bond [18] and heteroatom-H bond [19, 20]. Alongside the classical application in trifluoromethylation reactions, discovering new synthetic application in the synthesis of different fluoro-functionalized molecules has emerged as new trend in the chemistry of this reagent. For example, the CF3SO2Na has been found to be applicable in the trifluoromethylthiolation reactions by donating "CF3S" fragment via deoxygenation [21, 22]. Several novel synthetic reactions involving CF3SO2Na as thiocarbonyl (C=S) or carbonyl source have also been recently reported [23, 24]. Notably, the extensive interest in the CF3SO2Na participated organic synthesis has also led to the observation that CF3SO2Na could act as the trimethylsulfonyl source in the synthesis of molecules functionalized with CF3SO2-substructure. For example, Bi and Anderson et al. have reported the synthesis of trifluoromethylsulfonyl functionalized β-enaminones via an aryl migration wherein the methylsulfonyl free radical was proposed to trigger the cascade transformations [25]. In addition, the synthesis of a few other different organic molecules functionalized with trifluoromethylsulfonyl structure have also been reported via the coupling of CF3SO2Na to multiple or reactive single carbon-carbon bonds [26, 27]. Furthermore, the synthesis of trifluoromethyl sulfonylated aromatics have been accomplished via the coupling of CF3SO2Na to the reactive arenediazonium tetrafluoroborates [28] or aryl iodonium salts [29]. Despite these reported works, it can also be found that the trifluoromethylsulfonylation reactions by using CF3SO2Na is yet underdeveloped [30]. Especially, the trifluoromethylsulfonylation of stable C-H bond is hardly available, conveying the high urgency for developing practical C-H trifluoromethylsulfonylation protocols with CF3SO2Na.
Over the past decades, we and others have identified the functionalization of the vinyl C-H bond in enaminones as a powerful tool in the synthesis of poly-functionalized alkenes and diverse heterocyclic scaffolds via the construction of new C-C or C-heteroatom bonds. Particularly, the intramolecular and intermolecular C-C bond forming reactions [31-34], the intermolecular C-H acyloxylation [35, 36], sulfenylation [37, 38], amination [39], thiocyanation [40-44], as well as the cascade C-H coupling transformation and annulation providing various chromone derivatives [45-50] etc. have brought new perspective to the diversity-oriented synthesis based on enaminones. The coupling of enaminones and CF3SO2Na has also been investigated, and trifluoromethylated products resulting from the enaminones' C-H trifluoromethylation are provided in the known reports [51, 52]. Interestingly, the C-H trifluoromethylsulfonylation of enaminones has not yet been realized. Herein, based on our longstanding efforts in exploring novel synthetic application of enaminones [53-57] and the inherent advantages of stable chemical bond sulfur functionalization in the synthesis of diverse sulfur-containing molecules [58-70], we report the first method on the synthesis of trifluoromethylsulfonyl enaminones via the vinyl C-H trifluoromethylsulfonylation reactions of enaminones under transition metal-free conditions by simply using molecular iodine as the oxidant.
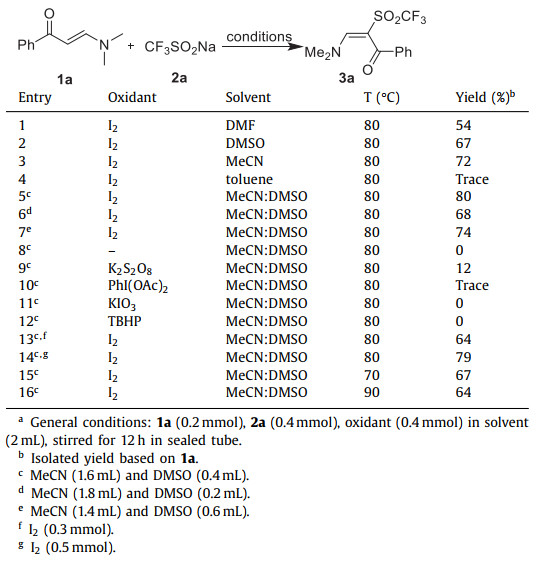
Initially, the reaction of enaminone 1a and CF3SO2Na (2a) was run in DMF in the presence of molecular iodine. Gratifyingly, the α-trifluoromethylsulfonyl functionalized enaminone 3a was afforded with fine yield by heating at 80 ℃ (entry 1). Other solvents such as DMSO, MeCN could also be used as medium for the practical formation of 3a (entries 2 and 3, Table 1). On the other hand, nonpolar medium such as toluene was found inapplicable (entry 4, Table 1). Delightfully, excellent yield was obtained when the mixed MeCN and DMSO with the volume ration of 8:2 was employed as reaction medium (entries 5–7, Table 1). The comparison of different oxidant additives and a blank entry proved that molecular iodine was the most proper oxidant for this reaction (entries 8–12, Table 1). Afterwards, the loading of molecular iodine and the reaction temperature was screened, respectively. But no further improved result on the yield of 3a was provided (entries 13–16, Table 1).
|
|
Table 1 Optimization on reaction conditions.a |
In the section of scope investigation, the reactions employing different enaminones were first conducted. According to the results (Scheme 1), outstanding application scope of the enaminone substrate was identified by fixing CF3SO2Na as the reaction partner. When the N, N-dimethyl functionalized, phenyl-based enaminones were employed, a broad variety of functional substituents in the phenyl ring, including methyl, methoxyl, halogen, cyano, trifluoromethyl and oxane etc., reacted with CF3SO2Na to provide correspondingly diverse products with fair to excellent yields under the optimized conditions (3b–3p). Substrates containing strong electron withdrawing group (EWG) in the phenyl ring of enaminones gave products with evidently lower yields (3d–3h). What's more, the enaminones featured with various amino structures such as pyrrolidine (3aa–3ai), piperidine (3aj–3al), diethylamino (3am–3an) were also used for the practical synthesis of corresponding products. Besides the phenyl groups, the successful synthesis using enaminones functionalized with other (hetero)aryl structures, such as naphthyl (3n and 3af) and thiophenyl (3o–3p and 3ai) indicated the broad tolerance of this method for enaminone substrates. More notably, the present C-H trifluoromethylsulfonylation protocol was also expanded to the reactions of β-substituted enaminone (3ao), enaminoester (3ap) as well as the enaminone derived from the natural product 16-dehydroprogesterone (3aq), confirming the high application potential of the present synthetic protocol. Interestingly, the configuration of the enaminone C=C double bond in the products was reversed as confirmed by the single crystal structures of 3a (CCDC: 2046334), 3c (CCDC: 2046335) and 3aj (CCDC: 2046336). The target transformation was not observed when an alkyl ketone derived enaminone (R = R1 = Me) was employed.

|
Download:
|
| Scheme 1. Scope on the enaminones for the CH trifluoromethylsulfonylation. General conditions: 1 (0.2 mmol), 2a (0.4 mmol), I2 (0.4 mmol) in mixed CH3CN/DMSO (1.6 mL/0.4 mL). Stirred at 80 ℃ for 12 h in sealed tube. Isolated yield based on 1. | |
Encouraged by the satisfactory results from the reactions of different enamines, the reactions employing different perfluoroalkyl sulfinites were then executed. Delightfully, the primary efforts proved that HCF2SO2Na and C2F5SO2Na could also be used for the C-H functionalization to give difluoromethylsulfonyl (4a–4c, Scheme 2) and pentafluoroethylsulfonyl (4d, Scheme 2) elaborated enaminones. The yields of these products obtained under the standard reaction conditions were lower than those trifluoromethylsulfonylated products. Further, the CF3CH2SO2Na was also used to react with 1a, but the C-H sulfonylation did take place, proving the full fluorinated alkyl sulfinites were uniquely suitable for this novel C-H transformation.

|
Download:
|
| Scheme 2. Reactions using different perfluoroalkyl sulfinites. | |
To disclose the synthetic application of the synthesized products, compound 3a was employed to react with different reaction patterners. Delightfully, the synthesis of nitrogen heteroaryls, including trifluoromethylsulfonyl functionalized pyrazole 6 and trifluoromethylsulfonyl functionalized pyrimidine 8 were successfully synthesized by reacting with sulfonyl hydrazine 5 (Scheme 3A) and amidine 7 (Scheme 3B) via facile operation, respectively.

|
Download:
|
| Scheme 3. Synthetic application of product 3a. | |
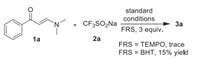
For the sake of probing possible reaction mechanism, the model reaction was then conducted in the presence of free radical scavenger (FRS). As shown in Scheme 4, the formation of product 3a was evidently suppressed in presence of either TEMPO or BHT, suggesting that the titled reactions proceeded via free radical process.
|
Download:
|
| Scheme 4. Control experiments with free radical scavenger. | |
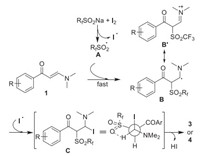
On the basis of the results from both the product synthesis and the control experiments, the plausible mechanism for the reactions is proposed. As shown in Scheme 5, the incorporation of RfSO2Na and molecular iodine provides perfluoroalkyl sulfonyl free radical A via thermo-induced iodine homo-cleavage. The free radical A adds quickly to the enaminone C=C double bond and give free radical intermediate B or its tautomeric species B'. The radical-radical coupling of B and iodine free radical takes places successively and led to the formation of intermediate C. As shown in the Neuman structure of C, the stronger hydrogen bond effect between the sulfonyl group and the β-H determines that the antiperiplanar elimination of HI from C affords the E-configurated perfluoroalkyl sulfonyl enaminones via formal reversion of the double bond configuration.
|
Download:
|
| Scheme 5. The plausible reaction mechanism. | |
In summary, by means of functionalizing the vinyl C-H bond in tertiary enaminones in the presence of perfluoroalkyl sulfonyl sulfinites, we have realized the synthesis of α-E-perfluoroalkyl sulfonyl enaminones by a novel C-H perfluoroalkyl sulfonylation and the conversion of the double bond configuration in the presence of only I2. This featured new protocol brings new perspective on the synthesis of polyfunctionalized alkenes possessing perfluoroalkyl structure without employing any metal catalyst or additive. In addition, the broad substrate tolerance as well as the relatively mild conditions are also favorable for its further application in organic synthesis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is financially supported by National Natural Science Foundation of China (No. 21861019) and Natural Science Foundation of Jiangxi Province (No. 20202ACBL203006).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.037.
| [1] |
M. Tordeux, B.R. Langlois, C. Wakeselman, J. Org. Chem. 54 (1989) 2452-2453. DOI:10.1021/jo00271a041 |
| [2] |
C. Zhang, Adv. Synth. Catal. 356 (2014) 2895-2906. DOI:10.1002/adsc.201400370 |
| [3] |
H. Mei, R. Pajkert, L. Wang, et al., Green Chem. 22 (2020) 3028-3059. DOI:10.1039/d0gc01025a |
| [4] |
P. Ghosh, S. Mondal, A. Hajra, J. Org. Chem. 83 (2018) 13618-13623. DOI:10.1021/acs.joc.8b02312 |
| [5] |
A. Murugan, N.B. Babu, A. Polu, et al., J. Org. Chem. 84 (2019) 7796-7803. DOI:10.1021/acs.joc.9b00676 |
| [6] |
L. Zhou, P. Li, B. Wang, L. Wang, Chem. Commun. 55 (2019) 3737-3740. DOI:10.1039/c9tb00289h |
| [7] |
X.H. Cao, X. Pan, P.J. Zou, et al., Chem. Commun. 50 (2014) 3359-3362. DOI:10.1039/c3cc49689a |
| [8] |
L. Wang, Y. Zhang, F. Li, et al., Adv. Synth. Catal. 360 (2018) 3969-3977. DOI:10.1002/adsc.201800863 |
| [9] |
L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21 (2019) 3362-3369. DOI:10.1039/c9gc00938h |
| [10] |
Z. Tan, S. Zhang, Y. Zhang, et al., J. Org. Chem. 82 (2017) 9384-9399. DOI:10.1021/acs.joc.7b01359 |
| [11] |
W.G. Shen, Q.Y. Wu, X.Y. Gong, et al., Green Chem. 21 (2019) 2983-2987. DOI:10.1039/c9gc00886a |
| [12] |
H. Hong, Y. Li, L. Chen, et al., J. Org. Chem. 84 (2019) 5980-5986. DOI:10.1021/acs.joc.9b00766 |
| [13] |
Y. Konik, M. Kudrjashova, S. Kaabel, et al., Org. Biomol. Chem. 15 (2017) 4635-4643. DOI:10.1039/C7OB00680B |
| [14] |
Z. Li, L. Jiao, Y. Sun, et al., Angew. Chem. Int. Ed. 59 (2020) 7266-7270. DOI:10.1002/anie.202001262 |
| [15] |
L. Zhao, P. Li, H. Zhang, L. Wang, Org. Chem. Front. 6 (2019) 87-93. DOI:10.1039/c8qo01079j |
| [16] |
K. Lu, X. Wei, Q. Li, Y. Li, et al., Org. Chem. Front. 6 (2019) 3766-3770. DOI:10.1039/c9qo00940j |
| [17] |
X.L. Yu, J.R. Chen, D.Z. Chen, W.J. Xiao, Chem. Commun. 52 (2016) 8275-8278. DOI:10.1039/C6CC03335K |
| [18] |
A. vander Werf, M. Hribersek, N. Selander, Org. Lett. 19 (2017) 2374-2377. DOI:10.1021/acs.orglett.7b00908 |
| [19] |
S. Soni, P. Pali, M.A. Ansari, M.S. Singh, J. Org. Chem. 85 (2020) 10098-10109. DOI:10.1021/acs.joc.0c01355 |
| [20] |
S. Liang, J. Wei, L. Jiang, et al., Chem. Commun. 55 (2019) 8536-8539. DOI:10.1039/c9cc03282g |
| [21] |
L. Jiang, J. Qian, W. Yi, et al., Angew. Chem. Int. Ed. 54 (2015) 14965-14969. DOI:10.1002/anie.201508495 |
| [22] |
X.L. He, S. Majumder, J. Wu, et al., Org. Chem. Front. 6 (2019) 2435-2440. DOI:10.1039/c9qo00350a |
| [23] |
Y.Y. Liao, J.C. Deng, Y.P. Ke, et al., Chem. Commun. 53 (2017) 6073-6076. DOI:10.1039/C7CC02373A |
| [24] |
X.F. Li, L.F. Shi, X.G. Zhang, X.H. Zhang, Org. Biomol. Chem. 16 (2018) 6438-6442. DOI:10.1039/C8OB01421C |
| [25] |
Y. Ning, A. Mekareeya, K.R. Babu, E. Anderson, X. Bi, ACS Catal. 9 (2019) 4203-4210. DOI:10.1021/acscatal.9b00500 |
| [26] |
J. Liao, W. Guo, Z. Zhang, et al., J. Org. Chem. 81 (2016) 1304-1309. DOI:10.1021/acs.joc.5b02674 |
| [27] |
Q.L. Wang, Z. Chen, Q. Zhou, Adv. Synth. Catal. 361 (2019) 2315-2320. DOI:10.1002/adsc.201801475 |
| [28] |
K. Zhang, X.H. Xu, F.L. J. Org. Chem. 80 (2015) 7658-7665. DOI:10.1021/acs.joc.5b01295 |
| [29] |
S.C. Cullen, S. Shekhar, N.K. Nere, J. Org. Chem. 78 (2013) 12194-12201. DOI:10.1021/jo401868x |
| [30] |
H. Guyon, H. Chachignon, D. Cahard, Beilstein J. Org. Chem. 13 (2017) 2764-2799. DOI:10.3762/bjoc.13.272 |
| [31] |
S. Würtz, S. Rakshit, J.J. Neumann, T. Dröge, F. Glorius, Angew. Chem. Int. Ed. 47 (2008) 7230-7233. DOI:10.1002/anie.200802482 |
| [32] |
R. Bernini, G. Fabrizi, A. Sferrazza, S. Cacchi, Angew. Chem. Int. Ed. 48 (2009) 8078-8081. DOI:10.1002/anie.200902440 |
| [33] |
X. Mao, T. Tong, S. Fan, et al., Chem. Commun. 53 (2017) 4718-4721. DOI:10.1039/C7CC00992E |
| [34] |
Y. Zhou, Y. Wang, Z. Song, et al., Org. Chem. Front. 7 (2020) 25-29. DOI:10.1039/c9qo01177c |
| [35] |
Y. Guo, Y. Xiang, L. Wei, J.P. Wan, Org. Lett. 20 (2018) 3971-3974. DOI:10.1021/acs.orglett.8b01536 |
| [36] |
F. Wang, W. Sun, Y. Wang, et al., Org. Lett. 20 (2018) 1256-1260. DOI:10.1021/acs.orglett.8b00222 |
| [37] |
J.P. Wan, S. Zhong, L. Xie, et al., Org. Lett. 18 (2016) 584-587. DOI:10.1021/acs.orglett.5b03608 |
| [38] |
Z. Shang, Q. Chen, L. Xing, et al., Adv. Synth. Catal. 361 (2019) 4926-4932. DOI:10.1002/adsc.201900940 |
| [39] |
Y. Yuan, W. Hou, D. Zhang-Negrerie, et al., Org. Lett. 16 (2014) 5410-5413. DOI:10.1021/ol5026525 |
| [40] |
Y. Gao, Y. Liu, J.P. Wan, J. Org. Chem. 84 (2019) 2243-2251. DOI:10.1021/acs.joc.8b02981 |
| [41] |
R. Chandran, A. Pise, S.K. Shah, et al., Org. Lett. 22 (2020) 6557-6561. DOI:10.1021/acs.orglett.0c02308 |
| [42] |
X. Duan, X. Liu, X. Cuan, et al., J. Org. Chem. 84 (2019) 12366-12376. DOI:10.1021/acs.joc.9b01722 |
| [43] |
G. Li, Q. Yan, X. Gong, et al., ACS Sustain. Chem. Eng. 7 (2019) 14009-14015. DOI:10.1021/acssuschemeng.9b02511 |
| [44] |
D. Ali, A.K. Panday, L.H. Choudhury, J. Org. Chem. 85 (2020) 13610-13620. DOI:10.1021/acs.joc.0c01738 |
| [45] |
For a review, see: L. Fu, J.P. Wan, Asian J. Org. Chem. 8 (2019) 767–776
|
| [46] |
J.P. Wan, Z. Tu, Y. Wang, Chem. Eur. J. 25 (2019) 6907-6910. DOI:10.1002/chem.201901025 |
| [47] |
S. Mkrtchyan, V.O. Iaroshenko, Chem. Commun. 56 (2020) 2606-2609. DOI:10.1039/c9cc09945j |
| [48] |
T. Luo, J.P. Wan, Y. Liu, Org. Chem. Front. 7 (2020) 1107-1112. DOI:10.1039/d0qo00065e |
| [49] |
H. Xiang, Q. Zhao, Z. Tang, et al., Org. Lett. 19 (2017) 146-149. DOI:10.1021/acs.orglett.6b03441 |
| [50] |
L. Fu, Z. Xu, J.P. Wan, Org. Lett. 22 (2020) 9518-9523. DOI:10.1021/acs.orglett.0c03548 |
| [51] |
H. Jiang, W. Huang, Y. Yu, et al., Chem. Commun. 53 (2017) 7473-7476. DOI:10.1039/C7CC03125D |
| [52] |
Q. Yu, Y. Liu, J.P. Wan, Org. Chem. Front. 7 (2020) 2770-2775. DOI:10.1039/d0qo00855a |
| [53] |
L. Gan, Q. Yu, Y. Liu, J.P. Wan, J. Org. Chem. 86 (2021) 1231-1237. DOI:10.1021/acs.joc.0c02431 |
| [54] |
D. Hu, L. Yang, J.P. Wan, Green Chem. 22 (2020) 6773-6777. DOI:10.1039/d0gc02806a |
| [55] |
G. Wang, Y. Guo, J.P. Wan, Chin. J. Org. Chem. 40 (2020) 645-650. DOI:10.6023/cjoc201912018 |
| [56] |
Y. Liu, J. Xiong, J.P. Wan, Adv. Synth. Catal. 362 (2020) 877-883. DOI:10.1002/adsc.201901234 |
| [57] |
L. Fu, X. Cao, J.P. Wan, Chin. J. Chem. 38 (2020) 254-258. DOI:10.1002/cjoc.201900417 |
| [58] |
X.M. Xu, D.M. Chen, Z.L. Wang, Chin. Chem. Lett. 31 (2020) 49-57. DOI:10.4236/aer.2020.84005 |
| [59] |
H. Zhang, H. Wang, Y. Jiang, F.et al., Chem. Eur. J. 26 (2020) 17289-17317. DOI:10.1002/chem.202001414 |
| [60] |
S. Tian, T. Luo, Y. Zhu, J.P. Wan, Chin. Chem. Lett. 31 (2020) 3073-3082. DOI:10.1016/j.cclet.2020.07.042 |
| [61] |
X. Xu, H. Yang, W. Li, Chin. J. Org. Chem. 40 (2020) 1912-1925. DOI:10.6023/cjoc201912044 |
| [62] |
S. Chen, Y. Li, M. Wang, X. Jiang, Green Chem. 22 (2020) 322-326. DOI:10.1039/C9GC03841H |
| [63] |
P.F. Zhong, H.M. Lin, L.W. Wang, et al., Green Chem. 22 (2020) 6334-6339. DOI:10.1039/d0gc02125c |
| [64] |
W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1895-1898. DOI:10.1016/j.cclet.2020.02.011 |
| [65] |
K. Sun, X.L. Chen, Y.L. Zhang, et al., Chem. Commun. 55 (2019) 12615-12618. DOI:10.1039/c9cc06924k |
| [66] |
L.Y. Xie, T.G. Fang, J.X. Tan, et al., Green Chem. 21 (2019) 3858-3863. DOI:10.1039/c9gc01175g |
| [67] |
D. Zeng, M. Wang, W.-.P. Deng, X. Jiang, Org. Chem. Front. 7 (2020) 3956-3966. DOI:10.1039/d0qo00987c |
| [68] |
Y. Meng, M. Wang, X. Jiang, CCS Chem. 3 (2021) 17-24. DOI:10.31635/ccschem.021.202000638 |
| [69] |
Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 8907-8911. DOI:10.1002/anie.202001589 |
| [70] |
M. Wang, X. Jiang, Chem. Rec. (2021) doi: 10.1002/tcr.202000162.
|
 2021, Vol. 32
2021, Vol. 32