CTP is an essential nucleotide that is required for the biosynthesis of nucleic acids [1] and protein glycosylation [2]. In addition, it also serves as a critical precursor during the synthesis of membrane phospholipids [3] and participates in cellular signal communication [4]. While ATP serves as the phosphate donor for most kinases, numerous studies have found CTP-dependent kinases that utilize CTP as phosphate donor, such as yeast diacylglycerol kinase [5]. Moreover, CTP synthase (CTPS1) has emerged as a potential therapeutic target for many diseases such as cancer [6]. CTP can bind and inhibit CTPS1 through competition with UTP for a shared 5′-triphosphate-binding pocket [7]. Furthermore, ParB-like proteins in bacteria are another important class of CTP-dependent molecular switches which are involved in DNA segregation during cell division [8]. Despite the importance of CTP-binding in critical metabolic processes, the proteome-wide study of CTP-binding proteins remains under investigation.
Recent advances in mass spectrometry (MS) instruments and analytical methods render MS-based proteomics a powerful tool for rapidly profiling the whole proteome from diverse biological samples [9]. Coupled with stable isotope labeling with amino acids in cell culture (SILAC) technique, MS can be used to identify and accurately quantify thousands of proteins from complex biological samples. So far, various affinity enrichment methods, such as biotin-based pulldown assay and activity-based protein profiling (ABPP), have been developed to capture specific group of proteins which have similar structure and function from complex biological system [10]. For instance, acyl phosphate-containing ABPP probes have been widely used for characterizing ATP- and GTP-binding proteins [11, 12]. Additionally, competition assays which use free ATP or different concentrations of ATP probes have also been employed to effectively eliminate the non-specific ATP-binding proteins from whole cell lysates [11, 13]. In this study, we report a proteome-wide analysis of CTP-binding proteins in human cells using a specific biotin-CTP probe coupled with SILAC-based mass spectrometry strategy, which provides new ideas about the biological roles of CTP.
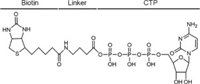
Many ATP- and GTP-binding proteins have a phosphate-binding loop (P-loop) which contains a glycine-rich sequence and a conserved lysine [14]. Acyl phosphate-containing ATP and GTP probes have been widely used for targeting the lysine residues in P-loop of target proteins [15, 16]. In this study, we designed a biotinylated CTP affinity probe which harbors three components: CTP, a binding moiety, which specifically recognizes the active sites of CTP-binding proteins; biotin, an enrichment moiety, which facilitates the enrichment of target proteins; γ-aminobutyric acid, a linker, which connects the enrichment moiety to CTP and acts as a spacer to minimize steric hindrance (Fig. 1). The biotinylated CTP affinity probe was synthesized and purified for the enrichment of CTP-binding proteins in human cells (Figs. S1 and S2 in Supporting information). We speculate that the CTP probe could play a role like ATP and GTP acyl-phosphate probe [17]: upon interacting with CTP-binding proteins, CTP moiety binds to the active sites and then the acyl-phosphate component of probe reacts with the conserved ε-amino group of lysine residue located at or near the binding pocket to form a stable amide bond (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 1. Chemical structure of the biotinylated CTP affinity probe. | |
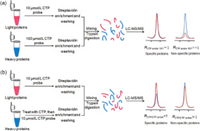
To verify this hypothesis, we incubated the CTP affinity probe with different concentrations of lysine, ranging from 100 µmol/L to 20 mmol/L. However, the formation of amide bonds were not observed until the concentration of lysine reached 20 mmol/L (Fig. S4 in Supporting information). This result may be caused by the distinctive structure of CTP. To overcome this limitation, we first enriched the CTP-binding proteins using streptavidin beads, eliminated non-specific proteins by extensively washing, and then digested the proteins with trypsin before LC-MS/MS analysis (Fig. 2). In this respect, the target proteins would be identified and quantified using the unmodified peptides. In addition, this method also allows the identification of those CTP targets which do not have lysine residues in their CTP-binding pockets.
|
Download:
|
| Fig. 2. SILAC-based quantitative proteomics approach for profiling CTP-binding proteins at the entire proteome scale. (a) The workflow for the competition experiment using different concentrations of CTP probe. (b) Workflow for the free CTP competition experiment. Shown is the workflow for the forward SILAC labeling experiments. | |
To exclude the non-specific targets, we employed low (10 µmol/L) and high (100 µmol/L) concentrations of CTP probe, along with SILAC-based quantitative proteomics approach, to identify CTP-binding proteins in human cells. Prior to incubation with the probe, cell lysates were passed through NAP-25 columns to remove the endogenous nucleotides. In forward SILAC experiment, equal amount of light and heavy cell lysates were incubated with low (10 µmol/L) and high (100 µmol/L) concentrations of CTP probe, respectively (Fig. 2a). In reverse SILAC experiment, labeling reaction was operated in an opposite way. Biotin-labeled proteins were enriched by streptavidin beads, digested with trypsin, and analyzed using LC-MS/MS as described above. The peak intensity ratios of heavy and light peptides were used to obtain the relative affinity ratios (RCTP probe 10/1, 100 µmol/L/10 µmol/L) of proteins towards different concentrations of CTP probe.
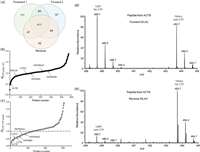
Similar as the previous quantitative affinity profiling of ATP-binding proteins, specific binding proteins would show RCTP probe 10/1 close to 1, whereas non-specific proteins would display RCTP probe 10/1 ≫ 1 due to concentration-dependent increase in peptides arising from non-specific proteins [18]. To include all the putative CTP-binding proteins, a criterion of RCTP probe 10/1 < 2 was used to select the specific CTP targets. With the criterion, a total of 575 proteins were identified and quantified in at least two SILAC experiments (Fig. 3a and Table S1 in Supporting information). Some known CTP-binding proteins, such as CTP synthase 1 (CTPS1) [19] and heat shock proteins HSP90s [20], were included in the list (RCTP probe 10/1 values of ~1, Fig. 3b and Table S1), demonstrating the feasibility of our strategy in profiling CTP-binding proteins.
|
Download:
|
| Fig. 3. SILAC-based competition assay for assessing CTP-binding proteins in human proteome. (a) A Venn diagram showing the overlap of the quantified proteins (RCTP probe 10/1 < 2) from forward and reverse SILAC labeling experiments. (b) The RCTP probe 10/1 ratios of proteins in probe concentration-dependent SILAC experiments. (c) The RCTP/CTP probe ratios of proteins in competitive SILAC experiments with and without free CTP treatment. (d, e) ESI-MS of the light- and heavy-labeled tryptic peptide AGFAGDDAPR from ACTB in the presence or absence of free CTP in forward (d) and reverse (e) SILAC experiments. | |
To further ascertain whether these proteins bind selectively to CTP, we performed competitive SILAC experiments in which free CTP acted as a competitor to compete with CTP probe (Fig. 2b). Cell lysates were pretreated with free CTP before incubation with CTP affinity probes. In this respect, CTP would occupy the binding pockets of specific targets, so that the labeling efficiency of CTP-binding proteins by CTP affinity probes would decrease. In forward SILAC experiment, the heavy and light cell lysates were incubated with and without free CTP, respectively, whereas reverse SILAC experiment was manipulated oppositely. CTP competitive ratio of protein (RCTP/CTP probe) was used to reflect its relative binding affinity towards CTP probe with vs. without free CTP. To include all the putative CTP targets, a criterion of RCTP/CTP probe < 1 was used to choose the candidate CTP-binding proteins. LC-MS/MS analysis identified and quantified 311 proteins in at least one SILAC experiment, 129 of which display RCTP/CTP probe < 1 (Fig. 3c and Table S2 in Supporting information). For example, when treating the heavy cell lysate using free CTP in forward SILAC experiment, the peak intensities of the heavy-labeled peptides from ACTB are lower than those of the light-labeled peptides without CTP treatment (Fig. 3d). A similar trend was also observed in reverse SILAC experiment (Fig. 3e, Fig. S5 in Supporting information showing the MS/MS).
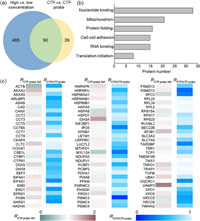
We analyzed the data from these two sets of SILAC experiments and identified 90 proteins with RCTP probe 10/1 < 2 in probe concentration-dependent SILAC experiments and RCTP/CTP probe < 1 in free CTP competition experiments (Fig. 4a, Table S3 in Supporting information). These proteins contain a small fraction of known CTP targets and a large proportion of newly discovered proteins. We further investigated the biological functions of these proteins by Gene Ontology (GO) analysis using DAVID database. The results showed that these proteins are involved in a variety of cellular processes, such as nucleotide binding, protein folding and cell adhension (Fig. 4b). We ranked these proteins according to their RCTP/CTP probe value (Fig. 4c and Table S3) and found that CTPS1 is among the top on the list, suggesting that this quantitative proteomics strategy could be employed for identifying new CTP-binding proteins.
|
Download:
|
| Fig. 4. Summary of CTP-binding proteins identified with the biotinylated CTP affinity probe. (a) Venn diagram depicting the overlap of the putative CTP-binding proteins quantified in two competitive SILAC experiments. (b) GO analysis showing the biological functions of candidate CTP-binding proteins. (c) A heat map displaying the quantification data of candidate CTP-binding protein in two competitive SILAC experiments. White and blue indicate a significant CTP-binding preference with a RCTP probe 10/1 ratio ≈1 and a low RCTP/CTP probe value, respectively. | |
In summary, we reported here a quantitative proteomics strategy using a biotinylated CTP affinity probe to profile CTP-binding proteins at the entire proteome scale. Previous studies showed that the identification of nucleotide-binding proteins by conventional bottom-up method is very challenging due to the relatively low abundance of nucleotide-binding proteins and the interference from other cellular proteins [11, 12], whereas the method used in this study can effectively capture nucleotide-binding proteins. We successfully synthesized the biotin-tagged CTP affinity probe and used it to enrich CTP-binding proteins by two sets of competitive SILAC experiments. We identified a total of 90 potential CTP-binding proteins which showed high affinity to CTP. In addition, we found that these candidate CTP-binding proteins are involved in multiple cellular pathways. These results deepen our understanding of CTP-binding proteins and lay a foundation for further revealing the biological functions of CTP. Moreover, the analytical approach used in this study could be employed for uncovering CTP-binding proteins in other organisms.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21807030, 21907028), the Science and Technology Innovation Program of Hunan Province (No. 2019RS2020), Natural Science Foundation of Hunan Province (No. 2020JJ5046), and the Fundamental Research Funds for the Central Universities (Nos. 531118010061, 531118010259).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.cclet.2021.05.057.
| [1] |
D.R. Evans, H.I. Guy, J. Biol. Chem. 279 (2004) 33035-33038. DOI:10.1074/jbc.R400007200 |
| [2] |
J. Denecke, C. Kranz, Biochim. Biophys. Acta 1792 (2009) 888-895. DOI:10.1016/j.bbadis.2009.01.013 |
| [3] |
Y.F. Chang, G.M. Carman, Prog. Lipid Res. 47 (2008) 333-339. DOI:10.1016/j.plipres.2008.03.004 |
| [4] |
M. Sellmeier, B. Weinhold, A. Munster-Kuhnel, Top. Curr. Chem. 366 (2015) 139-167. |
| [5] |
G.S. Han, L. O'Hara, S. Siniossoglou, G.M. Carman, J. Biol. Chem. 283 (2008) 20443-20453. DOI:10.1074/jbc.M802866200 |
| [6] |
D.E. Hansel, A. Rahman, M. Hidalgo, et al., Am. J. Pathol. 163 (2003) 217-229. DOI:10.1016/S0002-9440(10)63645-0 |
| [7] |
J.A. Endrizzi, H. Kim, P.M. Anderson, E.P. Baldwin, Biochemistry 44 (2005) 13491-13499. DOI:10.1021/bi051282o |
| [8] |
M. Osorio-Valeriano, F. Altegoer, W. Steinchen, et al., Cell 179 (2019) 1512-1524. DOI:10.1016/j.cell.2019.11.015 |
| [9] |
P. Scherp, G. Ku, L. Coleman, I. Kheterpal, Methods Mol. Biol. 702 (2011) 163-190. DOI:10.1007/978-1-61737-960-4_13 |
| [10] |
G.C. Adam, E.J. Sorensen, B.F. Cravatt, Mol. Cell. Proteomics 1 (2002) 781-790. DOI:10.1074/mcp.R200006-MCP200 |
| [11] |
Y. Xiao, Y. Wang, Mass Spectrom. Rev. 35 (2016) 601-619. DOI:10.1002/mas.21447 |
| [12] |
M. Huang, Y. Wang, Mass Spectrom. Rev. 40 (2021) 215-235. DOI:10.1002/mas.21637 |
| [13] |
J. Adachi, M. Kishida, S. Watanabe, et al., J. Proteome. Res. 13 (2014) 5461-5470. DOI:10.1021/pr500845u |
| [14] |
M. Saraste, P.R. Sibbald, A. Wittinghofer, Trends Biochem. Sci. 15 (1990) 430-434. DOI:10.1016/0968-0004(90)90281-F |
| [15] |
H. Qiu, Y. Wang, Anal. Chem. 79 (2007) 5547-5556. DOI:10.1021/ac0622375 |
| [16] |
M.P. Patricelli, A.K. Szardenings, M. Liyanage, et al., Biochemistry 46 (2007) 350-358. DOI:10.1021/bi062142x |
| [17] |
R. Cai, M. Huang, Y. Wang, Anal. Chem. 90 (2018) 14339-14346. DOI:10.1021/acs.analchem.8b03727 |
| [18] |
Y. Xiao, L. Guo, Y. Wang, Anal. Chem. 85 (2013) 7478-7486. DOI:10.1021/ac401415z |
| [19] |
E.M. Lynch, D.R. Hicks, M. Shepherd, et al., Nat. Struct. Mol. Biol. 24 (2017) 507-514. DOI:10.1038/nsmb.3407 |
| [20] |
C. Soti, A. Vermes, T.A. Haystead, P. Csermely, Eur. J. Biochem. 270 (2003) 2421-2428. DOI:10.1046/j.1432-1033.2003.03610.x |
 2021, Vol. 32
2021, Vol. 32 

