b State Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control, Guangdong Provincial Key Laboratory of Soil and Groundwater Pollution Control, School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China;
c Hubei Key Laboratory of Mineral Resources Processing and Environment, School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China
Inorganic heavy metal ions and organic dye pollutants are severe pollution sources in dyeing wastewater, which causes many negative impacts on human health and natural environment [1]. Among all the different types of dyes, inorganic Cr(Ⅵ) and organic RhB given the various dye wastewater treatment technologies, photocatalysis has attracted broad attention due to its economic feasibility and high efficiency [2]. Among the different types of photocatalysts, single-atomic semiconductor-based photocatalyst has become one of the most promising photocatalytic materials due to its maximum atom utilization and excellent redox catalytic properties, which has been developed very fast in clear energy catalysis recently and need to be urgently explored for wastewater treatment [3]. To construct a high efficient single-atomic semiconducting photocatalyst, engineering the single-atomic metal homogeneously dispersed on a high efficient semiconductor is of crucial importance. Among all the reported semiconducting materials, CuFeS2 quantum dots (QDs) are a kind of promising ultraviolet-visible-infrared (UV–vis-NIR) semiconductor photocatalytic material with a tunable bandgap, size-dependent quantum confinement effect, high surface area, as well as "mixed redox-couple of Cu(Ⅰ)−S−Fe(Ⅲ)" in its crystal structure [4]. It has been widely used in the field of photodetection [5], fluorescence labeling [6, 7], electrocatalysis [8, 9], and photocatalysis [10, 11]. It can be activated by photons with energy greater than the bandgap (Eg) to produce electron-hole pairs. These photogenerated electron-hole pairs can be further separated and migrated to the surface of the QDs and undergo a strong reduction/oxidation reaction with an electron acceptor/donor adsorbed on the catalyst surface [12]. At present, the photocatalytic performance of the photocatalysts is frequently limited by the fast recombination of the photogenerated electron-hole pairs. To overcome this disadvantage, various strategies have been developed, including the deposition of noble metal [13, 14], construction of heterojunctions [15], as well as the sensitization of the semiconductor with adsorbed dye molecules [16]. Besides, introducing active single atoms to strengthen the metal-support interactions is a widely used strategy that can maximize the atomic efficiency, change the adsorption and desorption properties of the surface, optimize the intrinsic activity of catalysts, and reduce the cost of noble metal catalysts [17]. Single atoms deposited on support surface act as electron acceptor to provide highly efficient electron transfer channels, hence prevent the fast recombination of the photogenerated electron-hole pairs, greatly boost the photocatalytic activity [18]. The selectivity and catalytic activity of the catalyst can be modulated by monatomic modification to selectively remove harmful substances in wastewater. However, studies on the formation and catalytic mechanism of single-atomic photocatalysts for environmental pollutant treatment are still rare up to now [19].
In this work, mixed-valence single-atomic Ag(Ⅰ) and Ag(0) co-decorated CuFeS2 QDs (Ag/CuFeS2 QDs) are reported firstly with a high Ag loading of 3.63 wt%. Based on the characterization results of aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS), we confirm that mixed-valence single-atomic Ag is uniformly distributed on the surface of the CuFeS2 QDs. Benefited from the formation of the mixed-valence single-atomic Ag/CuFeS2, the bandgap of CuFeS2 has been significantly lowered down to 1.21 eV. With the significant electron capture capability of metallic Ag single atoms, the charge carrier separation efficiency has been improved dramatically. The narrower bandgap of Ag/CuFeS2 promotes the photocatalyst performance by extending its absorption light spectrum up to the near-infrared (NIR) light range. Further, combine the density functional theory (DFT) calculations, the formation and catalytic mechanisms of monatomic catalysts are illustrated, which suggests that the introduction of Ag atoms can facilitate the engineering of the CuFeS2 QDs bandgap. Contributed from its small particle sizes and low surface states [20], this mixed-valence Ag(0) and Ag(Ⅰ) single-atom co-decorated semiconducting photocatalyst exhibits a stronger reduction power with its photoexcited electrons, which can give impetus to the photocatalytic treatment of diverse environmental pollutions in complex environmental media such as dyeing wastewater.
High-purity dark purple CuFeS2 QDs were prepared through a colloidal synthesis approach [4]. The Ag/CuFeS2 catalyst was prepared via the photoreduction method at room temperature, as shown in Text S1 (Supporting information). The obtained Ag/CuFeS2 catalysts showed a high metal loading of 3.63 wt% determined by inductive coupled plasma mass spectrometry (ICP-MS) (Table S1 in Supporting information). To investigate the crystal structure and electronic structure of Ag/CuFeS2, a series of characterizations, including XRD, XPS, and ultraviolet-visible diffuse reflectance spectra (UV–vis DRS) have been employed. Fig. 1a depicted the XRD patterns of CuFeS2 and Ag/CuFeS2 QDs. The predominant diffraction peaks in both samples were located at 2θ values of 29.3°, 33.8°, 48.6° and 57.8°, which could be assigned to the diffraction peaks from (112), (200), (220) and (400) lattice planes of the tetragonal phase CuFeS2 (JCPDS No. 37−0471). This observation indicated that CuFeS2 was crystallized in the tetragonal crystal system with a space group of I‒42d [21]. Moreover, no characteristic diffraction peaks of the metallic Ag were observed in Ag/CuFeS2 QDs, which supported that the Ag atoms were uniformly dispersed on CuFeS2 QDs support, and no particular-sized Ag was formed within Ag/CuFeS2 QDs. XPS was further used to study the valence state of Ag. As shown in Fig. 1b, characteristic peaks of Ag have been observed, the two doublet 3d peaks in the high-resolution XPS spectrum of Ag 3d (Fig. 1c) indicated that there were two different chemical states for Ag species. The Ag 3d5/2 and Ag 3d3/2 peaks with binding energies of 367.7 and 373.7 eV could be ascribed to mono valence state of Ag(Ⅰ); while the other doublet at 368.2 and 374.2 eV for Ag 3d5/2 and Ag 3d3/2, respectively, could be assigned to the metallic Ag(0) [22, 23]. The relative content of metallic Ag(0) to Ag(Ⅰ) was calculated to be 43.2%. These results suggested that Ag single atoms were located on QD surfaces as a mixed-valence state. Beyond that, the Cu 2p level in Ag/CuFeS2 QDs has been split into 2p3/2 and 2p1/2 peaks at 932.0 and 951.8 eV; while Fe 2p3/2 was characterized with binding energies of 711.6 eV; the Fe 2p1/2 peak was with binding energies of 723.9 eV. These observations suggested that the valence state of Cu in Ag/CuFeS2 remained as +1 state [24], and the Fe could be described with a +3 valence state (Figs. S1a and b in Supporting information) [25]. No variation in the valence states of Cu and Fe indicated that there was no redox reaction occurred after single-atomic Ag decorating on CuFeS2 QDs.

|
Download:
|
| Fig. 1. Crystal structure and electronic structure characterization of CuFeS2 and Ag/CuFeS2 QDs. (a) XRD patterns. (b) XPS survey spectra. (c) High-resolution XPS spectra of Ag 3d (d) Bandgaps. (e) Mott-Schottky curves. (f) EIS Nyquist plots of QDs electrodes measured under the open-circuit potential with natural light irradiation. | |
In terms of photocatalysis performance, bandgap engineering and charge-carriers have a significant influence on the performance of the semiconducting photocatalyst. As shown in Fig. 1d, the pure tetragonal phase CuFeS2 QDs exhibited a bandgap of 1.34 eV, when single Ag atoms were introduced, the bandgap of the material decreased to 1.21 eV. Furthermore, Mott-Schottky (MS) measurements, as shown in Fig. 1e indicated that the CuFeS2 and Ag/CuFeS2 QDs were both n-type semiconductors. It could conclude that the experimental flat-band potential of Ag/CuFeS2 was −0.07 V (vs. NHE). Furthermore, it was generally known that the bottom of the conduction bands in many n-type semiconductors was more negative by about −0.2 V than the flat-band potential, which indicated that the conduction band bottom of Ag/CuFeS2 was −0.27 V (vs. NHE) [26]. As the conduction band of CuFeS2 QDs was located at −0.11 V (vs. NHE) as shown in Fig. S1c (Supporting information), the conduction band of Ag/CuFeS2 was much more negative than that of the CuFeS2, which theoretically indicated that the Ag/CuFeS2 material was characterized with an enormous reduction potential. Moreover, the surface carrier-migration efficiencies could be characterized via electrochemical impedance spectroscopy (EIS) [27]. As shown in Fig. 1f, the semicircle diameter of the EIS Nyquist plot of Ag/CuFeS2 was smaller than that of CuFeS2 QDs, suggesting that the introduced Ag single-atomic species could lower the interfacial charge-transfer resistance, which could facilitate the separation and migration of the carriers. The above results suggested that Ag/CuFeS2 showed superior carriers separation ability and faster charge transfer efficiency.
The morphology together with the chemical composition in Ag/CuFeS2 was detected by high-angle annular dark-field (HAADF) image and energy-dispersive X-ray spectroscopy (EDS) under scanning transmission electron microscopy mode (STEM). The HAADF-STEM image of Ag/CuFeS2 in Fig. 2a indicated that the as-synthesized QDs were with a homogenous size distribution of an average diameter of ca. 6.8 nm. Individual Ag atoms in catalysts could be distinguished in the aberration-corrected HAADF-STEM images. As shown in Fig. 2b, Ag single atoms (marked with the white circles) were uniformly dispersed on the surfaces of CuFeS2 QDs, which revealed that only Ag single atoms were present; there were no Ag nanoparticles formed. The enlarged HAADF-STEM image (right) in Fig. 2b showed that the Ag/CuFeS2 had a lattice spacing of 0.304 nm, which corresponding to the d−spacing of the crystallographic (112) plane of tetragonal phase CuFeS2 QDs. The elemental compositions of Fe, Cu, S, and Ag were homogeneously distributed within the catalyst as verified by the STEM-EDS mapping images in Figs. 2c-g.

|
Download:
|
| Fig. 2. Morphology characterizations of Ag/CuFeS2. (a) HAADF-STEM image of Ag/CuFeS2. (b) Aberration-corrected HAADF-STEM image (left) of Ag single atoms (white dots in the circles) on CuFeS2 QD support and magnified HAADF-STEM image (right) of the square marked with red dots. STEM-EDS mapping of element distribution within (c) Ag/CuFeS2; (d) Fe; (e) Cu; (f) S; (g) Ag. | |
It was of great interest to further probe the accurate local structure of Ag single atoms on CuFeS2 QDs. Thus the highly-sensitive electronic and local coordinational structure probing techniques, such as the extended X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) spectroscopy were further used to confirm the valence state and local coordinational environment for Ag [28]. As shown in Fig. 3a, the Ag K-edge XANES of Ag/CuFeS2 was located between Ag foil and Ag2S, which double confirmed the mixed-valence Ag(Ⅰ) and Ag(0) in Ag/CuFeS2 and was consistent with the XPS observation. Moreover, Fig. 3b showed the Fourier transforms (FTs) of the k3-weighted EXAFS oscillations of the as-prepared Ag/CuFeS2, together with the standard references of Ag2S and Ag foil. According to the EXAFS fitting results, no Ag‒Ag coordination peak at 2.87 Å was observed, demonstrating that Ag in Ag/CuFeS2 did exist in the isolated single-atomic state, and there was no Ag nanoparticle formed. Moreover, the atomic distance of Ag‒S in Ag/CuFeS2 was ~2.48 Å, which was similar to the Ag‒S bond distance of ~2.51 Å in Ag2S, indicating that part of the single-atomic Ag(Ⅰ) was coordinated with S in Ag/CuFeS2 (Table S2 in Supporting information). Furthermore, an intensity maximum at 5.8 Å‒1 can be observed from the wavelet transform (WT) contour plots of Ag/CuFeS2 (Fig. 3c), which can be assigned to Ag‒S coordination without an Ag‒Ag signal. Therefore, it can be concluded that the single atomic Ag/CuFeS2 QDs were successfully synthesized.

|
Download:
|
| Fig. 3. X-ray absorption spectroscopy characterization of Ag/CuFeS2. (a) Ag K-edge XANES spectra of Ag foil, Ag2S and Ag/CuFeS2. (b) Fourier transform of k3-weighted Ag K-edge EXAFS fitting curve in R-space. (c) Wavelet transforms analysis of Ag foil, Ag2S and Ag/CuFeS2. | |
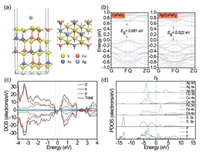
To further understand the electronic structure of Ag/CuFeS2, DFT calculations were employed. We have considered three different adsorption sites (Fe, Cu, S) for Ag to investigate the effects of single Ag adsorption on the CuFeS2 (112) surface structures, as shown in Fig. 4a. The adsorption energies of single Ag atom adsorption on the CuFeS2 (112) plane of the three sites were negative (Table S3 in Supporting information), indicating that the whole process was spontaneous exothermic transformation [29]. Besides, we have found the lowest adsorption energy was at the S center site; thus, we hypothesized that Ag was most likely to bind with S atoms to form Ag‒S [30, 31]. As shown in Fig. 4b, the calculated band structure showed that Ag/CuFeS2 was an indirect bandgap semiconductor with a bandgap of 0.522 eV, which was narrower than the 0.681 eV bandgap of CuFeS2 QDs. Due to the introduction of Ag single atoms, the conduction band minimum (CBM) edge moved to lower energy, which could contribute to a narrowing overall energy gap in Ag/CuFeS2 [32]. This tendency was consistent with the observed results from the Mott-Schottky curve and ultraviolet-visible (UV–vis) spectra. Moreover, an obvious impurity energy level between valence band maximum (VBM) and CBM in Ag/CuFeS2 could be observed, which was beneficial for the electron transition from the valence band (EVB) to the conduction band (ECB) and further promoted the separation of photogenerated electron-hole pairs. As shown in Figs. 4c and d, the orbital interaction between Ag atoms and Ag/CuFeS2 (112) plane was further investigated by the density of states (DOS) and the partial density of states (PDOS). After the introduction of Ag atoms, S 3p orbital and a hybrid orbital of Ag 4d, Fe 3d, Cu 3d contributed to the VBM, while the density state at the CBM was mainly attributed to Fe 3d and S 3p orbitals. Moreover, compared with CuFeS2 QDs (Fig. S2 in Supporting information), a hybrid state density appeared at the forbidden band. The PDOS showed that the hybrid density was formed via the hybridization of Ag 5s and Fe 3d orbitals. Hence, the theoretical calculation results suggested that the bandgap reduction could enhance the light absorption capacity in Ag/CuFeS2, and the presence of impurity energy levels formed a new charge transfer channel to improve the efficiency of the carrier separation, which was expected for the improvement of the photocatalytic activity [27].
|
Download:
|
| Fig. 4. DFT calculations of band structure and density of state. (a) Adsorption sites (left) of Ag on CuFeS2 (112) surface and top view (right) of Ag/CuFeS2 (112) surface. (b) Band structure of tetragonal phase CuFeS2 and Ag/CuFeS2. (c) DOS of Ag/CuFeS2. (d) PDOS of Ag/CuFeS2. | |
Considering the narrower bandgap feature within Ag/CuFeS2, it would be interesting to employ this material for environmental pollutants removal. The optical response spectrum range as well as the carrier separation efficiency, are expected to be enhanced compared to the original CuFeS2 QDs. Herein, RhB, which is a typical organic dye in dyeing wastewater [33], have been employed in the experiment. Prior to the degradation experiment, the initial pH value of the solution could influence the adsorption and degradation efficiency by affecting the surface complexation and the electrostatic interactions between the adsorbate and the QDs surface [34]. The degradation efficiencies of Ag/CuFeS2 were investigated under a broad range of pH from 2, 4, 6, 8 to 10. Under each pH, the reaction time was set to be 60 min. It has been observed that the fastest degradation rate occurred when the initial RhB solution (5 mg/L) was at pH 6, as shown in Fig. S3a (Supporting information). RhB is a cationic dye; the faintly acidic is favourable for the disassociation of protons, which facilitating the adsorption and photocatalytic degradation of RhB. Based on these pre-experimental results, the degradation performance of the Ag/CuFeS2 was investigated under the optimal reaction conditions. As shown in Fig. 5a, under the optimal pH condition, the degradation efficiency of Ag/CuFeS2 reached as high as 90% within the first 10 min, which was 3.55 times higher than that of CuFeS2 QDs. The decrease of concentration was evident by the reduction of the peak intensity at 554 nm in the UV–vis absorption spectra of RhB solution in the experiments (Figs. S3b and c in Supporting information). To understand the reaction kinetics, both the pseudo-first-order and pseudo-second-order kinetic models were applied to simulate the reaction process [35]. According to the fitting results, the pseudo-second-order kinetic model (Fig. 5b) was more suitable for describing the degradation process compared with the pseudo-first-order kinetic model (Fig. S3d in Supporting information) under different catalyst conditions. The fitting parameters indicated that Ag/CuFeS2 had a faster degradation rate (Table S4 in Supporting information). This could be attributed to a variety of interactions in the adsorption process, including the electrostatic interaction as well as the hydrogen bonding [36]. To further identify the active species and explore the photocatalytic mechanism of the high activities in Ag/CuFeS2 [37, 38], a series of species quenchers, including isopropanol (IPA, 1 mmol/L), benzoquinone (BQ, 1 mmol/L), and bicarbonate (NaHCO3, 1 mmol/L) were employed to scavenge the active species, such as hydroxyl radicals (·OH), superoxide radicals (O2·−), and photon-generated holes (h+). The degradation curve (Fig. 5c) indicated that h+ was the main reactive species in the degradation process, and the addition of BQ to capture O2·− also slightly inhibited the degradation efficiency, while ·OH could be ignored. Considering the variation of the positions of the conduction band and valence band, a possible electron and hole transfer process could be further explored. As the conduction band of Ag/CuFeS2 was more negative than the E0(O2/O2·−) = −0.046 V; therefore, the electrons in the conduction band could react with the oxygen dissolved in water to form O2·−. However, the potential of the Ag/CuFeS2 valence band was more negative than that of the E0(·OH/H2O) = +2.68 V, so h+ could not be oxidized to generate ·OH [39]. According to the structural mechanism of Ag/CuFeS2, the active species in the photocatalytic system were mainly h+ and O2·−, which was consistent with the conclusion obtained from the free radical quenching experiments. Combining with the active species explored, the photocatalytic mechanism could be described by the following equations (Eqs. 1‒4):

|
(1) |
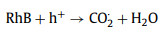
|
(2) |
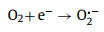
|
(3) |
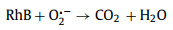
|
(4) |

|
Download:
|
| Fig. 5. Cr(Ⅵ) and RhB removal experimental data. (a) Photocatalytic degradation behaviours of RhB with different catalysts. (b) Pseudo-second-order fitting of RhB degradation. (c) The effects of different scavengers on the degradation of RhB over Ag/CuFeS2. (d) Photocatalytic removal behaviours of Cr(Ⅵ) with different catalysts. (e) Pseudo-second-order fitting of Cr(Ⅵ) removal. (f) Effects of different scavengers on the degradation of Cr(Ⅵ) over Ag/CuFeS2. | |
Besides organic dye, Cr(Ⅵ) salts are typical inorganic dyes that have also been frequently used in the leather industry [40]. They can easily enter into industry dyeing wastewater and causing heath problems severely. Benefited from the existence of the mixed redox-couple Cu(Ⅰ)‒S‒Fe(Ⅲ) inside the tetragonal phase CuFeS2 crystal structure, together with the rise of the CBM resulting in a larger reduction potential in Ag/CuFeS2 QDs, the efficiency and mechanism of Ag/CuFeS2 QDs on Cr(Ⅵ) removal has been studied. As shown in Fig. S4a (in Supporting information), Cr(Ⅵ) reduction under various initial pH values (pH 2, 4, 6, 8, 10) were explored. The results showed that the highest photocatalytic reduction efficiency for Cr(Ⅵ) was at pH 2. The removal rate was 95.7% within 250 min, as shown in Fig. 5d. The fast reaction was benefited from the acidic conditions, as sufficient protons (H+) could promote the transformation of Cr(Ⅵ) to Cr(Ⅲ) of the E0(Cr(Ⅵ)/Cr(Ⅲ)) = +1.33 V [41]. The mechanism of the reduction of Cr(Ⅵ) by Ag/CuFeS2 QDs could be explained by the following Eqs. 1, 5, 6:
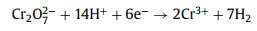
|
(5) |

|
(6) |
Moreover, comparing the performance of CuFeS2 QDs with the Ag/CuFeS2 QDs, the Cr(Ⅵ) removal efficiency of Ag/CuFeS2 QDs in the first 10 min was approximately 6.75 times higher than that of the CuFeS2 QDs. As the reaction progressed, the reaction rate was slower than that of the CuFeS2. The possible explanation was that the reaction rate of Ag/CuFeS2 was very high in the initial reaction period that led to the consumption of a large number of photoelectron (e‒) and thus resulted in the accumulation of h+ in the valence band, so the process of the reaction was hindered [42]. Comparing the two fitting curves by the kinetic models, the pseudo-second-order kinetic model (Fig. 5e) was more suitable than the pseudo-first-order fitting model (Fig. S4b in Supporting information) for the description of the removal process under different catalytic conditions. The fitting parameters were listed in Table S5 (Supporting information). To evaluate the active species during the reaction process, the radical quenching experiments were conducted under the optimal conditions (Fig. 5f). Based on the consequence of the quenching experiments, e‒ and O2·− were identified to be the main active species in Cr(Ⅵ) reduction [43], as molecular oxygen was found to consume part of the photogenerated electrons to produce O2·−. Thus the elimination of O2·− significantly suppressed the photoreduction of Cr(Ⅵ), indicating that O2·− mediated indirect reduction should govern the photoreduction of Cr(Ⅵ) [44]. The addition of NaHCO3 increased the pH, which inhibited the photocatalytic reaction efficiency, while the reaction rate was increased by adding IPA to capture ·OH. Because ·OH had oxidation property, the as-formed Cr(Ⅲ) during the reaction could be re-oxidized to Cr(Ⅵ), which was not conducive to the removal of Cr(Ⅵ); thus the reduction of ·OH by IPA increased the reaction rate. Moreover, the ·OH species may be generated by other pathways, because the potential of the Ag/CuFeS2 valence band was found to be more negative than that of E0(·OH/H2O) = +2.68 V, so h+ could not be oxidized to generate ·OH. The experimental results were also consistent with the structural mechanism.
Combined with the above reaction mechanism study, we further employed Ag/CuFeS2 QDs for the simultaneous Cr(Ⅵ) reduction and RhB degradation to reasonably use the photogenerated electron-hole pairs and maximization of the photocatalytic efficiency. As shown in Fig. S5a (Supporting information), the reduction efficiency of Cr(Ⅵ) and the degradation efficiency of RhB were significantly improved at neutral pH in the Cr(Ⅵ)/RhB mixture solution, in comparison with single pollutant systems at the same pH. In a single pollutant system that only contained RhB or Cr(Ⅵ), 95.2% of RhB and 30.2% of Cr(Ⅵ) were removed by Ag/CuFeS 2 after 2 h natural light radiation. However, in a mixture pollutants solution with both RhB and Cr(Ⅵ), the removal efficiencies for RhB and Cr(Ⅵ) in 2 h were as high as 97.3% and 79.4%, respectively, because the degradation of RhB could accelerate the separation of photogenerated electron-hole pairs to avoid the carriers aggregation and thus improve the reduction efficiency of Cr(Ⅵ). The quenching experimental results (Figs. S5b and c in Supporting information) indicated that O2·− and h+ were the main active species in the catalytic process. Moreover, EPR spectra exhibited high-intensity signals of O2·−, which was generated in the photocatalytic process (Eq. 3). The production of O2·− depended on e‒, and the number of e‒ was closely related to h+, thus the primary function of h+ was the degradation of RhB, whereas the consumption of h+ accelerated part of the electrons to migrate to the surface to carry the Cr(Ⅵ) reduction and the other part would react with O2 to form O2·− to facilitate RhB degradation. Based on the analysis mentioned above, it could be concluded that Ag/CuFeS2 could synergistically promote the photocatalytic efficiency of the Cr(Ⅵ) reduction and RhB degradation.
Furthermore, we used river water and tap water to simulate the environmental-related industrial dyeing wastewater to test the catalytical performance of Ag/CuFeS2 in real dyeing wastewater treatment applications. As shown in Fig. S6 (Supporting information), the efficiency of the Cr(Ⅵ) reduction and RhB degradation was similar to that in deionized water, which indicates that the complex contaminants such as dissolved organic matter, inorganic ions within tap water or natural river water would not significantly influence the photocatalytic performance of Ag/CuFeS2. Moreover, Fig. S7 (Supporting information) showed a well consistent activity remain by employing Ag/CuFeS2 for six consecutive catalytic cycles in Cr(Ⅵ)/RhB mixture solution. After six cycling catalytic tests, Cr(Ⅵ) removal efficiency was well preserved, which confirmed the excellent stability of Ag/CuFeS2. Fig. S5d (Supporting information) presented the XRD patterns of Ag/CuFeS2 before and after the pollutant removal reaction, where the prominent diffraction peaks of Ag/CuFeS2 remained invariable. Moreover, XPS spectra of Ag (Fig. S8 in Supporting information) after the reaction demonstrated that the valence states of Ag atoms were still mixed-valence (Ag(0) and Ag(Ⅰ)), which were steadily deposited on CuFeS2, suggesting significant phase stability of Ag/CuFeS2.
The mechanism of synergistic Cr(Ⅵ) reduction and RhB degradation over Ag/CuFeS2 was illustrated in Fig. 6. At different reaction stages, the Ag atom acted as an effective electron shutter to accept or release electrons [45]. Under sunlight irradiation, the electrons could be excited quickly from VBM to CBM of the CuFeS2 QDs. Subsequently, the electron transfer from the excited CuFeS2 QDs into Ag created an interfacial charge equilibrium and electron accumulation on Ag, which was beneficial for the photocatalysis reaction with contaminants. Also, it was demonstrated by the DFT results that the formation of the impurity level contributed to carrier separation. The excited electrons could be transferred from the CBM of CuFeS2 QDs to Ag(0), followed by the reaction with adsorbed on the surface of Ag/CuFeS2 to produce O2·−. The photogenerated holes left on the VBM of CuFeS2 QDs could react directly with the pollutants. According to the above discussion against the experimental results as well as theoretical calculation, a possible photocatalytic mechanism of the enhanced photocatalytic activity was proposed based on the energy band structures of Ag/CuFeS2 as follows: (1) With the reduction of the bandgap, the material could be excited by natural UV–vis-NIR light, which improved the efficiency of photon utilization; (2) the mixed-valence Ag(0) and Ag(Ⅰ) single atoms formed in Ag/CuFeS2 could effectively separate photon-generated carriers; (3) the metallic Ag(0) had excellent electrical conductivity, which could accelerate the movement of free-electron onto the semiconductor surface, which further enhanced the separation of photogenerated carriers, and thus more free electrons and holes could be obtained to react with the target contaminants; (4) the specific surface area of the spherical CuFeS2 QDs was relatively large, which could absorb more targeted pollutants, accelerating the reaction between photocarriers and target molecules to improve the photocatalytic efficiency.

|
Download:
|
| Fig. 6. Mechanism of simultaneous Cr(Ⅵ) reduction and RhB degradation over Ag/CuFeS2. | |
In this work, we developed a novel single-atomic Ag/CuFeS2 semiconducting photocatalyst for dyeing wastewater treatment. The catalyst consists of the tetragonal phase CuFeS2 QDs decorating with uniformly dispersed mixed-valence Ag single atoms. We discovered that the introduction of single-atomic Ag atoms could facilitate the engineering of the bandgap of CuFeS2 QDs, which is conducive for synergistically reduce the Cr(Ⅵ) and oxidize RhB in dyeing wastewater. Combined with the experimental results as well as DFT calculations, the formation and catalytic mechanisms of monatomic catalysts were illustrated. The narrow bandgap of Ag/CuFeS2 (1.21 eV) promotes the extension of the absorption spectrum from UV–vis to NIR. Meanwhile, the as-formed mixed-valence Ag(0) and Ag(Ⅰ) single atoms in Ag/CuFeS2 can effectively separate photon-generated carriers, thus enhancing the photocatalytic activity toward the degradation of organic dye or the reduction of inorganic dye. These findings provide new insights into the design of single-atomic catalysts for dye removal in dyeing wastewater.
Declaration of competing interestThe authors report no declaration of competing interests.
AcknowledgementsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21777045, 61875119), Distinguished Young Scholar Fund from Natural Science Funds of Guangdong Province, China (No. 2020B151502094), the program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Shanghai Rising-Star Program (No. 19QA1404000), and Shanghai Talent Development Fund. We would like to thank Ceshigo research service "www.ceshigo.com" for his contribution to the X-ray absorption spectroscopy (XAS) measurements. We are grateful to the analysis center at the school of environmental science, engineering, and SUSTech Core Research Facilities (SCRF) at the southern university of science and technology. We express our special thanks to Prof. Le-Le Duan for the help on the UV–vis absorption spectra measurement.
Supplementary materialsSupplementary data associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.040.
| [1] |
M. Ahmad, M. Yousaf, A. Nasir, et al., Sci. Technol. 53 (2019) 2161-2170. DOI:10.1021/acs.est.8b05866 |
| [2] |
S. An, G. Zhang, T. Wang, et al., ACS Nano 12 (2018) 9441-9450. DOI:10.1021/acsnano.8b04693 |
| [3] |
Y. Chen, J. Gao, Z. Huang, et al., Environ. Sci. Technol. 51 (2017) 7084-7090. DOI:10.1021/acs.est.7b00499 |
| [4] |
Q. Ge, X. Feng, R. Wang, et al., Environ. Sci. Technol. 54 (2020) 8022-8031. DOI:10.1021/acs.est.0c01018 |
| [5] |
N. Wu, X. Liu, M. Zeng, et al., J. Nanoparticle Res. 21 (2019) 268-278. DOI:10.1007/s11051-019-4709-9 |
| [6] |
T. Yan, Y. Li, X. Song, J. Wang, Z. Xie, D. Deng, J. Mater. Chem. C 7 (2019) 7279-7287. DOI:10.1039/C9TC01571J |
| [7] |
W. Girma, S. Tzing, P. Tseng, et al., ACS Appl. Mater. Interfaces 10 (2018) 4590-4602. DOI:10.1021/acsami.7b19640 |
| [8] |
J. Zou, Y. Chen, S. Liu, et al., J. Crittenden, Water Res. 150 (2019) 330-339. DOI:10.1016/j.watres.2018.11.077 |
| [9] |
U. Rajaji, K. Murugan, S. Chen, et al., Compos. Part B: Eng. 160 (2019) 268-276. DOI:10.1016/j.compositesb.2018.10.042 |
| [10] |
J. da Silveira Salla, K. da Boit Martinello, G.L. Dotto, et al., Colloids Surfaces A: Physicochem. Eng. Asp. 595 (2020) 124679-124687. DOI:10.1016/j.colsurfa.2020.124679 |
| [11] |
J. Qi, H. Lan, R. Liu, H. Liu, J. Qu, Water Res. 171 (2020) 115448. DOI:10.1016/j.watres.2019.115448 |
| [12] |
C. Zhou, Y. Zhou, B.E. Rittmann, Water Res. 119 (2017) 91-101. DOI:10.1016/j.watres.2017.04.044 |
| [13] |
J. Park, S. An, E.H. Jho, et al., Water Res. 185 (2020) 116242. DOI:10.1016/j.watres.2020.116242 |
| [14] |
J. Gao, H. Yang, X. Huang, et al., Chem 6 (2020) 658-674. DOI:10.1016/j.chempr.2019.12.008 |
| [15] |
Y. Wang, X. Shang, J. Shen, et al., Nat. Commun. 11 (2020) 3043. DOI:10.1038/s41467-020-16742-3 |
| [16] |
S. Woo, S. Choi, S. Kim, et al., ACS Catal 9 (2019) 2580-2593. DOI:10.1021/acscatal.8b03816 |
| [17] |
S. Ma, S. Zhan, Y. Jia, Q. Shi, Q. Zhou, Appl. Catal. B: Environ. 186 (2016) 77-87. DOI:10.1016/j.apcatb.2015.12.051 |
| [18] |
X. Fang, Q. Shang, Y. Wang, et al., Adv. Mater. 30 (2018) 1-7. DOI:10.1002/adma.201705112 |
| [19] |
S.K. Sahoo, Y. Ye, S. Lee, et al., ACS Energy Lett. 4 (2019) 126-132. DOI:10.1021/acsenergylett.8b01942 |
| [20] |
G. Li, Z. Lian, W. Wang, D. Zhang, H. Li, Nano Energy 19 (2016) 446-454. DOI:10.1016/j.nanoen.2015.10.011 |
| [21] |
T.E. Engin, A.V. Powell, S. Hull, J. Solid State Chem. 184 (2011) 2272-2277. DOI:10.1016/j.jssc.2011.06.036 |
| [22] |
M. Zhu, P. Chen, M. Liu, ACS Nano. 5 (2011) 4529-4536. DOI:10.1021/nn200088x |
| [23] |
X. Xiao, L. Ge, C. Han, et al., Appl. Catal. B: Environ. 163 (2015) 564-572. DOI:10.1016/j.apcatb.2014.08.037 |
| [24] |
A. Ghahremaninezhad, D.G. Dixon, E. Asselin, Electrochim. Acta 87 (2013) 97-112. DOI:10.1016/j.electacta.2012.07.119 |
| [25] |
X. Lv, J. Xu, G. Jiang, J. Tang, X. Xu, J. Colloid Interface Sci. 369 (2012) 460-469. DOI:10.1016/j.jcis.2011.11.049 |
| [26] |
A. Ishikawa, T. Takata, J.N. Kondo, et al., J. Am. Chem. Soc. 124 (2002) 13547-13553. DOI:10.1021/ja0269643 |
| [27] |
J. Yan, J. Liu, Y. Ji, et al., ACS Catal. 10 (2020) 8742-8750. DOI:10.1021/acscatal.0c02063 |
| [28] |
R. Shen, W. Chen, Q. Peng, et al., Chem 5 (2019) 2099-2110. DOI:10.1016/j.chempr.2019.04.024 |
| [29] |
W. Liu, Y. Li, F. Liu, et al., Water Res 151 (2019) 8-19. DOI:10.1016/j.watres.2018.11.084 |
| [30] |
T. Liu, B. Liu, L. Yang, et al., Appl. Catal. B: Environ. 204 (2017) 593-601. DOI:10.1016/j.apcatb.2016.12.011 |
| [31] |
Y. Chen, Z. Huang, M. Zhou, et al., Environ. Sci. Technol. 51 (2017) 2304-2311. DOI:10.1021/acs.est.6b04340 |
| [32] |
X. Xiao, Y. Gao, L. Zhang, et al., Adv. Mater. 32 (2020) 2003082. DOI:10.1002/adma.202003082 |
| [33] |
H. Xu, M. Prasad, X. He, L. Shan, S. Qi, Sci. China, Ser. E Technol. Sci. 52 (2009) 3054-3060. DOI:10.1007/s11431-009-0304-0 |
| [34] |
L. Wang, M. Cao, Z. Ai, L. Environ. Sci. Technol. 49 (2015) 3032-3039. DOI:10.1021/es505984y |
| [35] |
R. Chattot, T. Asset, P. Bordet, et al., ACS Catal. 7 (2017) 398-408. DOI:10.1021/acscatal.6b02356 |
| [36] |
I. Park, D.O. Atienza, A.M. Hofstead-Duffy, D. Chen, Y.J. Tong, ACS Catal. 2 (2012) 168-174. DOI:10.1021/cs200546f |
| [37] |
Y. Yu, C. Cao, H. Liu, et al., J. Mater. Chem. 2 (2014) 1677-1681. DOI:10.1039/C3TA14494A |
| [38] |
G.V. Buxton, C.L. Greenstock, W.P. Helman, A.B. Ross, J. Phys. Chem. Ref. Data. 17 (1988) 513-886. DOI:10.1063/1.555805 |
| [39] |
Y. Yin, L. Shi, W. Li, et al., Environ. Sci. Technol. 53 (2019) 11391-11400. DOI:10.1021/acs.est.9b03342 |
| [40] |
J. Ding, Y. Pan, L. Li, et al., Chem. Eng. J. 384 (2020) 123232. DOI:10.1016/j.cej.2019.123232 |
| [41] |
X. Yi, S. Ma, X. Du, et al., Chem. Eng. J. 375 (2019) 121944. DOI:10.1016/j.cej.2019.121944 |
| [42] |
Q. Xu, L. Zhang, B. Cheng, J. Fan, J. Yu, Chem 6 (2020) 1543-1559. DOI:10.1016/j.chempr.2020.06.010 |
| [43] |
D. Xia, W. Xu, Y. Wang, et al., Environ. Sci. Technol. 52 (2018) 13399-13409. DOI:10.1021/acs.est.8b03696 |
| [44] |
G. Dong, L. Zhang, J. Phys. Chem. C 117 (2013) 4062-4068. DOI:10.1021/jp3115226 |
| [45] |
L. Zhang, D.A. Blom, H. Wang, Chem. Mater. 23 (2011) 4587-4598. DOI:10.1021/cm202078t |
 2021, Vol. 32
2021, Vol. 32 

